In: Bisnrrrth: Characteristics, Production
andApplications
ISBN: 978-l-61470-640'3 Editors: K. P. Ghatak and S. Bhattacharya, pp. 145-156 @ 2012 Nova Science Publishers, Inc.Chapter 5
SyNTTTESIS
AI'{D PNOPNRTIES
OF
ND-DOPNN
Br4Tr3Orz
flstnc
rHE
Sorr
CoprsusrloN
TncnxIeuE
A.
(Imar
Al-Amani,
S.
Sreekuntan,
M,
N.
Ahmad
Fuuzi
and K.
A.
Ruzak-School of Materials and Mineral Resottrces Engineering, Universiti Sains Malaysia, 14300 Nibong Tebal, Penang, Malaysia
AssrRAcr
Nd-doped Bi+Ti:Orz ceramics, Bi4-Nd*Ti3O12 (BNT, x
-
0, 0.25, 0'50, 0'75 and l),*"r"
p."put"d using a soft combustion technique and their phase, structure, thermal behavior, grain morphology, dielectric and fenoelectric properties were investigated. The single phase of BIT was successfully obtained at different synthesis temperature, justafter combustion took place
at 450'C.
The thermal analysis confirmed the phase formation with corresponAing temperature. The single phase of BIT was also obtainedwith different Nd doping. It was also shown that Nd doping had a significance change in Raman and Fourier'ir.ansform Infrared spectroscopy (FTIR) spectra. Further increase the sintering ternperature at 900'C did not show the presence of secondary phase in X-ray diffraction
tXnp)
pattem. The average grain length and width decreasedwith
Ndcontent.
The
Curie temperatureof
BNT
also reducedto
434oC.The
remanent polarization, 2P. and coercive field, 2E" of BNT were higher than BIT, whereby the BNT075 ceramics exhibited the rnaximum 2P, and 28" valuei of aboutl8.4 pClcmz and 100.6 kV/cm, respectively.
Keyrvords : Bisrnuth titanate; Doping; Soft contbustion; Lanthanide.
146 A. Umar Al-Amani, S. Sreekantan, M. N. Ahmad Fauzi, et' al
INrnonucrIoN
Ion doping technique
is
a preferable wayto
enhance the electrical propertiesof
many ceramic materials to date. For instance, bismuth titanate (Bi+Ti:Orz or BIT) has been reportedto
exhibit low remanent polarizafion (2P,<
10 pC/crn';, high leakagecurent, low
fatigue resistant, high dielectric loss and high electrical conductivity whichlimit
its application in anon-volatile ferroelectric random access memory technology (NvFRAM)
ll-3].
In
order tosolve these issues, many researchem suggested that ferroelectric properties
of BIT
can be improved by tuning its composition using ion doping technique. Park et al. [4] found that La3*ion successfully doped in
BIT
film
which thefilm
had enhanced P, (16^- 20 pC/cm2), lowleakage
rurr*ni
110:7 A/cmz at 5V) and good fatigue resistant (3x
l0t0
switching.cycles)'Kojima et al. [5] reported that the P, up to
25
pClcm2 with fatigue free after 2x
1010 cycles could be achieved when Nd was partially doped in BIT thin fihn. Chon et al. [5] reported that the Bi: rsSm6 s5Ti3O12 thinfilm
has high P, and good fatigue endurance to reach about 29.5 pClcm2 and4.5x
1010 switching cycles. However, the increase in these values was stronglydependent
on
several things such asthe
annealing temperature andtype
of
annealing, substrate type, bottorn electrode, grain orientation, film thickness etc.Lanthanide doped BIT has been prepared using the conventional solid-state reaction [7-141, sol-gel rnethod [15-25], hydrolysis method [26,277, polymeric precursor method 128,
291, chemical solution deposition (CSD) [30-33], metal organic solution decomposition
(MOD)
[34-39], pulsed laser deposition(PLD) [40],
and metalorganic chemical vapordeposition (MOCVD) [41]. Each method has own merits and drawbacks. Conventionally prepared powder often uses high calcination temperature with repeated grinding in its process
which then produces hard powder and high agglomeration. As an alternative, wet chemical synthesis is beneficial to reduce the calcination temperature and improve the reactivity and
homogeneity
of
the
composition.For
deposition processof
thin
films, the PVD,
CVD, MOCVD andrf
sputtering are costly and the parameter settings are also complex. Sol-gelmethod
is
the simplest methodto
producethin
films. Sol-gel method also offers several advantages including pre- and post-depositionat
low
temperature, easier compositional control and better unifonnity of the films, economical compared to CVD and PVD techniques 13l.To the best of authors' knowledge, very least works on soft combustion technique have been reported. Previously, this technique has been used extensively to prepare a large number
of
technologically useful oxide materials such as refractories, magnetic, semiconductors,dielectric, catalyst, sensors, phosphors, etc. and none oxide materials such as carbides,
nitrides, borides
and
silicidesf42,
43]. This
approachis
convenient, requires simple experimental set up and save time as well as requires low energy consumption [44]. This is because this technique is characterized by self-sustaining solution combustion synthesis in which the exothermicity of the redox chemical reaction is used to produce the useful materials[45]. Recently, our group used glycine to produce praseodymium-doped bismuth potassium titanate and praseodymium-doped bismuth sodium titanate 146, 47). There also a study on praseodymium-doped bismuth titanate, however,
the fuel
agentwas
not
introduced insynthesis process [a8]. In the present study, Nd was partially doped in
BIT
using a noveltySynthesis and Propertis of No-Doped BiaTi3O12. ...
were investigated in terms of phase formation, thermal behavior, structnre, grain morphology, dielectric and ferroelectric properties.
ExpnnrprENTAL
Pnocnnune
Nd-doped
BIT
(Bi4-Nd*Ti3O12) were prepared according to the Nd contentx:
0, 0.25, 0.50, 0.75 and 1, the samples are denoted as BIT, BNT025, BNT050, BNT075 and BNTIO0, respectively. Firstly, bismuth nitrate(Bi
(NO3)3.5H2O, Sigma-Aldrich, 98 %) was dissolved at 40oC in distilled water. Separately, neodymium nitrate (Nd (Nq)3.6H2O, Sigma-Aldrich, 99.9 %) was dissolved in distilled water and citric acid (CoHsOz, Sigma'Aldrich,99.5 %) was used as a fuel agent. The neodymium solution together with citric aqueous were added into the bismuth solutionwith
continuousstining.
The titanium solution was prepared from titanium isopropoxide(Ti
IOCH (CHr)z]+,Aldrich
Chemistry, 97%)
which was initially dissolvedin
2-methaoxyethanol (Sigma-Aldrich, >99). Finally, the titanium solution wasslowly added into the bisrnuth neodymium solution
to
obtain the precursor solution. This solution was adjusted to pH7 with NH+OH (29 % solution). The prepared solution was stirred at 60oC for additional24hlo
obtain a homogeneous solution. After that, the temperature was increased to 80"C to form a dried precipitates. The precipitates were gently crushed using anagate mortar pestle.
The
crushed powderwas
heatedon
a
hot
plate
attached with thermocouple for the soft combustion process to take place, and the temperature was recorded-450"C. The reaction lasted less than 5 min and produced yellowish and greenish powders for BIT and BNT powders, respectively.
Thermal behavior
of
the
dried
powderwas
investigated using Themogravirnetry-Differential Therrnal Analysis, TG-DTA (Linesis). Phase identification was investigated by X-Ray Diffractionwith
Cu-Ku radiation, X-ray diffraction (XRD) (Bruker D8 Advanced). Raman spectrometer (Horiba Jobin-Yvon HRS00UV) and FTIR spectrophotometer (Perkin Elmer) were usedto
investigate the phonon mode and adsorption band, respectively. Themicrostructure
of
the
prepared samples was examined usinga
field
emission scanning electron microscopy, FESEM [Zeiss Supra 55VP PGT/HKL]. To investigate the dielectric and ferroelectric properties, the sintered pellet was coated with silver conductive paint andfired
at
700"Cfor
15 rnin.The
capacitance and loss tangent were measured using an Impedance analyzer (Hewlett Packard 4192A LF). The ferroelectric P-E hysteresis loop was investigated using Sawyer-Tower circuit.Rnsur-r
AND
DlscussloN
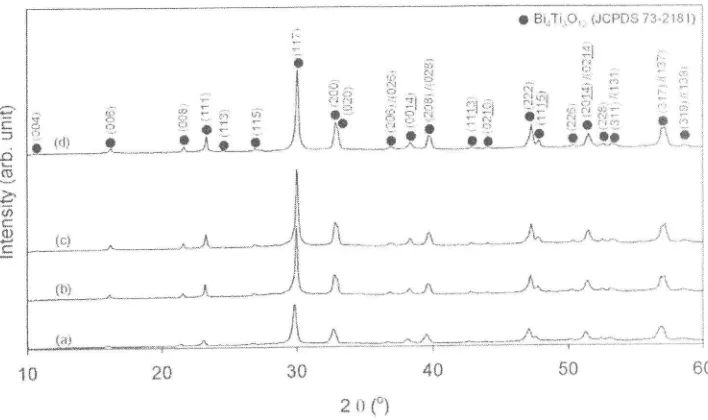
Figure
I
shows theXRD
patternsof
theBIT
powder prepared at different synthesis temperatures. The XRD pattems were compared with standard BIT data (JCPDS 73-2181).It
was found that a stable phase
BIT
with
layered perovskite structure could be obtained at temperatures between 40 and 60'C. Additionally, the stabilityof
phase formation was also achievedin
calcined powderat
500"C.This
indicates thatthe
synthesis temperature is sufficient to obtain the single phase of BIT without further calcination process.148 A. Umar Al-Amani, S. Sreekantan, M. N' Ahmad Fauzi, et. al
t
a.;l
:i!
a
;ll
t.
{'.,
.;,
:
i;
_:i
.i
t
l
I Pi,Ti,O,. {JCPr}$ ?3-?l8li-- * -**A*."---
**A*'n*'*
A
;
l.-^r-.A*^-
-.
-*
-,A-*-*-,,\*-*-A*"
i [image:4.518.94.448.68.278.2] [image:4.518.97.451.336.578.2]20f)
Figure 1. XRD patterns of BIT por,vder at differdnt synthesis temperatures: (a) 40'C, (b) 50"C. (c) 60"C and (d) calcination at 500"C for (c) samples.
,n
I
exoI
800
1000Temperature {oC}
Figure 2. TG and DTA of BIT polvder derived from 60"C.
Figure 2 shows the TG and DTA of BIT powder derived at 60oC. As shown in Figure 2,
the
TG
curve can be divided intofwo
distinct stages. Thefirst
weight loss took place at temperature rangeof
100-
355"C and second weight loss at temperature rangeof
480-505"C. Above 505oC, the reaction was completed as indicated
by
linear curve. The total weight lossof
abaut 42.4 Yo was obtainedfor
BIT
powder. TheDTA
curve showed theq
d
(g
,6
c
{l)
{fr)
s0 40
7A
1S
JU
eu5
16
c
'6
109
rcl
90 \o
;
(,-9
so-c .sl
o
B70
Synthesis and Properlis of No-Doped Bi+Ti:Orz
'..
endothermic peaks at approxirnate 60, 130 and 170"C, while exothermic peak at approxirnate
252,28A,307 and 501"C. The corresponding endotherrnic peaks could be ascribed
to
the vaporizationof
residual water and organic snbstances, which correspondto
a small weight loss. The exothermic peaks hetween 183 and 347"C could be explained by decompositionof
the nitrate and combustion/pyrolysis
of
the
Bi-Ti
organic chelating agent[49]. This
is supported by rnassive weight loss of about 39.8% on the TG curve.A
small exothermic peakat
final
weight loss couldbe
dueto
phase transitionfrom
amorphous oxidesto
BITcompound.
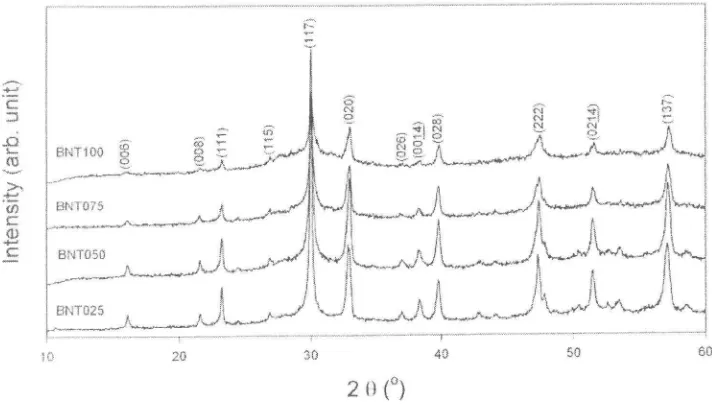
[image:5.509.68.424.191.395.2]2 {} {a) Figure 3. XRD patterns of BNT por'vders.
Figgre 3 shows the XRD patterns of BNT powders with various contents. The diffraction
peaks are inclexed using the standard data of BIT phase (JCPDS^73-2181). It was found that a
single phase was obtained for all compositions that indicates Nd3* ions were fully dissolved in the BIT solid solution. Additionally, the intensity of diffraction peaks were found to decrease ancl to have a broaden peak with Nd doping. Broadening of the peak indicates the decrease
of
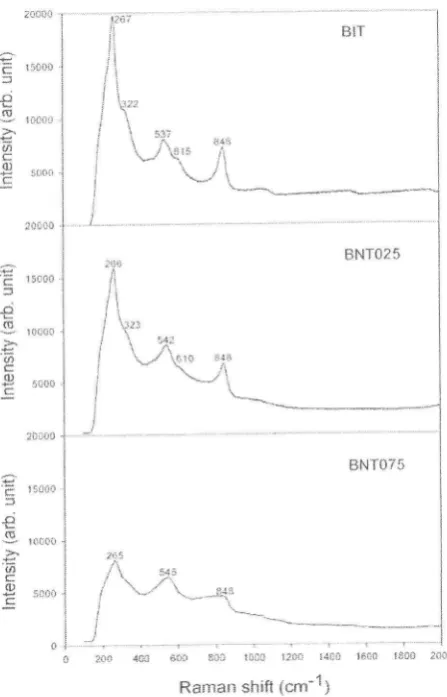
crystallite size [47].Figure 4 shows the Rarnan spectra of BIT, BNT025 and BNT075 at room temperature
from 100 to 2000 cm-1. The BIT exhibits a sharp and narrow peak with high intensity in five
Raman modes at267
,322,
537,615 and 848 cm-r.These modes characteristic changed with Nd content, which corresponded to the decrease
in intensiry and the increase in peak width. The peaks became broader and only showed three Raman modes as shown
in
BNT075. The interactionof
intemal modeof
TiOo octahedra appeared above 200 cm-1 dueto
large intragroup binding energyin
the octahedra and the mnch smaller lrass of Tia* ions [50, 5l ].In the case of BIT, the mode at 267 crn-l ascribed from the torsional bending of TiOo and those at 615 ancl 848 cm-l assigned to the stretching vibration. The mode at 322 cm-l was
from a combination
of
the stretching and bending vibrations. The modeat
537 cm-t was ascribed to the O-Ti-O stretching vibrations.149
=
J
.ciL
f0
z
qc
o
c
150 A. Umar Al-Amani, S. Sreekantan, M. N. Ahmad Fauzi, et' al
20css
'E l5*0t
-cj
I taoo,
>r
dt
c g scoo
c
eSttE
'* lsaoo
d
dl
+ t0060
>
'4 g 5oso
c
?*009
'E
''osd
€
*9 ,nooo
Et4
o
_--^=
cl-***?-
--"--t-.--o t4n ,{co 6t6 S$S 1883 t:tf l48e 1*fd l80s 200f
Raman shifl icnl'1)
Figure 4. Raman spectrum of BIT, BNT025 and BNT075 at room temperature.
G-t{
c0:
.co0Ffc-o lJ
Bi"o,
Ti-o
3r0$ 2t$0 ?006 150s
wsvenumher (cm't)
o #q a 6
I
$ C{
.aB F 1: :i: 1, t. i, ,{-::l f. :.ir*: .Sli ,{.i: .*,*
.l ;F,lt
iL: i.{, 1! ]la :g $ $ "t: $, .Yr a t; :1, i$. [image:6.516.153.377.67.416.2]'$::i:.,x,
[image:6.516.142.388.467.655.2]Synthesis and Propertis of No-Doped Bi+Ti:Orz ' '.
The FTIR spectra of BIT, BNT025 and BNT0?5 are shown in Figr.rre 5. Three adsolption bands appeared at 815, 587 and 418 cm-r were ascribed the perovskite-layered structure
of
BIT. The band at 815 and 587 crn-r belong to the Ti-O stretching vibrations while the peakaround
418
cm-r correspondedto
the
Ti-O
bending vibration.The
broad peak above 3000 cm-r and a small peak at 1645 cm-r were attributed to the stretching vibrationof
O-H groups and the bending vibrationsof
absorbed molecular water and 2-methaoxyethanol, respectively. The weak peak at 2365 crn-t belonged to the stretching vibrations of CO2, whilethe stretching vibration for carboxylic group (-COOH) was indicated by the presence of band at 1385
"*-t,
Th" bending vibrations of C-O were detected at 1052 cm-I, indicating that a few [image:7.508.82.420.291.482.2]organic groups were absorbed on the surface ofthe powders.
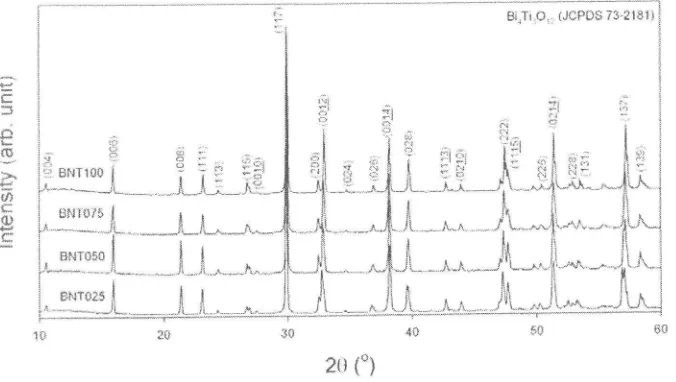
Figure 6 shows the XRD patterns of
BIT
and BNT ceramics sintered at 900oC for 3 h. Regardlessof
the Nd contents, all the ceramic samples were pure phase with no secondary phase.This
indicates thatthe
stabilityof BIT
phasewith
Nd
dopingat high
sintering temperature. Additionally, the intensityof
diffraction peaks was foundto
decrease with increasingNd
content.It
also suggested thatNd
doping hasa
significance effect on the crystallite size and lattice parameter of BIT.Bi,Ti.*,, (JcPos 73^:lsll
20 {o)
Figure 6. XRD pattems of BNT ceramics sintered at 900oC for 3 h.
Table 1. Average grain length and width of
BIT
and BNT ceramicsCompositions Average length (pm) Average width (pm)
BIT 10.66 t.5'7
BNTO25 8. l4 r.33
BNTO5O 6.88
l.l0
BNTO75 6.37 0.90
BNTlOO 5.
l9
0.78Figure 7 shows the SEM micrographs of
BIT
and BNT ceramics.All
the ceramics withdifferent Nd content exhibited rod-like grains. The grain size of BIT was larger than that
of
151
=
c
-;
L
{B
's
f,
0)
$
, gNftO0 t
t
Bri l{i 5 I
*.-
*--lL,
I
afiroso I
**. " _. ^it*.. .
I
8r.rr025
il
'.r.i
[image:7.508.62.449.552.636.2]t52 A. Umar Al-Arnani, S. Sreekantan, M. N. Ahmad Fauzi, et. al
BNT, both in terms of grain length and width. Additionally, the grain size of BNT ceramics decreased
with
increasing Nd content. The calculated average grain length and thickness is presented in Tablel.
This indicates that Nd could act as grain growth inhibitor.gli I0t5
:r:
,l
[image:8.514.94.433.114.643.2]Synthesis and Propefiis of No-Doped Bi+Ti:Orz ...
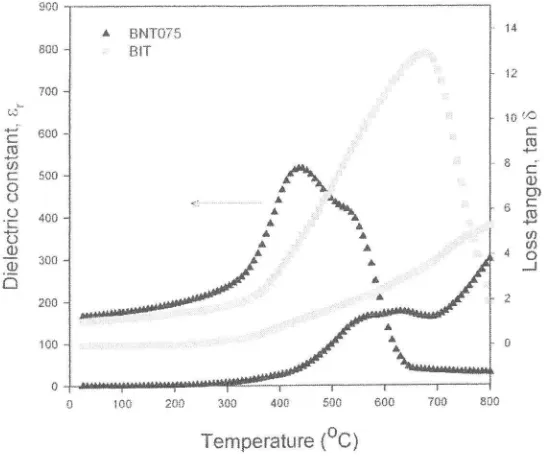
Figure
8
shows the temperature dependenceof
the
dielectric propeftiesof BIT
andBNT075 ceramics, measured at
I
MHz. With increasing temperature, the dielectric constant increases and has a maximum peak at the Curie temperature T.. The T" ofBIT
is 675"C. On the other hand, BNT075 has lorver T" valueof
434"C. This indicates that theT.
decreasedwith
Nd
doping. Additionally, the dielectric peak broadens with Nd doping, indicating thediffusing
of
the ferro-paraelectric phase transition. The paftial substitutionof
Ndfor
Bi
in BNT significantly reduced the loss tangent as shown in Figure 8.Temperature {oC)
Figure 8. Dielectric properties of BIT and BNT075 as a function of temperature.
153
ft,a
c
(osc
(u o) ac -nt a .A 4O J 2d
600($
a c 500
o o .g L 400
()
(l)
E
300(]
i1u 15, ttl I li: s (l ., "it fl;-5 I
:
l
l{
-1i i
{a) ilij
*
,il
*
.t
- i ...1
'.
.I..-1'-a'.
*
.'ir
t
e{t b
3
is uJ
*'
'|'i
Eir BtlrCzS FilrtlFC SNT0?$ tsri11{i0
{js*rps:r'!Jon
;i .50 .25 0 2$ 53 j5 103 F {kviint
154 A. Umar Al-Amani, S. Sreekantan, M' N' Ahmad Fauzi, et. al
The ferroelectric hysteresis loops of BIT and BNT ceramic measured at the applied field of 75 kVlcm are illustrated in Figure 9(a). The remanent polarization, 2P, and coercive field, 2E. were plotted
in
Figure 9(b).As
shownin
both figures, the 2P, and 2E" are strongly dependent on Nd content. The 2P, and 2E. increase from 0 to 0.75 and decrease beyond-that. Then, the BNT075 ceramics shows the maximum 2P, and 2E, valuesof
18.4pClcnz
and 100.6 kV/cm, respectively. These values are higher than that of BIT ceramicof
10.9 pC/cm2 and 50.6 kV/cm. This indicates that Nd doping improved ferroelectricity'CoNcr.usron
The single phase of
BIT
was obtained at different synthesis temperatures using the soft combustion technique. Nd-doped Bi+Ti:Orz was successfully investigated in tennsof
phase formation, structure,thermal
behavior,grain
morphology,dielectric
and
ferroelectric properties. Thermal analysis showed the reaction was highly exothermic and completed at 500"C. The influence of Nd doping on Raman mode and FTIR spectra was clearly observedin both analysis. Additionally, the grain size both the length and width exhibited in small size at higher Nd content. The maximum dielectric peak was found
to
be broaden and to have a reduced Curie temperature,T"
with
Nd
doping.The
ferroelectric properties was also improved with Nd content.ACXNOWLEDGMENT
The authors appreciate the technical support provided by School of Materials and Mineral
Resources Engineering, Universiti Sains Malaysia, Prof. Uematsu Keizo and Assoc' Prof. Tanaka Satoshi of Nagaoka University of Technology, Japan. This research was supported by
USM-RU grant 1001/PBahan/8042018 and l00liPBahan/81 1069.
RgrnRENcrs
t1]
Sedlar, M., Sayer, M., Ceramics International,22,3,1996.t2)
Yamaguchi,M.,
Kawanabe,K.,
Nagatomo,T.,
Omoto, O., Mrtlerials Science and Engineering B, 41,l,
1996.t3]
Madeswaran, S., Giridharan, N. V., Jayavel, R., Materials Chemistry and Physi$, $$,1,2003.
t4]
Park, B. H., Kang,B.
S., Bu, S. D., Noh,T. W',
Lee, J., Jo, W', Nature, 401,6754,
1999.
15]
Kojima,T.,
Sakai,T.,
Watanabe,T.,
Funakubo,H.,
Saito,K.,
Osada,M.,
AppliedPhysics Letters,80, 15, 2002.
t6l
Chon, U., Kim, K. 8., Jang, H. M.,Yi,
G. C', Applied Physics Letters, 79,2001'Synthesis and Propertis of No-Doped Bi+TirOrz
...
155t8]
Kim,
J. S., Lee, S.Y.,
Lee, H. J., Ahn, C. W.,Kim,
I.
W., Jang,M.
S., Journalof
Electroceramics, 21, 1-4 SPEC. ISS., 2008.
19]
Chen, M., Liu, Z. L., Wang, Y., Wang, C. C., Yang,X.
S., Yao, K. L., Physica StatusSolidi (A) Applied Reseqrch, 2A0, 2, 2A03.
[10]
Chen,M.,
Litt,Z.
L.,
Wang,Y.,
Wang,C.C.,
Yang,X.
S., Yao,K.L.,
Physica B: Condensecl Matter, 352, l -4, 2004.[11]
Mao, X. Y., Mao, F. W., Chen,X.8.,
Integrqted Ferroelectrics,79,1,2006.Uzl
Tang,Q. Y.,
Kan,
Y.
M.,
Li, Y.
G.,
Zhang,G.
J.,
Wang,P.
L.,
Solid
StateC ommun icati on s, 1 42, 1 -2, 2007 .
[3]
Wang, W., Zhu, J., Mao, X. Y., Chen,X.8.,
Journal of Physics D: Applied Physics,39, 2,2006.114]
Pineda-Flores, J. L., Chavira, E., Reyes-Gasga, 1.,Gonzai
lez, A. M., Huanosta-Tera,A,
Jow'nal of the European Ceraffiic Society, 23 , 6, 2003 .[15]
Chen, X. Q., Qi, H. Y., Qi, Y. J., Lu, C. J., Physics Letters, SectionA:
General, Atomic qnd Solid State Physics,346, l-3,2005.[16]
Cui, L.,Hu, Y. J., Physica B: Condensed Matter, 444,1,2009.[17]
Fan, S., Zhang, F., Wang, P., Ren, Y.,Journal of Rure Earths,26,4,2008.t18]
Kim, J., Kim, J. K., Heo, S., Lee, H.S.,Thin Solid Films,503,l-2,2Q06.[19]
Ke,H.,
Wang,W.,
Chen,L.,
Xu, J.,
Jia,D., Lu,
Z.,Zhou,Y'
Journalof
Sol-Gel Science and Technology, 53,l,
l20l
Cuo, D. Y., Li, M. Y., Liu, J., Fu, L., Wang, J., Yu, B. F., Yang, 8., Materials Science nnd Engineering B: Solid-State Materialsfor
Advanced Technology, 142,2'3,2007.l2l)
Bae, J. C., Kim, S. S., Choi, E. K., Song, T. K., Kim, W. J., Lee, Y .1., Thin Solid Filns,472.l-2,20A5,
1221
Tajiri, T.,
Sumitani,K.,
Haruki, R., Kohno,A.,
IEEE Transactionson
Ultrasonics, Ferroelectrics, and Frequency Control, 54, 12, 2007.l23l
Lu, C. J., Qiao, Y., Qi, Y. J., Chen, X. Q.,Zhu,I.S.,Applied
Physics Letters,87,22, 2005.l24l
Tomar, M. S., Melgarejo, R. E., Singh, 5.P., Microelectronics Journal,36,3-6,20A5.I25l
Bae, J. C.,Kim,
S. S., Park,M.
H., Jang,K.
W., Lee,Y.
I.,
Song,l.5.,
Journalof
Cr1,s 701 Grow lh, 268, 1 -2, 2004.
[26]
Kan,Y.M.,
Zhang, G. J., Wang, P. L., Cheng,Y.B., Jottnql
of the European Ceramic Society,28, 8, 2008.t27l
Kan,Y.,
Jin,X.,
Zhang, G., Wang, P., Cheng,Y. 8.,
Yan, D., Journal af MaterialsC hem is try, I 4, 24, 2004.
l2S]
SimApres,A. 2.,
Quinelato, C., Ries,A.,
Stojanovic,B.
D., Longo, E', Varela, J. A., Materials Chemistry and Pltysics, 98, 2-3, 2006.129) SirnApes, A. 2., Stojanovic, B. D.,Zaghete, M. A., Riccardi, C. S., Ries, A., Moura, F', Longo, E., Varela, J. A,, Integrated Ferroelectrics, 60,2004.
[30]
Yang,8.,
Zhang,D.M.,
Zhou, B., Huang, L.H.,Zheng, C.D., Wu, Y. Y., Gtto, D. Y,, Yu, J., Journal of Crystal Grov,th, 310, 21, 2008.[3 I
]
Chen, Y. C., Hsinng, C. P., Chen, C. Y., Gan, J. Y., Sun, Y. M., Lin, C. P., Thin SoliclFilms,5l3,l-2,2AA6.
I32l
Chen,Y.
C., Sun,Y.M.,
Lin, C. P., Gan,J.Y.,
Journal of Crystal Growth,268,1-2, 2004.1s6 A. Umar Al-Amani, S. Sreekantan, M. N' Ahmad Fauzi, et. al
t34l
Giridharan, N.,Supriya, S.,Thin SolidFilms,5l6,
16, 2008.t35]
He, H., Huang, J., Cao, L., Wang, L., Microelectronic Engineering, 85, 3, 2008't36]
Liu,W.L.,Xia,H.R.,Han,H.,Wang,X.Q,MaterialsResearchBulletin,39,9,2A04.
t37]
Liu,
w.
L.,
Xia, H. R., Han, H., Wang,X.
Q., Journal ofcrystal
Growth,264,l-3,
2004.l3S]
Liu, W. L., Xia, H. R., Han, H., Wang, X. Q., Materials Letters,58,24,2004'[39]
Liu, W. L., Xia, H. R., Han, H., Wang, X. Q., Iournal of Solid State Chemistry, 177,9,
2004.t40]
Zhang, S. T., Zhang, X. J., Cheng, H. W., Chen,Y'
F', Liu,Z'
G', Ming'N'
B', Hu, X' B., Wang,LY
., Applied Physics Letters, 83,21,2003 't41]
Kojima,T.,
Sakai,T.,
watanabe,T.,
Funakubo,H.,
Saito,K.,
osada,M.,
AppliedPhysics Letters, 80, 2002.
t42l
Patil,K. c.,
Aruna,s.
T.,Mimani,T.,
current opinion in solid stqte and Materials Science, 6, 6,2002.l43l
Lno, s., Tang,Z.,Yao, W., Zhang,z' Microelectronic Engineering, 66, l-4,2043.t44l
Berger, D., Matei, C., Papa, F., Voicu, G., Fruth, Y., Progress in Solid State Chemistry,35,2-4 SPEC. ISS., 2007.
t45]
Patil, K.C,
Bulletin of Materials Science, 16, 6, 1993'[46]
Ng, C. Y.,Razak, K. A., Journal of Alloys and Compotrnds,509,3,I47)
Razak, K. A., Yip, C. J., Sreekantan, S., Journal of Alloys and Compounds, 509' 6,t4g]
Goh, P. Y., Razak,K.A.,
Sreekantan, s., Journal of Alloys andcompounds,475,l'2,
2009.
I4gl
Du, X., Xu, Y., Ma, H., Wang, J., Li,x.,
Journal of the American ceramic society,9A, 5,2007.t50]
Kojima, 5., Ferroelectrics, 239,