Efficacy of Artesunate-Amodiaquine
and Quinine-Azithromycin Combination in Treatment
of Uncomplicated Falciparum Malaria in Children
Ayodhia Pitaloka Pasaribu, Elvina Yulianti, Munar Lubis, Syahril Pasaribu, Chairuddin P. Lubis
Department of Child Health, Medical School - USU/H. Adam Malik Hospital, Medan
Abstract: Resistance of malaria treatment is a major problem. Because of the highly incidence of malaria falciparum resistance in Indonesia, Department of Health, Republic of Indonesia since end of 2004 change the treatment standard into artesunate - amodiaquine combination. The aim of this study was to compare the efficacy of artesunate-amodiaquine with quinine-azithromycin combination as treatment for uncomplicated malaria falciparum in children. We conducted a randomized, open label, clinical trial since August 2006 until September 2006 in Panyabungan, Kabupaten Mandailing Natal, North Sumatera Province, in school age children between 5 – 18 years. Group I received artesunate 4mg/bw/po combined with amodiaquine 10mg/bw for 3 days, Group II received quinine oral for 7 days, 10mg/bw/divided in 3 doses for 4 days continued with 5mg/bw/divided in 3 doses for 3 days combined with azithromycin 10mg/bw/po for 3 days. Parasitemia counted at the day of 0, 2, 7 and 28. Peripheral blood smear was done in 700 children with suspected malaria, 232 were positive for falciparum malaria. Then, randomly separated into two groups, 116 received artesunate-amodiaquine and 116 received quinine-azithromycin. End of study, we found 114 children in group I and 106 children in group II. Peripheral blood smear at day 2, 7 and 28 showed 100% cure rate for both group (p = 0,001), respectively. Tinnitus adverse event was found in 6 children in Group II (p = 0,042). Quinine-azithromycin combination can be use as an alternative treatment for uncomplicated falciparum malaria in children.
Keywords: artesunate-amodiaquine, quinine-azithromycin, falciparum malaria, parasitemia, uncomplicated
Abstrak: Resistensi pada pengobatan malaria merupakan hal yang penting. Oleh karena tingginya angka resistensi malaria falsiparum di Indonesia, Departemen Kesehatan Republik Indonesia sejak akhir tahun 2004 mengubah standar pengobatan malaria falciparum dengan menggunakan gabungan artesunat-amodiakuin. Tujuan dari penelitian ini untuk membandingkan efikasi gabungan artesunat-amodiakuin dengan kinin- azitromisin sebagai pengobatan malaria falsiparum pada anak. Suatu penelitian uji klinis, acak, terbuka dilakukan sejak bulan Agustus 2006 hingga September 2006 di Penyabungan, Kab. Madina Propinsi Sumatera Utara, pada anak penderita malaria antara usia 5 tahun sampai 18 tahun. Kelompok I mendapat pengobatan per oral artesunat 4mg/kgbb digabung amodiakuin 10mg/kgbb selama 3 hari, Kelompok II mendapat pengobatan kinin per oral selama 7 hari dengan dosis 10mg/kgbb/3dosis selama 4 hari dilanjutkan 5mg/kgbb/3dosis selama 3 hari digabung azitromisin per oral selama 3 hari dengan dosis 10mg/kgbb. Parasitemia dinilai pada hari ke-0, 2, 7 dan 28. Pemeriksaan darah tepi dilakukan pada 500 anak yang diduga malaria,didapatkan 232 anak positif malaria falsiparum. Kemudian secara acak dibagi menjadi dua kelompok, 116 anak mendapat artesunat-amodiakuin dan 116 anak mendapat kinin-azitromisin. Pada akhir penelitian didapatkan 114 anak pada kelompok I dan 106 anak pada kelompok II. Dari hasil pemeriksaan darah tepi pada hari ke-2,7 dan 28 pada kedua grup didapatkan angka kesembuhan 100% pada kedua kelompok (p = 0.0001), secara berurutan. Efek samping telinga berdenging dijumpai pada 6 orang anak pada kelompok kedua (p = 0.042). Gabungan kinin-azitromisin bisa digunakan sebagai alternatif pengobatan pada malaria falsiparum tanpa komplikasi.
INTRODUCTION
Malaria has become a priority for the international health community that affecting deaths in children and pregnant women and also reduce work productivity. Estimates of annual mortality caused by malaria ranging from 1,5 – 2,7 million people, especially children and pregnant women.1-4
The spread of chloroquine-resistant Plasmodium falciparum has had a major impact on both the health of residents in areas where the parasite is endemic. Chloroquine treatment is now ineffective in most areas of the world. The usual replacement, pyrimethamine-sulfadoxine, is rapidly losing efficacy in many areas.5,6
New antimalarial regimens, urgently needed because of the worldwide development of multi drugs resistance, should aim to cure patients no longer responding to standard therapy.7
Artemisinin based Combination Therapy (ACT) is increasingly being advocated to replace chloroquine as standard treatment. The concept of ACT is based on the use of two drugs with different modes of action: an artemisinin-derivative that causes rapid and effective reduction of parasite biomass and gametocyte carriage and a partner drug that has a longer duration of action. The hypothesis is that these two drugs together will achieve effective clinical and parasitological cure, protect each other from the development of resistance by P. falciparum, and reduce the overall rate of malaria transmission.8
Department of Health, Republic of Indonesia since end of 2004 had changed the standard treatment for falciparum malaria with artesunate-amodiaquine as the first line drug replacing chloroquine.1
Artesunate is a water soluble derivative of dihydroartemisinin that has ability to reduce gametocyte carriage rate and used to treat uncomplicated falciparum malaria.9
Amodiaquine is a 4-aminoquinoline similar in structure and activity to chloroquine in preventing malaria.10
A study in Burkina Faso revealed combination of artesunate-amodiaquine is very effective and can be used as first-line treatment for uncomplicated malaria.11
Effective quinine monotherapy for malaria has been available for more than 350 years, quinine is also inexpensive and widely available, but its side effects are not
insignificant, and, in many countries, resistance has precluded its use as monotherapy.6,7
Azithromycin, the most potent macrolide antimalarialwith a long half-life (68 hours), demonstrates synergismwith quinine against P. falciparum in vitro, suggesting clinical utility as a combination therapy.12
In this study we compare the efficacy of artesunate-amodiaquine as treatment for uncomplicated falciparum malaria in children.
METHODS
Place and Time. This study was conducted from August to September 2006 in 6 districts, Panyabungan Jae, Adian Jior, Gunung Manaon, Pagarantonga, Gunung Baringin and Purba, Kabupaten Mandailing Natal, Sumatera Utara Province.
Study Design. This is a randomized, open label clinical trial which done in primary to high school children in those area. It has been approved by Ethic Committee of University of Sumatera Utara and it had got the inform consent from the sample’s parents before it was commenced.
Study Population. Seven hundred children from primary to high school whose 5-18 years old which suspected positive malaria were screen through thick and thin blood smear examination. If falciparum malaria was founding the blood smear, will be put in inclusion criteria. After filling the inform consent, anamnesis, physical examination and blood smear were taken. Exclusion criteria include 1) cannot finish the study completely, 2) severe malaria, 3) do not take drugs properly. Participants who fulfill the inclusion criteria randomly selected into one of the two groups by using sample random sampling, the first group is artesunate plus amodiaquine and second group is quinine plus azithromycin. Group I (A) recieved oral treatment of artesunate 4mg/kgbw combine with amodiaquine 10mg/kgbw for 3 days. Group II (B) recieved oral quinine for 7 days 10 mg/kg/3 doses for 4 days then continued with 5 mg/kg/3 doses for 3 days combine with oral azithromycin for 3 days 10 mg/kgbw. All antimalarial drugs given after meal.
were done during study. Physical examination and repeated blood smear examination were done on day 2,7 and 28. The body weight was measured with MIC scale (sensitivity 0,05 kg) and height MIC scale (sensitivity 0,1 cm). Nutritional status was measured with standard anthropometry technic based on CDC NCHS-WHO. Thick and thin blood smear was prepared with giemsa based on procedure and read by trained analyst. Sexual and asexual parasites was counted in 200 leucocyte. Main result of the study was cure, define as lost of parasites without recrudescence in 28 days of observation.
Statistical Analysis. Data was processed with SPSS for windows 14 (SPSS Inc, Chicago). Data analysis to found out the results before and after treatment in both groups were test with Wilcoxon rank. Characteristic data and adverse events was analysed with Pearson chi square. Tests were significant if p<0.05.
RESULTS
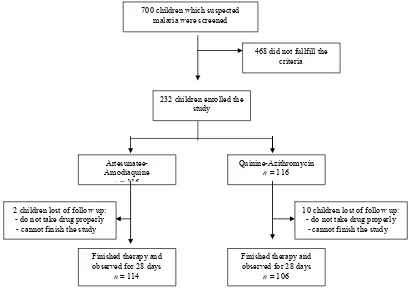
From the beginning of the study measurement were done in 700 children who suspected with malaria. During study we
found 232 children with positive falciparum malaria, which divided into two groups: 116 children in group I (artesunate-amodiaquine) and 116 children in group II (quinine-azithromycin). After treatment only 220 children completed the study. (Figure).
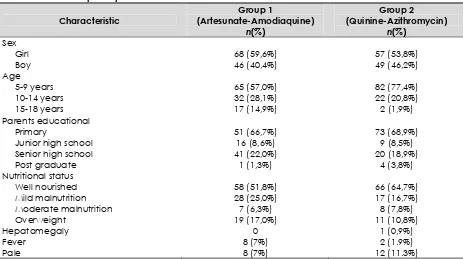
Distribution and characteristic sample is similar in both groups (Table 1). Hepatomegaly only found in 1 person and splenomegaly in 3 persons in group II, eventhough this is an endemic area. Theoritically, from endemic malaria area we can found unspecific clinical symptoms such as cough, diarrhea, headache or even dyspnea but in this study we only found pale and fever (Table 2).
Cinchonism, like tinnitus after the use of quinine found in 6 persons in group quinine-azithromycin, meanwhile nausea and vomiting more common in group artesunate-amodiaquine (8 persons) like showed in Figure 2.
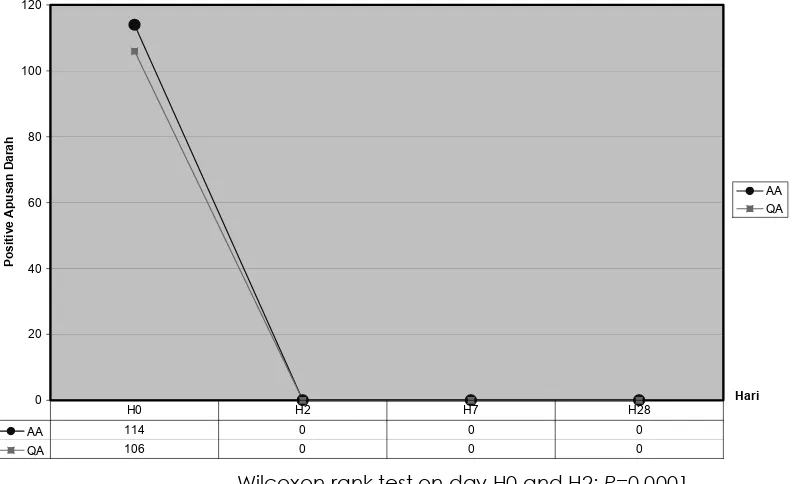
After observation for 28 days we found the same cure rate in both groups, from blood smear examination we found lost of parasites on day 2 and still negative since day 28 (Figure 3).
468 did not fullfill the criteria
232 children enrolled the study
Artesunatee-Amodiaquine
n= 116
Quinine-Azithromycin
n = 116
10 children lost of follow up: - do not take drug properly - cannot finish the study 2 children lost of follow up:
- do not take drug properly - cannot finish the study
Finished therapy and observed for 28 days
n = 114
Finished therapy and observed for 28 days
n = 106 700 children which suspected
malaria were screened
Table 1.
Characteristic of the participant
Characteristic
Group 1
(Artesunate-Amodiaquine)
n(%)
Group 2
(Quinine-Azithromycin)
n(%)
Sex
Girl 68 (59,6%) 57 (53,8%)
Boy 46 (40,4%) 49 (46,2%)
Age
5-9 years 65 (57,0%) 82 (77,4%)
10-14 years 32 (28,1%) 22 (20,8%)
15-18 years 17 (14,9%) 2 (1,9%)
Parents educational
Primary 51 (66,7%) 73 (68,9%)
Junior high school 16 (8,6%) 9 (8,5%)
Senior high school 41 (22,0%) 20 (18,9%)
Post graduate 1 (1,3%) 4 (3,8%)
Nutritional status
Well nourished 58 (51,8%) 66 (64,7%)
Mild malnutrition 28 (25,0%) 17 (16,7%)
Moderate malnutrition 7 (6,3%) 8 (7,8%)
Overweight 19 (17,0%) 11 (10,8%)
Hepatomegaly 0 1 (0,9%)
Fever 8 (7%) 2 (1.9%)
Pale 8 (7%) 12 (11.3%)
Table 2.
Blood smear in preliminary observation
Group 1
(Artesunate-Amodiaquine)
n(%)
Group 2
(Quinine-Azithromycin)
n(%)
P
Parasitemia
≤ 5 000/µl 32 (28.1%) 33 (31.1%) 0.585
5 000 – 10 000/µl 67 (58.8%) 55 (51.9%)
10 000 – 15 000/µl 14 (12.3%) 15 (14.2%)
15 000 – 20 000/µl 1 (0.9%) 3 (2.8%)
P<0.05
17
8
1
13
6 6
0.567 0.68 0.042
0 2 4 6 8 10 12 14 16 18
Kelompok I Kelompok II P
Efek Samping setelah Pengobatan
Sakit Kepala
Mual Muntah Tinnitus
*P<0.05
0
Figure 3. Successful of treatment after day 2, 7 and 28 from blood smear examination
DISCUSSION
The results of combination artesunate (4mg/kgbw/day for 3 days) and amodiaquine (10mg/kgbw/day for 3 days) in children with falciparum malaria from multicentre study in Africa, cure rate were varies between 91% to 98%.13
This is the first open label clinical trial conducted in school age children. From this study, we found 100% cure rate in artesunate-amodiaquine group with the same dose during 28 days of observation.
A study by Mutabingwa in Tanzania reported recrudescence rates by day 28 was 11,2% in artesunate-amodiaquine therapy,14
meanwhile from our study we did not found any recrudescence from artesunate-amodiaquine group. One study monitored the in vitro sensitivities of P. falciparum isolates pre deployment and during the deployment of artesunate plus amodiaquine treatment in Mlomp, Casamance (Southwestern Senegal) during 2000 to 2004, parasites remained susceptible to both drugs.15
Another study found adverse events after consuming combination of this drugs are rash, anemia, convulsion, dehydration and respiratory problem.16
Adverse events that appear during observation of artesunate-amodiaquine in our study were 17 headache
(14,9%), 8 nausea/vomiting (7,0%), 1 tinnitus(0,9%).
Quinine is an anti malarial which is widely used but with dangerous side effect and already resistance as monotherapy.6
For children with uncomplicated malaria in areas where parasites remain sensitive, the WHO recommend to treat patients with an oral regimen (8 mg/kg of body weight three times per day) for 7 days.17
The in vitro data reported that azithromycin will have clinical utility in combination with other anti malarial agents.5
Azithromycin is an optimizing combination because its in vivo activity with longer half life (68 hours) and has synergic effect with quinine for treating falciparum.18
In 1995, daily azithromycin showed an efficacy of 83% in preventing P. falciparum malaria in highly immune adult males in Western Kenya.19
When used in combination, quinine-azithromycin have the role of quickly reducing the initial parasite biomass, whereas azithromycin with its longer half-life has to reliably eliminate the remaining parasites.20
(72%; 95% CI, 50;84), and Kenya (83%; 95% CI, 68.5;91.1).5
From this study we found 100% cure rate in quinine-azithromycin group and no recrudescence was found during 28 days of observation. In 56 (88%) of the 64 participants in azithromycin-chloroquine study in India, resolution of fever and parasitemia occurred by day 3, and, in 61 (97%) of 63 participants, it occurred by day 7.6
Adverse events more common in quinine-azithromycin group, mostly related to cinchonism and electrocardiogram changes (QT intervals).20
In this study we found adverse events in quinine-azithromycin group such as: 6 tinnitus (5,7%), 13 headache, 6 nausea/vomiting.
We concluded that, artesunate-amodiaquine combination therapy are well tolerated and had high cure rate in uncomplicated falciparum malaria treatment in children. Artesunate-amodiaquine group has less adverse events compare to quinine-azithromycin group and lost of follow up more common in quinine-azithromycin group. It is probably because the use of this drug takes longer times so the obeidity is lower. Further study is needed in children to observe the optimum dose and duration of quinine-azithromycin combination therapy.
REFERENCES
1. Gebrak malaria. Pedoman tatalaksana kasus malaria di Indonesia. Departemen Kesehatan RI, 2005.h.1-16
2. Penatalaksanaan kasus malaria di Indonesia. Departemen Kesehatan RI, 2002.h.2-18
3. Greenwood BM, Bojang K, Whitty CJ, Targett GA. Malaria. Lancet 2005; 365:1487-98
4. Bell D, Winstanley P. Current in the treatment of uncomplicated malaria in Africa. Br Med Bull. 2004; 71:29-43 5. Ohrt C, D George, Willingmyre, Lee P,
Knirsch C, Milhous W. Assessment of azithromycin combination with other antimalarial drugs against plasmodium falciparum in vitro. Antimicrob Agents and Chemother. 2002; 2518-24
6. Dunne MW, Singh N, Shukla M, Valecha N, Bhattacharyya PC, Dev V, et al. A multicenter study of azithromycin, alone and in combination with chloroquine, for the treatment of acute uncomplicated plasmodium falciparum malaria in India. J Infect Dis. 2005; 191:1582-8
7. Kremsner PG, Krishna S. Antimalarial combinations. Lancet. 2004; 364:285-294
8. Martensson A, Stromberg G, Sisowath C, Msellem MI, Gil JP, Montgomery SM, et al. Efficacy of artesunate plus amodiaquine versus that of artemether – lumefantrine for the treatment of uncomplicated childhood plasmodium falciparum malaria in Zanzibar, Tanzania. Clin Infect Dis. 2005; 41:1079-86
9. The use of animalarial drugs, Report of an Informal Consultation. WHO. Geneva 2001.h.1-12
10. Antimalarial drug combination therapy. Report of a WHO technical consultation, 2004.h.5-36
11. Barennes H, Nagot N, Vale I, Koussoube-Balima T, Ouedraogo A, Sanou T, et al. A randomized trial of amodiaquine and artesunate alone and in combination for the treatment of uncomplicated falciparum malaria in children from Burkina Faso. Trop Med Int Health 2004; 9:438-44
12. Miller RS, Wongsrichanalai C, Buathong N, McDaniel P, Walsh DS, Knirsch C, et al. Effective treatment of uncomplicated plasmodium falciparum malaria with azithromycin-quinine combinations: A randomized, dose-ranging study. Am J Trop Med Hyg 2006;401-6
14. Mutabingwa TK, Anthony D, Heller A, Hallet R, Ahmed J, Drakeley C, et al. Amodiaquine alone, amodiaquine+ sulfadoxine-pyrimethamine, amodiaquine+ artesunate, and artemether-lumefantrine for outpatient treatment of malaria in Tanzanian children: a four-arm randomised effectiveness trial. Lancet. 2005; 365:1474-80
15. Agnamey P, Brasseur P, Pecoulas PE, Valliant M, Olliaro P. Plasmodium falciparum in vitro susceptibility to antimalarial drugs in Casamance (Southwestern Senegal) during the first five years of routine use of artesunate-amodiaquine. Antimicrob Agents and Chemother. 2006; 1531-4
16. Yeka A, Banek K, Bakyaita N, Staedke SG, Kamya MR, Talisuna R, et al. Artemisinin versus nonartemisinin combination therapy for uncomplicated malaria: randomized clinical trials from four sites in Uganda. PLos Med. 2005; 190-19
17. Jouan ML, Jullien V, Tetanye E, Tran A, Rey E, Treluyer JM, et al. Quinine pharmacokinetics and pharmacodynamics in children with malaria caused by plasmodium falciparum. Antimicrob Agents and Chemother. 2005; 3658-62
18. Andersen SL, Ager A, P McGreevy, Schuster BG, Wesche D, Kuschner R, et al. Activity of azithromycin as a blood schizonticide against rodent and human plasmodia in vivo. Am J Trop Med Hyg. 1995; 52(2):159-61
19. Heppner DG, Walsh D, Uthaimongkol N, Tang DB, Tulyayon S, Permpanich B, et al. Randomized, controlled, double-blind trial of daily azithromycin in adults for the prophylaxis of plasmodium vivax malaria in western Thailand. Am. J Trop Med Hyg. 2005; 73(5):842-9
20. Noedl H, Krudsood S, Chalermratana K, Silachamroon U, Leowattana W, Tangpukdee N, et al. Azithromycin combination therapy with artesunate or quinine for the treatment of uncomplicated plasmodium falciparum malaria in adults: A randomized, phase clinical trial in Thailand. Clin Infect Dis. 2006; 43:1264-71