RATNA YUNIATI
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
I hereby declare that this dissertation entitled “Morphological and Molecular
Analysis of Jatropha curcasL. Related to Aluminum Stress as a Preliminary Study to Develop Transgenic Plant” is a true record of the original research work of my
supervisor and me except for quotations and citations which have been duly
acknowledged. I also declare that it has not been previously or concurrently
submitted to any other University either in whole or in part for the award of any
degree, fellowship or any other similar titles.
Bogor, August 2012
Related to Aluminum Stress as a Preliminary Study to Develop Transgenic lant. Supervised by SUHARSONO, UTUT WIDYASTUTI SUHARSONO, DIDY SOPANDIE, and AKIHO YOKOTA.
As a response to a progressive price increase of fossil based oil in the world, various initiativesto develop a reliable source of renewable energy become a major global attention. One potentially promising option is biofuel since these are derived from biomass. Biofuel crops should be planted on land that is considered marginal. A crop that is often cited as ideal for growing on marginal land including acid soil with high Al solubility, is the physic nut,Jatropha curcas. However, the major problem of the plant cultivation in acid soil is the decreasing productivity. Jatropha plants are not, by nature, tolerant to Al stress. A promising approach to overcome this limitation is to develop a transgenic Jatropha plant that can withstand to severe Al-stress. Thus it is possible to use TaALMT (Triticum aestivum Al-activated malate transporter 1) gene which encodes the member of a novel membrane protein family that functions as an anion channel to mediate malate efflux from plant roots cells in the presence of extracellular Al3+. Investigating the physiology and molecular basis of Al-stress tolerance during seedling development is an essential prerequisite before developing Al-tolerant Jatropha plant by genetic engineering. Among six populations of Jatropha curcas L., IP-2P population could be categorized as slightly tolerant to Al stress, but it had uniform seed viability, and high productivity character which became the reason to use it as a plant material to develop transgenic Jatropha plant. For preliminary study, J. curcas IP-2P was choosen to be used for gene expression analysis by Quantitative Real-Time Polymerase Chain Reaction (qRT PCR). To normalize mRNA expression in the gene expression analysis we used actin gene. Because there is no information of actin gene nucleotide sequence from J. curcaswe isolated Jatrophaactin (JcACT) by PCR. Three cDNA of actin genes from J. curcas L. IP-2P had been isolated. The analysis revealed that Al stress induced ALMT transcript accumulation both in roots and leaves of non transgenic J. curcas IP-2P. Low pH treatment alone could also induce transcript accumulation both in the root apices and leaf but at lower rates than that observed for Al. This result indicated that response of Jatropha seedlings were not specific to Al stress but also low-pH stress. The TaALMTgene was successfully introduced into Jatropha curcasL. IP-2P via Agrobacterium tumefaciens-mediated transformation. Two putative transformant shoots were regenerated following selection on the bispyribac-containing medium. The presence of the transgene in the genome of transgenic plants was confirmed by PCR. The results indicated that the exogenous TaALMT1 gene was successfully integrated into the genome of J. curcas IP-2P plants.
Related to Aluminum Stress as a Preliminary Study to Develop Transgenic Plant. Supervised by SUHARSONO, UTUT WIDYASTUTI SUHARSONO, DIDY SOPANDIE, and AKIHO YOKOTA.
Aluminum toxicity is the primary factor limiting crop production on strongly acidic soils. Plants have evolved different mechanisms to overcome Al stress. In a number of plant species it has been shown that Al tolerance appears to be mediated by Al-activated release of organic acid anions such as malate, oxalate, or citrate, which chelate Al3+in the rhizosphere and prevent its entry into the root apex. A gene, TaALMT1 (Triticum aestivum Al-activated malate transporter 1), which is responsible for malate release, has been identified in wheat. ALMT gene encodes the member of a novel membrane protein family that functions as an anion channel to mediate malate efflux from plant roots cells in the presence of extracellular Al3+. Therefore it has an important role in the detoxification of Al3+ ion to increase Al tolerance in plants.
Jatropha is a large perennial shrub, which produces non-edible seeds that contain 30-40% oil that ideal for biodiesel production. Jatropha plants can grow in marginal lands such as acid soils, and therefore do not compete with the existing agricultural resources. However, the major problem of the plant cultivation in acid soil is the decreasing productivity.
One promising approach to overcome this limitation is to develop a transgenic Jatropha plant that can withstand to severe Al-stress.In most plant species there is considerable genotypic variation for the ability to withstand to Al toxicity. Investigating the physiology and molecular basis of Al-stress tolerance during seedling development is an essential prerequisite before developing Al-tolerant Jatropha plant by genetic engineering. Six populations of Jatropha curcas L. were different under low pH and Al-stress. The Al treatment affected all growth parameters. There was no single population that showed the best performance on every growth parameter. This information suggests that the adaptability and tolerance to Al stress among the six populations were nearly the same following the one month treatment duration. Although based on the growth parameter, IP-2P could be categorized as slightly tolerant to Al stress, it has high productivity and those became the reason to use it as plant material to develop transgenic Jatrophaplant.
For preliminary study, J. curcasL. IP-2P was choosen to be used for gene expression analysis by Quantitative Real-Time Polymerase Chain Reaction (qRT PCR). Quantitative RT-PCR analysis revealed that Al stress induce ALMT transcript accumulation both in roots and leaves of the wild type J. curcasL. IP-2P. The gene expression was higher in the 24 h exposure rather than 7 days. Low pH treatment alone could also induce transcript accumulation both in the root apices and leaf but at significantly lower rates than that observed for Al. This result indicated that response of Jatropha seedling were not specific to Al stress but also the response occurred to other factors, such as low-pH stress.
cDNA as template and actin primer designed from conserved region of Arabidopsis thalianaactin gene sequences. Three cDNA of actin genes from J. curcasIP-2P had been isolated, cloned and characterized. Those cDNA clones which were 610, 534, and 701 nucleotides in length, shares 98% similarity with actin mRNA of Hevea brasiliensisand 99% with actin mRNA of Ricinus communis. Those three sequences had already registered in the GenBank databases under the following accession numbers: HM587793 for JcACT1, HM587794 for JcACT2, and HM587795 for JcACT3. These actin cDNA nucleotide sequences are the first J. curcas actin information from Indonesia reported.
The TaALMT1 gene from wheat Triticum aestivum L. was introduced into Jatropha curcas L. IP-2P. The binary vector pA2-ALMT construction carrying the ALMT gene had been introduced to Jatropha via Agrobacterium tumefaciens -mediated transformation. This method involved the use of cotyledon explants that co-cultivated with disarmed Agrobacterium strain LBA4404, which on the T-DNA region carries CaMV 35S promoter driven the ALMT coding region, and AtALS (Arabidopsis thaliana Acetolactate synthase) encoding bispyribac resistance. Two putative transformant shoots were regenerated following selection on the bispyribac containing medium. The presence of the transgene in the genome of the three months old transgenic plants was confirmed by PCR. The results indicated that the exogenous TaALMT1gene was successfully integrated into the genome of J. curcas L. IP-2P plants.
Key words: actin gene,Agrobacterium tumefaciens,aluminum stress,ALMTgene,
Neither this paper nor any part may be reproduced or transmitted in any form or by any means, including electronic, mechanical, photocopying, translating, recording, or by any information storage and retrieval system without including or mentioning the source and without the prior written permission from Bogor Agricultural University.
A dissertation submitted to The Graduate School of
Bogor Agricultural University, in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the field of plant biology
By
RATNA YUNIATI
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
Name of student : Ratna Yuniati Registered number : G363070031
Approved by
1. Advisory committee
_________________________
Prof. Dr. Ir. Suharsono, DEA (Chairman)
_________________ _____________________
Dr. Ir. Utut Widyastuti, M.Si. Prof. Dr. Ir. Didy Sopandie, M.Agr (Member) (Member)
_________________
Prof. Akiho Yokota (Member)
2. Plant Biology Study Program Graduate School
__________________
__________________
Dr. Ir. Miftahudin, M.Si. Dr. Ir. Dahrul Syah, M.Sc.Agr. (Head) (Dean)
Date of Examination Date of graduation:
This dissertation entitled “Morphological and Molecular Analysis of
Jatropha curcas L. Related to Aluminum Stress as a Preliminary Study to Develop Transgenic Plant” consists of the research results on Jatropha transgenic for marginal land. It is presented as a partial fulfillment of the requirements for the
Doctoral Degree in the Graduate School of Bogor Agricultural University.
This dissertation is the “stop by” of my long journey in obtaining my degree
in Plant Biotechnology. This dissertation would not have been possible without the
guidance and the help of several individuals who in one way or another contributed
and extended their valuable assistance in the preparation and completion of my
study.
All Praises only to Allah, The Lord of the universe. Shalawat and salam
always devote to our Prophet Muhammad (Peace be upon Him), all of His families
and His disciples, who bring the world hijrah from jahiliah period to akhlakul
karimah period. A figure whom teaches us the meaning of life.
First of all, I am expressing my deepest gratitute to ALLAH S.W.T., the
Almighty for answering my prayers, for giving me the strength to carry out this work
with sound health and mind.
I would like to express my deep and sincere gratitude to my supervisor,
Professor Suharsono. His wide knowledge and his logical way of thinking have been
of great value for me. His understanding, encouraging and personal guidance have
provided a good basis for the present work.
I also express my sincere thanks to members of my thesis committee Dr. Ir.
Utut Widyastuti, and Prof. Dr. Ir. Didy Sopandie, M.Agr. for their valuable
suggestions during the research preparation, lab works, and writing of this
dissertation.
I owe my most sincere gratitude to Professor Akiho Yokota, and Dr. Kinya
Akashi who gave me a valuable opportunity to conduct some part of my research in
Graduate School of Biological Sciences in Nara Institute of Science and Technology
scholarship and financial support. I also thank Sandwich-likeProgram, The Bilateral Exchange Program JSPS-DGHE Joint Research projects 2009, Hibah Kompetensi
2010, International Research Collaboration and Scientific Publication under contract
number 203/SP2H/PL/Dit.Litabmas/IV/2012 on behalf of Prof. Suharsono who
financially supported this research.
I thank Dr. Sasaki (Okayama University, Japan) who provided the ALMT
gene to the research group of Prof. Suharsono. I warmly thank Dr. Kajikawa
Masataka and Dr. Saki Hoshiyasu for their valuable advice and friendly help. Their
extensive discussions around my work have been very helpful for this study.
My sincere thanks are due to Dr. Ir. Ence Darmo Jaya Supena, Dr. Ir. Ulfah
Siregar, Prof. Dr. Ir. Iskandar Zulkarnain Siregar, M.For., and Dr. Susiani
Purbaningsih, DEA as the examiner for their detailed review, constructive criticism
and excellent advice for the revision of this dissertation. I also wish to thank
Dr. Ir. Miftahudin, M.Si. as the Head of Plant Biology Study Program who
contributed to the revision of this dissertation, as well as for his continuous support
in the Ph.D. program.
Also my sincere thank to all post graduate student in IPB, for their help in all
my experiments. Thank you to the numerous dear friends who gave me a second
home in Bogor, to all of my co-workers in the BIORIN (Biotechnology Research
Indonesia-The Netherland) lab, Research Center for Bioresources and Biotechnology
(RC Bio), Bogor Agricultural University for their invaluable support, help and
friendship throughout the study.
I highly appreciate to all my colleagues at the Department of Biology
University of Indonesia, for sharing their expertise in different fields.
My special gratitude is due to my parents, H. Muhammad Noer Burhanuddin,
Hj. Suwartini, H. Muchsin and Hj. Masrifah for teaching me that even the largest
task can be accomplished if it is done one step at a time. They have been an
inspiration throughout my life. They have never failed to give me financial and moral
Finally, to my lovely husband Zakaria and my lovely sons Taufiqi Akmal and
Atariki Naufal, thank a million for all the prayers, for your sincere understanding,
moral support, and being patience during the period of my study. Certainly, without
their support, and constant encouragements, I would not be able to accomplish this
study.
This research could not have been accomplished without a strong belief in the
ideas being tested. To all the scientist whose challenging and educational comments
inspired me and greatly contributed to my way of scientific thinking, I owe you a
great deal.
Bogor, August 2012
Ratna Yuniati was born on June, 24, 1967 in Banjarmasin, South Kalimantan.
She spent her childhood with her parent H. Muhammad Noer Burhanuddin, B.A.E
and Hj. Suwartini in Jakarta until she graduated from senior high school in 1986. She
is married with Ir. Zakaria and having two son, Taufiqi Akmal and Atariki Naufal
She pursued her study at University of Indonesia and she graduated from
Department of Biology, The Faculty of Mathematics and Natural Sciences in 1992.
Since 1993 she has been working as teaching staff in the Laboratory of Plant
Physiology, Department of Biology, Faculty of Mathematics and Natural Sciences,
University of Indonesia. In 1999, she received her Master of Science degree in
Biotechnology from Graduate School of Bogor Agricultural University sponsored by
Tim Manajemen Program Doktor (TMPD).
In 2007 she admitted as a Doctoral Student in the Plant Biology Study
Program, Graduate School Bogor Agricultural University sponsored by Beasiswa
Pendidikan Pascasarjana (BPPS). In May to December 2009 she was a recipient of a
Postdoctoral Fellowship from the Japan Society for the Promotion of Science (JSPS)
in the frame of the Bilateral Exchange Program, JSPS – DGHE Joint Research
Project entitled: “Molecular adaptation of Jatropha curcas to acid soil for
reforestation of tropical wastelands”. In September 2010 to December 2010 she got
a grant scholarship for Sandwich Program from DGHE. During those time, she
carried out research in the Graduate School of Biological Sciences at Nara Institute
of Science and Technology, Nara, Japan.
The research topic III entitled “Isolation, Cloning, and Characterization of
Page
LIST OF TABLES ……….. xvi
LIST OF FIGURES ……….. xvii
CHAPTER I INTRODUCTION ………... 1
Background ……… 1
Objectives……… 4
Approach………. 5
Novelty ………. 6
II LITERATURE REVIEW ……….. 7
Jatropha curcasL. ………. 7
Acid Soils ………... 10
Aluminum Toxicity ……… 11
General Effects and Symptoms of Al Toxicity in Plants ………... 13
Uptake and Distribution of Al at the Whole Plant and Root Level …… 14
Aluminum Tolerance Mechanisms ……… 16
Effect of Al on Organic Acid Anions Exudation ……….. 17
Aluminum Stress-induced Genes ……….. 22
Aluminum-activated Malate Transporter (ALMT) Gene ………... 24
ALMT Function ………. 26
The ALMT Family ………. 27
Structure of Membrane Topology of ALMT1 Protein ……….. 28
Quantitative Real Time Polymerase Chain Reaction ………. 30
Actin ………... 32
Agrobacterium-mediated Plant Transformation ……… 33
Agrobacterium tumefaciensT-DNA Transfer Process ……….. 38
III TOLERANCE ANALYSIS AND EXPRESSION ANALYSIS OF Jatropha curcasL. ALMTGENES UNDER LOW pH AND ALUMINUM STRESS ……….. Abstract ……….. 43
Introduction ……… 43
Materials and Methods ………... 45
Results and Discussion ………... 49
Conclusion ……….. 60
IV ISOLATION, CLONING AND CHARACTERIZATION OF ACTIN-ENCODING cDNAs FROM Jatropha curcasL. IP-2P …….. 63 Abstract ……….. 63
Introduction ……… 63
Materials and Methods ……….. 64
Results and Discussion ……….. 67
Abstract ……….. 73
Introduction ……… 73
Materials and Methods ……….. 76
Results and Discussion ………... 83
Conclusion ……….. 92
VI GENERAL DISCUSSION ………. 93
VII GENERAL CONCLUSION AND SUGGESTION ……….. 99
REFERENCES ……….. 101
1. Effect of Al treatment on growth parameters of six Jatropha populations……….
54
2. Abbreviations of various media used for Jatropha regeneration and transformation ………
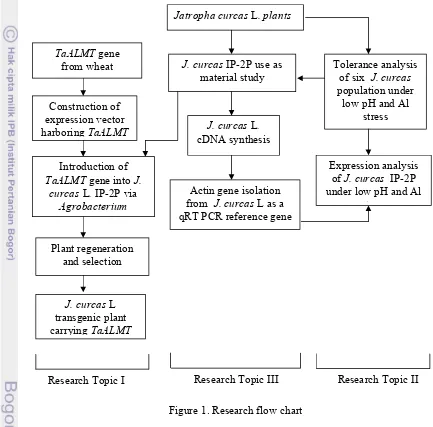
1. Research flow chart ………. 5
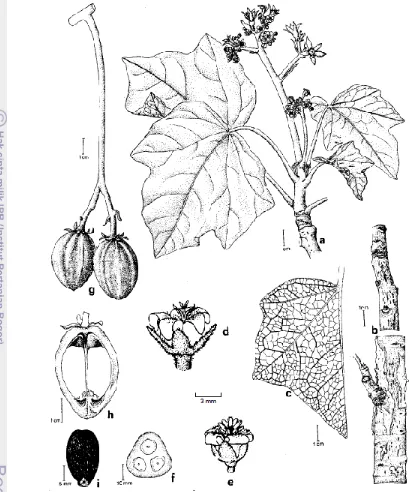
2. Important parts of the physic nut: a - flowering branch, b - bark, c - leaf veinature, d – pistillate flower, e - staminate flower, f - cross-cut of immature fruit, g - fruits, h - longitudinal cut of fruits (Source: Heller 1996) ………
9
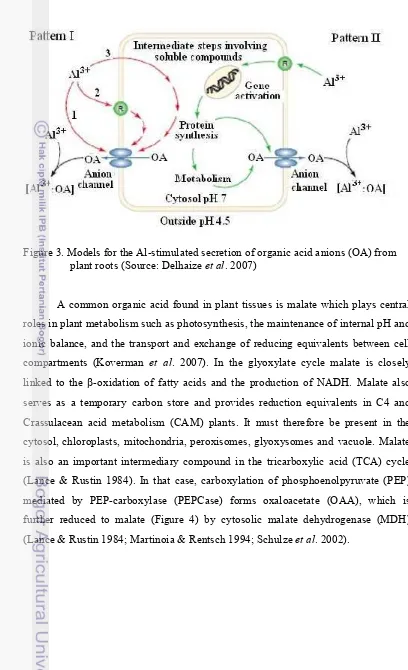
3. Models for the Al-stimulated secretion of organic acid anions (OA) from plant roots (Source: Delhaize et al.2007) ………
20
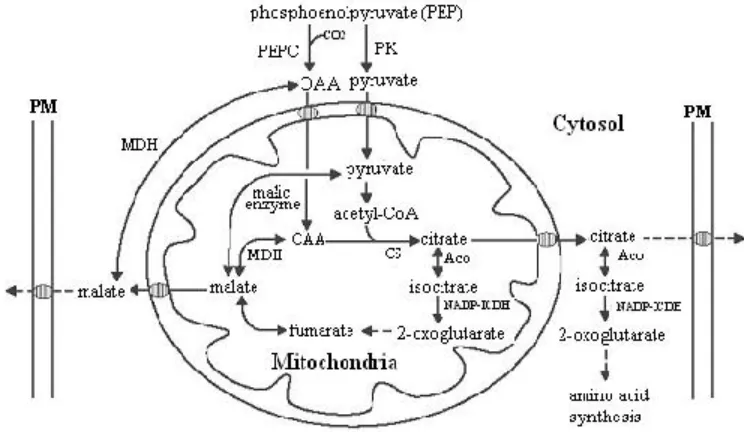
4. Diagrammatic representation of carbon pathways in plant cells related to malate and citrate metabolism. Aco, aconitase; CS, citrate synthase; MDH, malate dehydrogenase; NAD-ICDH, NAD specific isocitrate dehydrogenase; NADP-ICDH, NADP specific isocitratedehydrogenase; OAA, oxaloacetate; PEPC, phosphoenolpyruvate carboxylase; PK, pyruvate kinase; PM, plasma membrane. Hatched ellipses on the plasma membrane and mitochondria denote membrane transporter (Source: Mariano et al. 2005) ………
21
5. Hypothetical models (a) Pattern I and (b) Pattern II for the Al3+
-activated efflux of organic anions by members of the ALMT and MATE families of proteins (Source: Delhaize et al.2007)………..
26
6. Topological model of ALMT1. The model depicts the membrane topology of ALMT1 (Source: Motoda et al. 2007) ……….
29
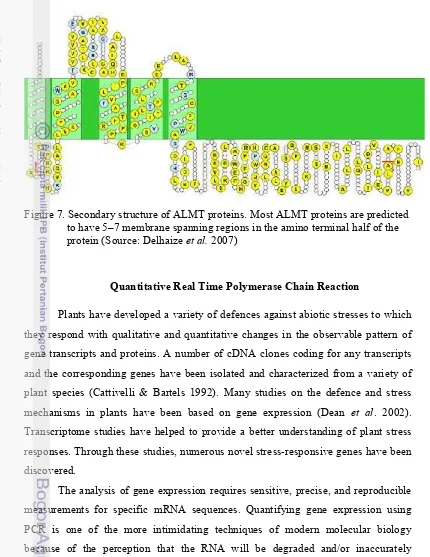
7. Secondary structure of ALMT proteins. Most ALMT proteins are pre-dicted to have 5–7 membrane spanning regions in the amino terminal half of the protein (Source: Motoda et al. 2007)...
30
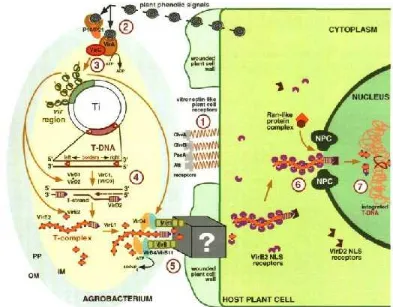
8. Agrobacterium-plant cell interactions. This diagram summarizes all major cellular reactions involved in T-DNA transport. Steps 1 through 7 indicate sequential processes that occur during Agrobacteriuminfection. Step 1, binding of Agrobacterium to the host cell surface receptors; Step 2, recognition of plant signal molecules by the bacterial VirA/VirG sensor-transducer system; Step 3, activation of the bacterial vir genes; step 4, production of the transferable T-strand; step 5, formation of the T-complex and its transport into the host plant cell; step 6, nuclear import of the T-complex; and step 7, T-DNA integration. IM, bacterial inner membrane; NPC, nuclear pore complex; OM, bacterial outer membrane; PP, bacterial periplasm (Source: Sheng & Citovsky 1996)..
39
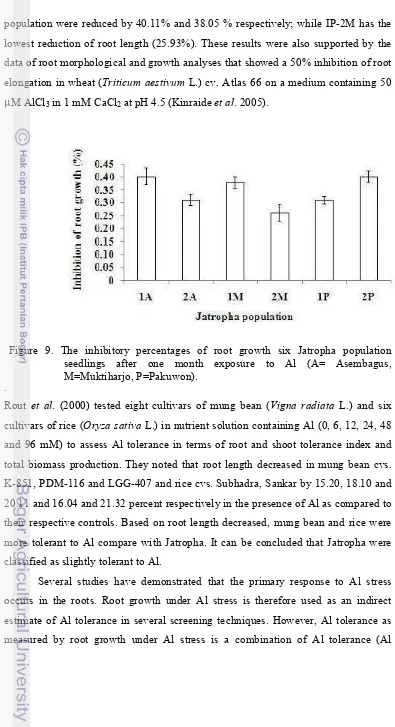
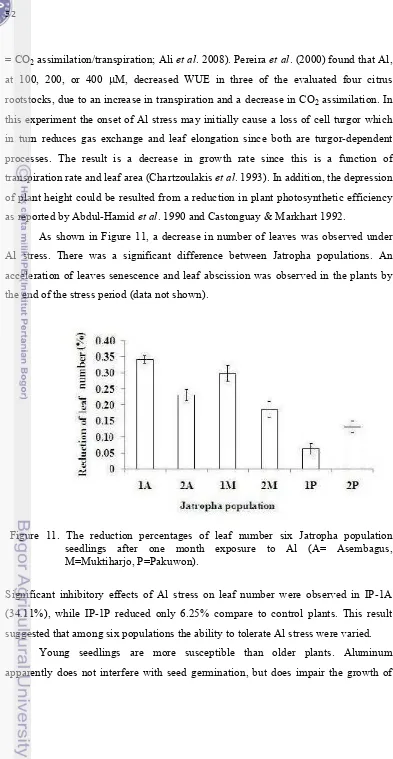
9. The inhibitory percentages of root growth six Jatropha population seedlings after one month exposure to Al (A= Asembagus, M=
Muktiharjo, P=Pakuwon) ………..
11. The reduction percentages of leaf number six Jatropha population seedlings after one month exposure to Al (A= Asembagus, M=
Muktiharjo, P=Pakuwon) ………..
52
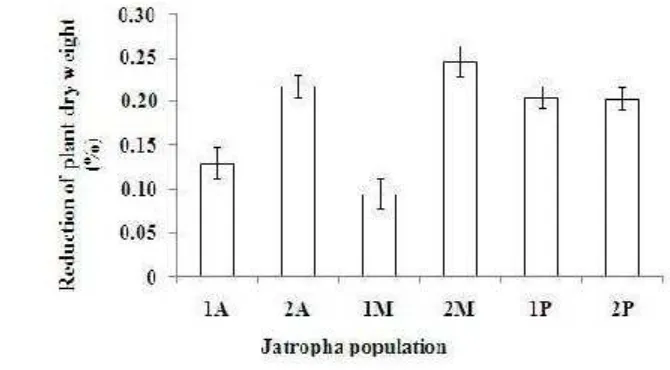
12. The reduction percentages of plant dry weight six Jatropha population seedlings after one month exposure to Al (A= Asembagus,
M=Muktiharjo, P=Pakuwon) ………
53
13. Total RNA of J.curcas L.IP-2P from (A) root tips and (B) leaves .…… 55 14. Expression level of JcALMT transcript relative to actin in the root and
leaf tissues ofJ.curcastreated by Al for (A) 24 hour and (B) 7 days……
57
15. Expression level of JcALMT transcript relative to actin in the root and leaf tissues of J.curcastreated by pH for (A) 24 hour and (B) 7 days…...
59
16. pGEM®T Easy vector for carrying actin gene fragment from J. curcas L. IP-2P...
66
17. Total RNA from J. curcasroot ……… 68 18. PCR amplification to isolate actin fragments using cDNA as a template
and degenerate primer for actin genes, P1Ac12S and P1Ac284N. Lane 1, 2 = actin fragments from root, Lane 3= actin fragments from leaf, M = 1 kb DNA ladder markers ………..
68
19. Location ofJatrophaactin fragment inside the general structure of the A. thalianaactin genes. (Source: McDowell et al. 1996) ……….
69
20. Restriction analysis of three positive recombinant clones withEcoRI, 700 bp (1), 600 bp (2,3), M = 1 kb DNA ladder marker ...
70
21. Phylogenetic tree describes relationship among Jatropha actin with another actins. Tree built with the neighbor-joining method, formed well-defined three separate groups: I = dicotyledonous-woody plants, II = monocotyledonous plants, III = dicotyledon-herbaceous plants. The bootstrap values shown………..
71
22. pGEM-ALMT1-1 plasmid which carried the ALMT1-1 coding region (Source: Sasaki et al. 2004) ……….
76
23. Schematic representation of pMSH1 vector. RB=Right Border, LB=Left Border of T-DNA; NPTII=neomycin phosphotransferase II gene; HPT=hygromycin phosphotransferase gene, P35S =cauliflower mosaic virus 35S promoter; PNOS=promoter of the nopalin synthase gene; T=terminator of the nopalin synthase gene ………
25. Agarose gel profile of the PCR product the 1500 bp fragment for the ALMT1 gene (Lane 1) M = 1 kb DNA ladder………..
84
26. Agarose gel profile of pMSH1 (lane 1), digested pMSH1 (lane 2), 1500 bp ALMT1insert (lane 3 and 4), digested-pA2 (lane 5). (M1=λ Sty marker, M2 = 1 kb DNA ladder).……….
84
27. PCR amplification of the cloned 1500-bp fragment of the ALMTgene inside (A) pMSH1 and (B) pA2 vector was performed by direct colony PCR using specific primer for ALMT. (M = 1 kb DNA ladder, Lane 1-3 transformed E. colicarrying ALMT inside the binary vector) …………
85
28. Agarose gel electrophoresis of M = 1 kb DNA ladder, lane 1, positive control ALMTfragment; lane 2, E.colicolonies PCR harboring pA2-ALMT construct, lane 3-4, A. tumefaciensLBA4404 colonies PCR transformed with pA2-ALMT construct. Arrows indicate a 1500 bp fragment of ALMTgene. Resolved on 1% agarose gel………
86
29. (A) Calli induced on callus-inducing medium containing bispyribac.and (B) Non-infected explants. Bar equals 1.0 cm ………..
87
30. Three months-old selection of regenerated adventitious shoots on bispyribac containing medium. Bar equals 1.0 cm.. ………
89
31. (A) Non-transformed shoot on root elongation media. (B) Arrow points to root of non-transformed plant after two weeks on root elongation media (Inset: picture taken from the bottom side of the culture bottle). Bar equals 1.0 cm. ……….
90
32. PCR confirmation of four bispyribac-resistant regenerated shoots. Arrows indicates the amplification of ALMT gene (1500 bp). M= 1 kb DNA ladder. Lane 2 and 3 were putative transgenic shoots……….
1. Area and distribution of acid soils based on soil type in Indonesia …….. 127
Background
Global energy supply is currently mainly based on fossil fuels such as coal,
oil and gas. Increased use of fossil fuels causes environmental problems both locally
and globally. Developing a reliable source of renewable energy is therefore attracting
a major global attention (Jain & Sharma 2010). One potentially promising option is
biofuel, since these are derived from biomass and do not contribute to the greenhouse
effect (Eijck & Romijn 2008; Jain & Sharma 2010).
Indonesia started to develop the biofuel industry in 2006 as a response to a
progressive price increase of fossil based oil in the world, declining domestic crude
oil production and considerable progressive increase in domestic oil consumption. In
promoting the production of biofuel, the Indonesian government already had a
number of legal instruments, including the Presidential Decree No. 5/2006 on
national policies for optimizing energy use and the Presidential Instruction No.
1/2006 on the use of biofuel. Since then, various initiatives both from the
government and private sectors underline efforts to develop biofuel industry in
Indonesia.
Indonesia has the potential to become a major producer of biofuel. There are
many crops that could be used as raw materials, including palm, Jatropha curcas, sweet sorghum, sugar cane and cassava. Crude Palm Oil (CPO) can be used to
produce biodiesel, a replacement for diesel, while sugar and cassava can be used to
produce bioethanol to replace gasoline. CPO and molasses are the current primary
feedstock used in Indonesia’s biofuel production. Cassava, Jatropha and sweet
sorghum are other potential feedstocks. However, the development of those
commodities is still in the early stage. To increase biofuel production, the
government encourages private companies to build more processing plants.
Partly in order to respond to accusations that biofuel compete with food
production, some policy makers have proposed that biofuel crops should be planted
on land that is considered marginal or idle. There are millions of hectares of such
land around the world, which play no role in food production. In Indonesia marginal
all provinces (Adimihardja et al. 2004; Directorate of Land Rehabilitation and Soil Conservation 2000). According to the Indonesian government, there are three types
of land not used for agriculture: marginal land, critical land and sleeping land. In
Indonesia, “marginal” land is considered to be less productive land, whether dry or
wetland with high acidity due its formation process and its nature and properties.
Indonesian marginal land therefore includes swampland, wetlands and peat forests,
as well as dry land on acidic soil. “Critical” land is land that has been ecologically
degraded as a result of intensive agricultural practices, and which is no longer
suitable for farming. These degraded lands were once important for maintaining food
security in the country, and priority should be given to improving food security for
example through better paddy field irrigation. “Sleeping” land is temporarily
uncultivated or neglected land, that does not match its previously allocated land use
planning classification such as for agriculture, housing, industry and public services
(Anonim 2008).
Acid soils cover a large area of cultivated land in Indonesia. The pH of these
soils ranges from 4 to 5. On highly acidic soils (pH 4.0), the rhizotoxic aluminum
species, Al3+, is solubilized to ionic form, which is toxic to all living cells at low
concentrations. In plants, ionic Al rapidly inhibits root elongation by targeting
multiple cellular sites and subsequently the uptake of water and nutrients (Kochian et al. 2005; Ma 2007), resulting in poor growth. Al toxicity has therefore been recognized as a major factor limiting crop production on acid soils, which account
for 30% to 40% of the world’s arable soils (von Uexküll & Mutert 1995).
However, some plant species have developed mechanisms to detoxify Al,
both internally and externally (Ryan et al. 2001; Ma et al. 2001; Kochian et al. 2005). The most-documented and general mechanism of Al tolerance in both
monocots and dicots is release of organic acid anions, including malate, citrate, and
oxalate, from the roots in response to Al (Kochian et al. 2005; Ma 2007). The Al-dependent stimulation of organic acid efflux from roots has been associated with an
increase in Al resistance. Those anions of organic acids released by roots are thought
to chelate the toxic Al cations, to form non-phytotoxic Al form, and thus prevent
Al-induced malate in wheat, citrate in barley and sorghum have been identified (Sasaki
et al. 2004; Magalhaes et al. 2007).
One crop that is often cited as ideal for growing on marginal land including
acid soil with high Al solubility, is the physic nut, Jatropha curcas.The problem of great concern regarding the Jatropha plant is these plants are not, by nature, tolerant
to Al stress. One promising approach to overcome this limitation is to develop a
transgenic Jatropha plant that can withstand severe Al-stress. Thus it is possible to
use TaALMT (Triticum aestivum Aluminum-activated Malate Transporter) cDNA to generate transgenic Jatropha with enhanced tolerance to Al-stress.
Jatropha is one of such renewable oils, an important multipurpose plant
belonging to the family Euphorbiaceae. Jatropha is grown as a boundary fence to
protect field from the grazing animals and as a hedge to prevent soil erosion. It is a
native of the central America and occurs mainly at low altitudes in areas with annual
temperature of well above 30°C. Among the various oil seeds, Jatropha has been
found more suitable for biodiesel production on the basis of various characteristics.
This oil-bearing tree produces seeds that contain 30-40% oil which ideal for
biodiesel production. Jatropha oil has higher cetane number (51) compared to other
oils, which is compared to diesel (46–50) and make it an ideal alternative fuel (Jain
& Sharma 2010).
In 2006 The Plantation Research and Development Center (Puslitbangbun),
Indonesian Ministry of Agriculture through selection mechanism, has obtained six
lines/ population of Jatropha i.e. IP-1A, IP-2A, IP-1M, IP-2M, IP-1P and IP-2P. Those plant materials were a composite result of superior individuals which selected
from different provenances. From those IP-1 populations the next selected were IP-2
populations which is the derivation of plant material IP-1 (Hasnam 2007a). There is
wide genetic variation, both within and between species, in the resistance of plants to
Al. Such variation provides breeders with a strategy for improving the ability of crop
plants to cope with Al toxicity.
One of the requirements in developing Al-tolerant Jatropha transgenic that
can adapt to acid soil with high level of soluble Al is the availability of a good
selection tool that is able to find parental resources based on tolerant and
typical traits and could be used as a criterion to evaluate Al-tolerance of plant. It may
be expected that the higher tolerance may associate with higher plant morphological
traits values. Besides investigating morphological characteristics, plant stress study
based on gene expression is also required. Up until now, there has been no data about
ALMT gene expression analysis on J. curcas under Al-stress. The analysis of gene expression requires sensitive, precise, and reproducible measurements for specific
mRNA sequences. Quantitative Real-Time PCR (qRT PCR) is, at present, the most
sensitive method. To avoid bias, qRT-PCR is typically referenced to an internal
control gene which should not fluctuate during treatments. Mainly housekeeping
genes were used for the quantification of mRNA expression. Currently, at least nine
housekeeping genes are well described for the normalization of expression signals.
One of the most common are actin (Sturzenbaum & Kille 2001). Until recently the
actin gene has never been isolated from J. curcas.
Objectives
For all the above reasons, the general objective of the present study was
to introduce the TaALMTgene from wheat into J. curcas L. IP-2P plants. While the specific objectives were:
1. To evaluate the low pH and Al-tolerance of six Jatropha population in the acid
soil with Al-stress treatment.
2. To examine the ALMTgene expression from the roots and leaves of wild type J. curcasL. IP-2P during Al-stress treatment.
3. To isolate, clone and characterize actin gene from J.curcasL. IP-2P.
To achieve these objectives, the research was conducted as described by Figure 1.
Approach
Transgenic plant generating experiments was initiated with selected
population. Six populations of Jatropha are used for starting materials of this study.
The required data include information on plant tolerance under low pH and Al-stress
and Al-induce gene expression. Analysis of these data allows distinguishing more or
expression study of the Jatropha population was done as the preliminary research
before developing transgenic Jatropha plants carrying ALMT gene. The gene expression study by using Quantitative RT PCR needs actin gene as an internal
control, thus it needed to isolate from Jatropha cDNA.
Figure 1. Research flow chart
Novelty
This dissertation involved developing a transgenic J. curcas L. IP-2P plants capable of growing in acid soil with high aluminum solubility. This dissertation is
the first report on J. curcas transgenic plants carried the ALMT gene. The second novelty of this dissertation is the isolation of cDNA encoding actin (JcACT) from J.
Tolerance analysis of six J. curcas population under
low pH and Al stress
Expression analysis ofJ. curcas IP-2P under low pH and Al
stress Jatropha curcas L. plants
J. curcas IP-2P use as material study
J. curcas L. cDNA synthesis
Actin gene isolation from J. curcas L as a qRT PCR reference gene TaALMTgene
from wheat
Construction of expression vector harboring TaALMT
Introduction of TaALMTgene into J.
curcas L. IP-2P via Agrobacterium
Plant regeneration and selection
J. curcas L transgenic plant carrying TaALMT
curcas L. IP-2P as internal control in the gene expression analysis by using qRT PCR. The three actin cDNA nucleotide sequences included in this dissertation are the
CHAPTER II
LITERATURE REVIEW
Jatropha curcasL.
Jatropha curcas L. is a deciduous, multipurpose shrub belonging to the family Euphorbiaceae. It is distributed naturally in the equatorial Americas, from
where it has been introduced and become naturalized in many parts of the tropical
and subtropical regions of the world (Heller 1996). J. curcas plant has medicinal values and is commonly grown as hedges to protect gardens and fields from animals.
However, in the recent years, the species has gained tremendous significance as a
potential biodiesel plant, which is characterized as a safe, healthy environment and
renewable resource, alternative to petrodiesel.
J. curcas is very adaptable to a wide range of soils and climates and grows well without any special nutrition regime (Patil & Singh 1991). It can be grown in
arid zones (20 cm rainfall) as well as in higher rainfall zones. It is a quick yielding
species even in adverse land situations, viz., degraded and barren lands under forest
and non-forest use, dry and drought prone area, marginal lands even an alkaline soils
and also as agro forestry crops. Jatropha can be a good plant material for
eco-restoration in all types of wasteland. It can be propagated from seed or cuttings of
stem or branch, and produces large quantity of oil-seed within 2–3 years after
planting.
Jatropha, the physic nut, by definition, is a small tree or large shrub which can reach a height of up to 5 m, but can attain a height of 8 or 10 m under favorable
conditions. The plant shows articulated growth (Kumar & Sharma 2008) straight
trunk, thick branchlets with a soft wood and a life expectancy of up to 50 years
(Achten et al. 2008). The branches contain latex. Normally, five roots are formed from seedlings, one central and four peripheral. A tap root is not usually formed by
vegetatively propagated plants (Kobilke 1989). Jatropha has 5 to 7 shallow lobed leaves with a length and width of 6 to 15 cm, which are arranged alternately. The
plant produces flowers in racemose inflorescences. Inflorescences are formed
terminally. The flowers are unisexual, male and female flowers are produced in the
same inflorescence; occasionally hermaphrodite flowers occur (Dehgan & Webster
group of male flowers. In a few, the expected places of female flowers are
substituted by male flowers. Numerically, 1–5 female flowers and 25–93 male
flowers are produced per inflorescence. The average male to female flower ratio is 29
: 1. Both sexes have five nectar glands produced nectar located at the base of flower.
Male flower has ten stamens performed a circle patterns. Pollen is yellow, globular.
The female flowers has three cell ovary that terminated by three styles. Each
inflorescence, once it begins flowering, flowers daily, and the flowering lasts for 11
days. Jatropha flowers during the rainy season with concentrated flowering from late
July to late October. Pollination is by insects. The rare hermaphrodite flowers can be
self-pollinating. During pollination, sepal and petal will protect the fruit
development. After pollination, a trilocular ellipsoidal fruit is formed. The exocarp
remains fleshy until the seeds are mature. A fruit contain 3-4 black seedwhich 2 cm
long and 1 cm thick. Fruits will ripe after 40-50 days after pollination follows with
fruit color change from green to yellow (Raju & Ezradanam 2002; Bhattacharya et
al. 2005). Gupta (1985) investigated the anatomy of other plant parts. Relevant parts
of the plant are shown in Figure 2.
Carvalho et al.(2008) found that the genome of J. curcasis relatively small (C = 416 Mb) and in the same size range as that of rice and an average base
composition of 38.7% GC. The karyotype of J. curcas is made up of 22 relatively small metacentric and submetacentric chromosomes whose size range from 1.71 to
1.24 mm. In addition, the morphometric similarities observed between chromosomes
from heterologous pairs suggest that J. curcasis an autotetraploid species.
Seed storage behaviour of Euphorbiaceae is generally orthodox according to
Ellis et al. (1985) (one exception, Hevea). Orthodox seed storage behaviour means “mature whole seeds not only survive considerable desiccation (to at least 5%
moisture content) but their longevity in air-dry storage is increased by reduction in
seed storage moisture content and temperature (e.g. to those values employed in
Figure 2. Important parts of the physic nut: a. flowering branch; b. bark; c. leaf veinature; d. pistillate flower; e. staminate flower; f. cross-cut of immature fruit; g. fruits; h. longitudinal cut of fruits (Source: Heller 1996).
Two- or sixmonth-old seeds stored in unsealed plastic bags at ambient temperatures
(approximately 20°C) for 5 months and germinated on average by 62% (ranging
Kobilke (1989) investigated the viability of seeds of different ages (1 to 24
months) that were collected directly from the sites or stored for a certain time. Seeds
older than 15 months showed viabilities below 50%. One explanation for this rapid
decrease is that these seeds remained at the site, having been exposed for long
periods to extreme changes in levels of humidity and temperature. High levels of
viability and low levels of germination shortly after harvest indicate innate (=
primary) dormancy. This behaviour has also been reported for other Euphorbiaceae
(Ellis et al. 1985).
Acid Soils
Acid soils, which are soils with a pH of 5.5 or lower, are one of the most
important limitations to agricultural production worldwide. Approximately 30% of
the world’s total land area consists of acid soils and as much as 50% of the world’s
potentially arable lands are acidic (von Uexkull & Mutert 1995). Furthermore, the
large areas of acidic soils in the tropics and subtropics are critical food-producing
regions for developing countries. Thus, acid soils limit crop yields in many
developing countries (Kochian et al. 2004).
Most of the total area land available in Indonesia is 190 million ha, for
agricultural land is classified as ultisols (47 million ha) (See Appendix 1). Those
soils are considered acid with high Al content, low cation exchange capacity and low
nutrient content (Mulyadi & Soepraptohardjo 1975; Santoso 1991). The most
widespread dry acid soil in Indonesia is in Sumatera, Borneo, and Papua
(Widjaja-Adi et al.1992). Acid soils in Indonesia are characterized by low pH (4.0-5.50), cationexchange capacity (CEC) 10.17 10.89 me/100 g (low), base saturation 19
-21 % (very low); and Al exchangeable 3.43 - 3.80 me/100 gram (Pusat Penelitian
Tanah 1983).
Mineral acid soils result from parent materials that are acidic and naturally
low in the basic cations (Ca, Mg, K and Na), or because these elements are leached
from the soil, reducing pH and the buffering capacity of the soil. As soil pH
decreases, aluminum (Al) is solubilized and the proportion of phytotoxic aluminum
ions increases in the soil solution. In most mineral soils there is sufficient Al present
to buffer the soil to around pH 4. Organic acid soils, consisting of large amounts of
and the pH of these soils can fall below pH 4 (Kidd & Proctor 2001). Superimposing
agricultural production on an ecosystem, especially ammonium fertilization,
accelerates soil acidification due to nitrification. Furthermore, acid rain, containing
nitric and sulfuric acids, is increasing the rate of soil acidification.
Slightly acidic soils (pH of 6.5) are considered most favorable for overall
nutrient uptake. Such soils are also optimal for fixing legumes and
nitrogen-fixing soil bacteria. Some plants are adapted to acidic or basic soils due to natural
selection of species in these conditions. Potatoes grow well in soils with pH <5.5
while cotton, garden pea, and many grasses grow well in alkaline soil (>7.5). Root
growth in spinach was significantly inhibited at pH 4.5 compared with growth at
more neutral conditions. Soil pH also affects the soil in other ways. For example, soil
microbe activity; particularly nitrogen-fixing bacteria may be reduced in acid soil. In
the case of nitrogen-fixing plants, soil acidity is even more problematic since their
symbiotic bacteria are also sensitive to Al and acidity (Hungria & Vargas 2000).
A number of factors contribute to acid soil toxicity depending on soil
composition. In acid soils with a high mineral content, the primary factor limiting
plant growth is Al toxicity (Kinraide 1991). But Al toxicity is not the only stress in
acid soils; among others, proton and manganese toxicity as well as phosphorus
deficiency are also common (Marschner 1995).
Plants in acid soils also suffer from deficiencies in nitrogen, calcium
magnesium and potassium, with phosphorus being the major limiting nutrient. This
acid soil “syndrome” includes toxic levels of Al, manganese, and iron. Although
chemical amendments such as lime can neutralize soil acidity, this option is not
economically feasible, nor is it an effective strategy for alleviating subsoil acidity
(Raoet al. 1993).
Aluminum Toxicity
Aluminum is the most abundant metal in the earth’s crust and occurs in a
number of different forms in the soil. In neutral and basic soils, Al is mostly found as
oxide or silicate precipitates that are not toxic to plants. The neutral Al hydroxide
species, Al(OH)30 is the predominant form of Al at neutral and slightly acidic pH
soils (pH 5.0), aluminum is solubilized into toxic trivalent cation, Al3+ which is
believed to be the primary phytotoxic Al species (Kochian 1995).
The adverse effect of acid soil on plant growth is strongly related to the
toxicity of Al. Aluminum toxicity is the most widespread form of metal toxicity to
plants and its occurrence is rivaled only by salinity. Because of its pH-dependent
solubility, Al toxicity occurs only at soil pH values below 5.5 and is most severe in
soils with low base saturation, poor in Ca and Mg (von Uexkull & Mutert 1995).
Aluminum toxicity has frequently been linked to Ca2+ (Rengel 1992) either
because of Al-induced perturbations in cellular Ca2+metabolism or because of Ca2+
amelioration of Al toxicity (Kinraide & Parker 1987). Several investigations found
Al-induced alterations in the structure of calmodulin, the chief mediator of
intracellular Ca2+ signaling and initiated considerable research on the role of
calmodulin in Al toxicity (Haug & Vitorello 1996). More recently,
phosphoinositide-mediated signal transduction, a pathway which also involves Ca2+as an intracellular
messenger, has been investigated as a primary site of Al toxicity in mammalian
(Haug et al. 1994) and plant cells (Jones & Kochian 1995; Jones et al. 1995). In both cases Al treatment presumably inhibit the activity of phospholipase C or the action of
the trimeric G-protein.
Although most research has focused on the effects of Al on plant root system
because inhibition of root growth is one of the earliest symptoms of Al injury and the
most easily recognized symptom in solution culture (Delhaize & Ryan 1995),
accumulating evidence shows that Al affects photosynthesis (Pereira et al. 2000), photoprotective systems (Chen et al. 2005), water relations (Simon et al. 1994), carbohydrate content (Graham 2002), mineral nutrition (Lidon et al. 1999), organic acid metabolism (Watanabe & Osaki 2002), and nitrogen metabolism (Xiao 2002) in
plant shoot. Recently, attention has been paid to those plant species that can
accumulate Al to high levels in the above-ground parts and their mechanisms of Al
General Effects and Symptoms of Al Toxicity in Plants
The symptom of Al toxicity has been related to the linkage of Al to
carboxylic groups of pectins in root cells (Klimashevsky & Dedov 1975) or to the
switching of cellulose synthesis into callose accumulation (Teraoka et al. 2002). Callose deposition in plasma membranes and plasmodesmata is widely used as an
indicator of Al toxicity in plants because callose accumulates in root tips after
exposure to toxic levels of Al (Zhang et al. 1994; Sivaguru et al. 2000). Aluminum also inhibit mitosis in the root apex (Rengel 1992; Delhaize & Ryan 1995; Liu &
Jiang 2001) implicating blockage of DNA synthesis (Horst et al. 1983), aberration of chromosomal morphology and structure (Liu & Jiang 2001) occurrence of anaphase
bridges and chromosome stickness (Liu & Jiang 2001). It has been reported that
fragmentation of DNA molecules occurs after exposure to Al, and Al-induced
programmed cell death in the root-tip triggered by reactive oxygen species (Pan et al. 2001).
Cells which have been reported to be affected by Al are the root cap,
meristem, elongating cells, root hairs and branch initials (Foy et al. 1978; Rengel 1996). Root tips are the most Al-sensitive region (Ryan et al. 1993), and the distal region of the transition zone was shown to be most Al-sensitive root apical region
(Sivaguru & Horst 1998). Intracellular Al appeared to accumulate in nuclei of
differentiated cells 1 to 2 mm behind the root tip, close to the undifferentiated
meristematic zone within 30 min to several hours of exposure (Delhaize et al. 1993a; Lazof et al. 1994; Vasquez et al. 1999). In the series of experiments, Silva et al. (2000) attempt to determine whether Al enters meristematic cells and binds to nuclei
when roots are exposed to a low Al3+activity in solution. The results are consistent
with others in the literature from experiments with higher Al concentrations in
solution. Collectively, they indicate that Al is able to penetrate the cell symplasm
relatively fast (Lazof et al. 1994; Vitorello & Haug 1996) and bind to nuclear molecules (Matsumoto 1991), presumably leading to decreases in mitotic activity
(Clarkson 1965; Matsumoto 1991). It has been shown in many experiments by
Matsumoto (1991) and others that Al ions is capable of binding tightly to DNA and
RNA, presumably to its phosphate backbone or alternatively to associated histones
interactions finally inhibit cell division and cell elongation in root tips. The more
recent finding that Al causes inhibition of basipetal auxin transport in maize roots
was also interpreted as a mechanism of Al-induced inhibition of root cell elongation
(Kollmeier et al. 2000).
Direct Al interference with nuclear activities is one of the primary controlling
events in the restriction of root growth. Aluminum-injured roots become stubby and
frequently acquire a brownish coloration. Fine branching and root hairs are reduced
and the root system often takes on a “corraloid” appearance. In the root apex, cracks
can easily be observed in the epidermis. Uneven and radial expansion of cells of the
cortex cause root thickening and mechanical stress on the epidermis (Ciamporova
2002).
Uptake and Distribution of Al at the Whole Plant and Root Level
In higher plants the analysis of transport and sequestration of transition
metals is complex because of tissue- and cell-specific differences and organ-specific
transport. The processes that are assumed to be influencing metal accumulation rates
in plants are mobilization and uptake from the soil, compartmentalization and
sequestration within the root, efficiency of xylem loading and transport, distribution
between metal sinks in the aerial parts, sequestration and storage in leaf cells
(Clemens et al.2002). At each level of the transport within the plant, concentrations and affinities of metal chelators as well as the presence and selectivity of transporters
may influence metal accumulation rates.
The apoplast continuum of the root epidermis and cortex is readily permeable
for solutes. In general, solutes have to be taken up into the root symplasm to cross
the endodermis before they can enter the xylem (Tester & Leigh 2001). The cell
walls of the endodermal cell layer act as a barrier for apoplastic diffusion into the
vascular system. Subsequent to metal ion uptake into the root symplasm, three
processes govern the movement of metals from the root into the xylem: sequestration
of metals inside root cells, symplastic transport into the stele and release into the
weight chelators, free hydrated metal cations and metal chelates in the mobile
transpiration stream, and stationary metal-binding sites in the cell wall material
surrounding the xylem vessels (Senden & Wolterbeek 1990). Xylem-unloading
processes are the first step in controlled distribution and detoxification of metals in
the shoot, as well as in a possible re-distribution of metals via the phloem (Schmidke
& Stephan 1995).
Transition metals reach the apoplast of leaves in the xylem sap, from where
they have to be scavenged by leaf cells (Marschner 1995). Transporters mediate
uptake into the symplast, and distribution within the leaf occurs via the apoplast or
the symplast (Karley et al. 2000). Trafficking of metals occurs inside every plant cell, maintaining the concentrations within the specific physiological ranges in each
organelle and ensuring delivery of metals to metal-requiring proteins. Chelation with
certain ligands, for example histidine, nicotianamine (Pich et al. 1994) and citrate (Senden et al. 1995), appears to route metals primarily to the xylem. By contrast, chelation with other ligands, such as phytochelatins or metallothioneins, might route
metals predominantly to root sequestration (Evans et al. 1992). Excess essential metals, as well as non-essential metals, are sequestered in leaf cell vacuoles
(Vögeli-Lange & Wagner 1990; Krämer et al. 2000). Intriguingly, different leaf cell types show pronounced differential accumulation. The distribution pattern varies with
plant species and element.
In most plant species, especially Al-sensitive and crop species, Al ions are
taken up mostly through the root system, where it accumulates predominantly in the
epidermis and in the outer cortex (Wagatsuma et al. 1987; Delhaize et al. 1993; Matsumoto et al. 1996). Transport systems and intracellular high-affinity binding sites then mediate and drive uptake across the plasma membrane. Uptake of Al ions
is likely to take place through secondary transporters such as channel proteins and/or
H+-coupled carrier proteins. The membrane potential, which is negative on the inside
of the plasma membrane and might exceed −200 mVin root epidermal cells (Hirsch
et al. 1998) provides a strong driving force for the uptake of cations through secondary transporters.
There have been many studies concerning the Al uptake mechanism in Al
pattern, with an initial rapid, non-linear phase (<30 min), followed by a slower, linear phase. The non-linear phase and the linear phase might consist mainly of
passive accumulation in the apoplast and uptake across the plasma membrane,
respectively. Since most of Al in the rapid phase was removed by 30-min desorption
with citrate (Zhang & Taylor 1989), the rapid phase appears to consist mainly of
passive accumulation into apoplast. In contrast, the linear phase was interpreted as
uptake into symplasm. However, it was suggested that the linear phase includes
metabolism-dependent binding of Al (Zhang & Taylor 1990) or accumulation of
insoluble Al species in apoplast (Archambault et al.1996; Tice et al. 1992).
Lazof et al. (1994) reported that Al penetrates into the symplast within 30 min. It is considered that Al disturbs the intracellular metabolism by binding to
intracellular components such as nuclei (Aniol 1984; Tice et al. 1992), mitochondria (Aniol 1984), or protein (Aniol 1984). Recent study by Jones and Kochian (1995)
suggested that Al-induced specific inhibition of phospholipase C caused the disorder
of 1,4,5-trisphosphate-mediated signal transduction pathway and/or cytoskeleton
dynamics.
There are many plants species that accumulate Al to a considerable extent in
the shoot (Jansen et al. 2002; Watanabe & Osaki 2002). Such plants, frequently called hyperaccumulators, are mainly woody plants from tropical or subtropical
regions. Classic examples of hyperaccumulators are the tea plant (Camellia sinensis), hydrangea and melastoma (Melastoma malabathricum L.). It was elucidated by Watanabe et al.(2001) that melastoma, accumulated Al in excess of 10,000 mg kg−1 in mature leaves, and that the forms of Al in the leaves were Al3+, oxalate,
Al-(oxalate)2 and Al-(oxalate)3in order of the amount present (Watanabe et al. 1998). It
was also reported that the main Al form accumulated in tea leaves was Al-catechin
(Nagata et al. 1992, 1993), Al-citrate inHydrangea macrophylla leaves (Ma et al. 1997a) and Al-(oxalate)3in buckwheat leaves (Ma et al. 1997b).
Aluminum Tolerance Mechanisms
Due to the many hypotheses trying to explain the mechanisms of Al toxicity,
numerous associated resistance mechanisms have been proposed and demonstrated
crossing the plasma membrane, entering the symplast, and reaching sensitive
intracellular sites (Al exclusion) are mechanisms of Al resistance while those conferring the ability of plants to tolerate Al in the root (and/or shoot) symplast are
mechanisms of Al tolerance (Taylor 1991; Kochian 1995). Al resistance could involve the immobilization of Al at the cell wall, selective permeability at the
plasmalemma, production of root mucilage, changes in the rhizosphere pH, the
exudation of Al-chelating compounds into the apoplasm/rhizosphere (e.g.organic
acid), and the active efflux of Al3+across the plasma membrane. While Al tolerance
involve chelation of Al in the cytosol, vacuolar compartmentation, complexation by
Al-binding proteins, or Al-tolerant enzymes.
Effect of Al on Organic Acid Anions Exudation
Organic acids are carbon compounds with at least one carboxyl group
(-COOH), and play a fundamental role in cellular metabolism. Some of them are
involved in energy production as intermediates in the tricarboxylic acid (TCA) cycle
(e.g. citrate, malate), while others are directly or indirectly involved in other
metabolic processes including the assimilation of carbon and nitrogen, the regulation
of cellular pH and osmotic potential, the maintenance of electroneutrality during
excess nutrient cation uptake, and the supply of energy to symbiotic bacteria (e.g.
malate, malonate, oxalate) (Haynes 1990; Marschner 1995; Taiz & Zeiger 2002).
Organic acids of low molecular weight have often been mentioned as playing
an important role in certain mechanisms evolved by plants to cope with
environmental stress like Al toxicity, and phosphorus (P) and iron (Fe) deficiency
(Jones 1998; Ryan et al. 2001; Kochian et al. 2004). Root exudation of organic acid anions (OA) which can chelate and detoxify Al in the rhizosphere has been
consistently reported in several studies. At the near-neutral pH of the cytosol, most
of these acids exist there as deprotonated anions (Taiz & Zeiger 2002). Once
transported out of the root, they carry a varying negative charge and can effectively
chelate Al3+ in the rhizosphere to form complexes with Al thereby reducing its
Al-induced secretion of organic acids has been characterized in several plant
species or cultivars. Citric and malic acids are well-known as strong Al chelators and
they seem to be involved in tolerance mechanisms in different species. Because of
their high stability constants with Al and/or their high concentrations in the plant
tissue, citric and malic acids might reduce the impact of toxic Al ions in plant root
tissue, especially in the Al-tolerant lines. Malic acid has been reported to be secreted
from the roots of Al-tolerant cultivars of wheat (Triticum aestivum; Delhaize et al. 1993; Basu et al. 1994) in response to Al stress, citric acid from Al-tolerant cultivars of snapbean (Miyasaka et al. 1991) and maize (Pellet et al. 1995), and Cassia tora (Ma et al. 1997c), and oxalic acid from buckwheat (Ma et al. 1997b) and taro (Ma & Miyasaka 1998). The amount of organic acids secreted increases with increasing
external Al concentrations (Delhaize et al. 1993; Ma et al. 1997c). The site of organic acid secretion has been localized to the apex of the roots which is consistent
with the targeting site of Al toxicity (Ryan et al.1993).
Plants differ in the species of organic acid anions secreted, temporal secretion
patterns, temperature sensitivity and dosage responses to Al (Ma 2000). Organic acid
anions release in response to Al can be categorized into two different types,
designated by Ma et al. (2001) as pattern I and II. Pattern I is typified by immediate (15 to 30 min) OA release in response to Al exposure of the root and the rate of
release remains constant with time. This pattern suggests that Al3+ ions activate an
existing mechanism of OA transport in the plasma membrane and therefore the
activation of genes is not necessary. Wheat and buckwheat (Fagopyrum esculentum) are exemplars of pattern I (Delhaize et al. 1993; Zheng et al. 1998). Immediate activation of OA release in wheat is consistent with the molecular characterization of
ALMT1, an aluminum-activated malate transporter, in wheat (hereafter TaALMT1), which is constitutively expressed in wheat roots, and the activation of OA transport
activity can be followed at the biophysical level (Sasaki et al.2004). TaALMT1gene expression is not induced by Al stress but is constitutive; expression levels correlate
well with the amount of OA release and overall Al tolerance (Sasaki et al. 2004; Raman et al. 2005). In this case, it was supposed that activation of the malate channel plays a critical role in the quick response of malate release to Al treatment
agents can block OA release from intact wheat roots, suggesting that reversible
phosphorylation of TaALMT1 may be involved in its transport activity (Osawa & Matsumoto 2001). Pattern II OA release requires an induction period for OA release
after Al treatment (Ma et al. 2001). The lag phase between the moment of addition of Al and the onset of OA release by roots, together with the gradual increase of release
with time, indicate that activation of genes related to the metabolism and membrane
transport of these compounds might be required. Citrate release from C. tora is a typical example of pattern II, which requires 4 h for OA release after the Al exposure
(Ma et al. 1997). Sorghum (Sorghum bicolor) also exhibits pattern II and requires days to reach full OA release (Magalhães et al. 2007). In sorghum, it is clear that induction of the SbMATE OA transporter protein, a sorghum homolog of the
multidrug and toxic compound extrusion transporter, occurs during the lag between
onset of Al stress and OA release (Magalhães et al.2007). Induction of Al-activated OA transporters has also been reported in oilseed rape (Brassica napus), which is consistent with the observation that rape has pattern II OA release (Ligaba et al. 2006). The delay in OA release may also be due to a lag in OA production. Al stress
does not affect OA metabolism in triticale while pattern II OA release is observed
there (Hayes & Ma 2003).
Figure 3 describe models for the aluminium-stimulated secretion of organic
acid anions (OA) from plant roots. For Pattern I-type responses, Al activates an anion
channel on the plasma membrane that is permeable to organic acid anions. This
stimulation could occur in one of three ways: (1) Al3+ interacts directly with the
channel protein to trigger its opening; (2) Al3+ interacts with a specific receptor (R)
on the membrane surface or with the membrane itself to initiate a
secondary-messenger cascade that then activates the channel; or (3) Al3+ enters the cytoplasm
and activates the channel directly, or indirectly via secondary messengers. In the
Pattern II response, Al interacts with the cell, perhaps via a receptor protein (R) on
the plasma membrane, to activate the transcription of genes that encode proteins
involved with the metabolism of organic acids or their transport across the plasma
membrane. Organic acid anions form a stable complex with Al, thereby detoxifying
Figure 3. Models for the Al-stimulated secretion of organic acid anions (OA) from plant roots (Source: Delhaize et al. 2007)
A common organic acid found in plant tissues is malate which plays central
roles in plant metabolism such as photosynthesis, the maintenance of internal pH and
ionic balance, and the transport and exchange of reducing equivalents between cell
compartments (Koverman et al. 2007). In the glyoxylate cycle malate is closely linked to the -oxidation of fatty acids and the production of NADH. Malate also
serves as a temporary carbon store and provides reduction equivalents in C4 and
Crassulacean acid metabolism (CAM) plants. It must therefore be present in the
cytosol, chloroplasts, mitochondria, peroxisomes, glyoxysomes and vacuole. Malate
is also an important intermediary compound in the tricarboxylic acid (TCA) cycle
(Lance & Rustin 1984). In that case, carboxylation of phosphoenolpyruvate (PEP)
mediated by PEP-carboxylase (PEPCase) forms oxaloacetate (OAA), which is
further reduced to malate (Figure 4) by cytosolic malate dehydrogenase (MDH)
Figure 4. Diagrammatic representation of carbon pathways in plant cells related to malate and citrate metabolism. Aco, aconitase; CS, citrate synthase; MDH, malate dehydrogenase; NAD-ICDH, NAD specific isocitrate dehydrogen-ase; NADP-ICDH, NADP specific isocitrate dehydrogendehydrogen-ase; OAA, oxaloacetate; PEPC, phosphoenolpyruvate carboxylase; PK, pyruvate kinase; PM, plasma membrane. Hatched ellipses on the plasma membrane and mitochondria denote membrane transporters (Source: Mariano et al. 2005).
Physiological studies have shown that the secretion of organic acid anions is
mediated through anion channels or transporters. Anion channels have been
characterized in the plasma membranes of a range of different plant cells where they
are known to be involved in several important cellular functions. These include
turgor regulation, stomatal movement, nutrient acquisition, and control of membrane
potential (Barbier-Brygoo et al. 2000).
Two types of anion channels in the plasma membrane have been identified in
protoplasts derived from wheat roots. One is the outwardly rectifying anion channel
that is activated at membrane potentials more positive than the equilibrium potential
for the permeant anion (Skerrett & Tyermann 1994). This channel has been
<