PRODUCTION OF TOMATO AND POTATO IN THE FIELD
ADEEL ABD ALKARIM FADHL
GRADUATE SCHOOL
With this I declare that this thesis the effects of biofertilizer with different drying system and storage period on growth and production of tomato and potato in the field is my own work under the direction of an advisory committee. It has not yet been presented in any form to any Education institution. The sources of information which is published or not yet published by other researchers have been mentioned and listed in the references of this thesis.
ADEEL ABD ALKARIM. Pengaruh pupuk hayati dengan perbedaan sistem pengeringan dan lama penyimpanan terhadap pertumbuhan dan produksi tomat dan kentang di lapangan. Dibimbing oleh HAMIM dan ARIS TJAHJOLEKSONO .
Pupuk hayati merupakan salah satu alternatif pengganti pupuk anorganik yang dapat meningkatkan kesuburan tanah dan produksi tanaman. Penelitian ini bertujuan menguji efektifitas pupuk hayati hasil berbagai metode pengeringan dan lama penyimpanan untuk meningkatkan serapan hara, produksi tanaman tomat dan kentang di lapangan. Dua percobaan dilakukan di lapangan Laboratorium Balitsa Lembang, Bandung. Percobaan pertama menggunakan Rancangan Acak Kelompok dengan satu faktor (aplikasi pupuk hayati) yaitu (B0) tanpa pupuk hayati (kontrol), (B1) pupuk hayati cair, (B2) pupuk hayati hasil freezedryer tanpa penyimpanan, (B3) pupuk hayati hasil freezedryer dengan 3 bulan penyimpanan, (B4) pupuk hayati hasil sentrifugasi tanpa penyimpanan dan (B5) pupuk hayati hasil sentrifugasi dengan 3 bulan penyimpanan. Percobaan ini dilakukan 3 ulangan dengan total 18 satuan percobaan. Percobaan kedua juga disusun menggunakan Rancangan Acak Kelompok dengan dua faktor. Faktor pertama adalah dosis NPK anorganik yaitu (A1) 50% NPK dan (A2) 100% NPK. Faktor kedua adalah aplikasi pupuk hayati yaitu (B0) tanpa pupuk hayati (kontrol), (B1) pupuk hayati cair, (B2) pupuk hayati hasil freezedryer tanpa penyimpanan, (B3) pupuk hayati hasil freezedryer dengan 3 bulan penyimpanan untuk tomat, sedangkan untuk kentang, (B2) pupuk hayat hasil freezedryer tanpa penyimpanan, (B3) pupuk hayati hasil sentrifugasi tanpa penyimpanan. Selain itu tanaman dipupuk dengan pupuk kandang dosis 20 ton / Ha. Dalam percobaan ini dilakukan 3 ulangan dengan total 24 satuan percobaan. Hasil penelitian menunjukkan bahwa viabilitas bakteri cenderung menurun selama penyimpanan pupuk hayati. Penyimpanan pupuk hayati selama 3 bulan tidak berbeda nyata mengurangi pertumbuhan dan produksi tanaman. Aplikasi pupuk hayati meningkatkan serapan hara makro dan mikro, pertumbuhan vegetatif dan produksi tanaman. Pada tanaman tomat, aplikasi pupuk hayati meningkatkan serapan hara makro dan mikro serta parameter pertumbuhan tanaman. Aplikasi pupuk B4 meningkatkan parameter bobot buah per tanaman sebesar 112.1% dibandingan dengan kontrol. Aplikasi pupuk hayati yang dikombinasikan dengan NPK 100% hampir sama dengan pupuk NPK 50% dalam meningkatkan pertumbuhan dan produksi tanaman. Semua aplikasi pupuk hayati berbeda nyata mengurangi serangan penyakit yang disebabkan oleh cendawan Alternaria solani. Pada tanaman kentang, aplikasi pupuk hayati nyata meningkatkan serapan hara makro dan mikro serta bobot kering akar, kecuali pada parameter tajuk. Produksi tanaman meningkat sebagai respon aplikasi pupuk hayati. Aplikasi pupuk hayati hasil sentrifugasi (B4) meningkatkan bobot umbi per tanaman sebesar 41.6%, sedangkan aplikasi pupuk hayati hasil sentrifugasi + 50% NPK (A1B3) meningkatkan produksi umbi per plot sebesar 79.5%.
ADEEL ABD ALKARIM. The effects of biofertilizer with different drying system and storage period on growth and production of tomato and potato in the field. Supervised by HAMIM and ARIS TJAHJOLEKSONO .
Biofertilizer is one alternative of fertilizer that is able to increase soil fertility and crop production. The purpose of this research was to investigate the effect of biofertilizer dried by different methods and exposed to different period of storage on nutrient uptake, vegetative growth and production of potato and tomato plant grown in the field. Two experiments were carried out in the field Laboratory of Baltsa Lembang, Bandung. The first experiment was prepared using Randomized Block Design with one factor (biofertilizer application) including i.e (B0) without biofertlizer (control), (B1) liquid biofertilizer, (B2) freezedried biofertilizer without storage, (B3) freezedried biofertilizer with 3 months storage, (B4) centrifuged biofertilizer without storage and (B5) centrifuged biofertilizer with 3 months storage. In this experiment 3 replications (blocks) were applied with total of 18 combination blocks. The second experiment was also prepared using Randomized Block Design with two factors. The first factor was anorganic NPK dosage by (A1) 50% NPK and (A2) 100% NPK and the second factor was biofertilizer application i.e (B0) without biofertlizer (control), (B1) liquid biofertilizer, (B2) freezedried biofertilizer without storage, (B3) freezedried biofertilizer with 3 months storage for tomato, whereas for potato, the factors of (B2) freezedried biofertilizer, (B3) centrifuged biofertilizer were applied. Besides that the plant were fertilized with manuare by about 20 ton/Ha. In this experiment 3 replications (blocks) were applied with total of 24 combination blocks. The results showed that viability of bacterium tended to decline during storage of biofertilizer. The storage of biofertilizer during 3 months did not significantly reduce the effect on growth and production of plant. Application of biofertilizer increased macro and micro nutrient absorption, vegetative growth and plant production. In tomato plant application of biofertilizer increase total macro and micro nutrient as well as plant growth parameters. Plant production also increased as response to biofertilizer by about 112.1% that was indicated by application of B4 for fruit weight per plant. Application of biofertilizer in combination with 100% NPK was almost similar to that of 50% NPK in improving growth and production of plant. All application of biofertilizer significantly reduced disease attack caused by alternaria solani fungus. In potato plant, macro and micro nutrient absorption and root dry weight increased significantly by application of biofertilizer but not on shoot parameters. Plant production, also increased as response to biofertilizer by about 41.6 % indicated by application of centrifuged biofertilizer (B4) for tuber weight per plant and increase plant production per plot by about 79.5 % that was indicated by application of 50 % NPK+ centrifuged biofertilizer (A1B3).
Lower soil fertility and crop production is major problem that has been facing by all farmers. The low soil fertility is caused by continues cropping and heavy application of inorganic fertilizer or chemical fertilizer. Further, the biofertilizer was introduced to decrease chemical fertilizer application and increase the crop production. Thus the objective of this study is to investigate the effect of biofertilizer dried by different methods and exposed to different period of storage on vegetative growth and productivity of potato and tomato plant grown in the field. This research was conducted in Laboratory of Plant Physiology and Laboratory of Microbiology Department of Biology Bogor Agricultural University and in the Research Institute for Vegetables (Balitsa) in Lembang, West Java.
Potato and tomato were grown in field conditions with two experiment. The first experiment was prepared using Randomized Block Design with one factor (biofertilizer application) including i.e (B0) without biofertlizer (control), (B1) liquid biofertilizer, (B2) freezedried biofertilizer without storage, (B3) freezedried biofertilizer with 3 months storage, (B4) centrifuged biofertilizer without storage and (B5) centrifuged biofertilizer with 3 months storage. In this experiment 3 replications (blocks) were applied with total of 18 combination blocks. The second experiment was prepared using Randomized Block Design with two factors. The first factor was anorganic NPK dosage by (A1) 50% NPK and (A2) 100% NPK and the second factor was biofertilizer i.e (B0) without biofertlizer (control), (B1) liquid biofertilizer, (B2) freezedried biofertilizer without storage, (B3) freezedried biofertilizer with 3 months storage for tomato, whereas for potato, the factors of (B2) freezedried biofertilizer, (B3) centrifuged biofertlizer were applied. Besides that the plant were fertilized with manuare by about 20 ton/Ha. In this experiment 3 replications (blocks) were applied with total of 24 combination blocks. The results showed that the viability of bacteria tended to decline during storage of biofertilizer. The viability of centrifuged biofertilizer showed higher than freezedried biofertilizer either before or after storage. Viability of Azotobacter and
Azospirilum slightly declined after 1 month storage, while Bacillus sp and
Pseudomonas spdid not. Meanwhile the storage of biofertilizer during 3 months was not significantly different to that of fresh (without storage) in viability and both of them had positive effect on growth and production of plant.
caused increased vegetative growth of tomato plant when compared to control (A2B0). Leaf number was the parameter that mostly affected by application of either biofertilizer and anorganic fertilizer, while plant height and steam diameter slightly affected. Consequently this caused significantly increased of total dry weight by the treatment. Even though not significantly different, application with 50% tended to have higher response than 100% NPK. Application with 100 of NPK resulted in almost the same dry weight and plant height for combination with B1, B2 as well as B3. Plant production also increased as response to biofertilizer by about 112.1% with the maximum result showed by application of B4 for fruit weight per plant and increase production per plot by about 118.7 % that was indicated by application of B2. All application of biofertilizer significantly reduced disease attack caused by alternaria solani fungi.
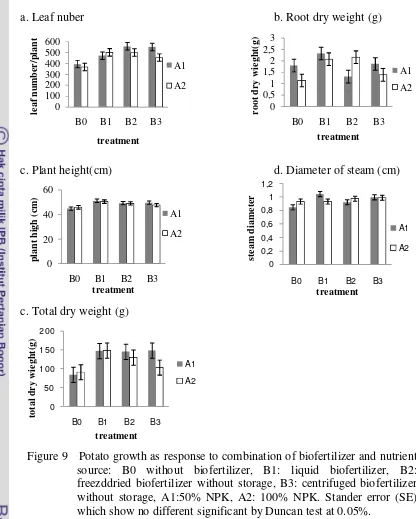
In potato plant, macro and micro nutrient absorption and root dry weight increased significantly by application of biofertilizer but not on shoot parameters. Total macro nutrient absorption tended to increase by application of liquid biofertilizer (B1) by about of 53.9% and increase of total micro nutrient absorption was obtained by centrifuged biofertilizer with 3 months storage (B5) by about of 91.3%. Vegetative growth of potato such as plant height, steam diameter, leaf number and total dry weight showed no significant improvement but tended to increase as response to biofertilzer. The improvement was due to increase of root indicated by higher root dry weight. On the other hand, application of biofertilizer with nutrition source caused an increase of vegetative growth of the plant. Leaf number was the parameter that affected by application of either biofertilizer and anorganic fertilizer, while plant height and steam diameter were only slightly affected. Plant production, also increased as response to biofertilizer by about 41.6 % indicated by application of centrifuged biofertilizer (B4) for tuber weight per plant and increase plant production/plot by about 79.2 % indicated by application of 50% NPK + centrifuged biofertilizer (A1B3)
Keywords: biofertilizer, tomato, potato, nutrients absorption.
Copyright© 2010 Bogor Agricultural University
Copyright are protected by law,1. It is prohibited to cite all or part of this thesis/dissertation without referring to and mentioning the source.
a. Citation only permitted for the sake of education, research, scientific writing, report writing, critical writing or reviewing scientific problem.
b. Citation doesn’t inflict the name and honor of Bogor Agricultural University.
PRODUCTION OF TOMATO AND POTATO IN THE FIELD
ADEEL ABD ALKARIM
A thesis as part of the requirements for achieving the degree of Master of Science in
Plant Biology at the Department Biology
Graduate School
of tomato and potato in the field.
Name : Adeel Abd Alkarim
Registration number : G353088111
Major : Biology
Approved
Advisory Committee
Dr.Ir.Hamim, M.Si. Dr.Ir.Aris Tjahjoleksono, DEA Chairman Member
Agreed
Coordinator of Major Plant Biology Dean of Graduate School
Dr.Ir.Miftahudin, M.Si Prof. Dr.Ir. Khairil A.Notodiputro, M.Si
First and foremost, my most earnest gratitude to our Lord, Allah, for blessing me with the ability to undertake this study and granting me the strength to complete it. Several people in one way or another contributed to the success of this work. I would like to convey my sincere and special thanks to my main supervisor Dr.Ir.Hamim, M.Si for his guidance, encouragement, advice and constructive criticisms during the preparation, laboratory analysis and finalizing this thesis. I would like also to thank him for allowing me carry out my research using his material, otherwise this work would not have been completed in time.
I wish also to thank my second supervisor Dr.Ir.Aris Tjahjoleksono, DEA for his valuable time and comments on this work. I am very grateful to the
Indonesian government that gave me the opportunity to do a Master of Science in Botany from this prestigious Bogor Agricultural University under the Developing Countries Partnership Program (DCP).
It’s also with sincere gratitude that I acknowledge the contributions of Dr.Ir.Miftahudin,M.Si in his capacity as the coordinator of major of Botany for his guidance and encouragement throughout my study period. Special thanks also go to the following people:
1. The technicians and staff in the laboratory of Microbiology Department of Biology, who were always there to help and advise me in all laboratory. To my friends who have stood with me during difficult time. Their contributions ranged from counseling and moral support during the entire academic period. I have to remember my friends, Gani, Jeni, Hermas, Nurul Alhidayati , Malik, Andro, and to all my friends.
2. To my Yemeni friends living in Indonesia: , Abdulraman Alhuthi, Ali Obadi, Mohmed Munis and all staff of the Yemen Embassy in Jakarta.
Special thanks go to my Parents, my wife, my brother and sisters for their continued prayers.
Bogor, April 2010
Page
LIST OF TABLES ... ….iii
LIST OF FIGURES ... iv
LIST OF APPENDICES ... v
INTRUDUCTION ... 1
Background ... 1
Purpose of Research... 2
Benefit of Research ... 3
LITERATURE REVIEW ... 4
Nutrition and Plant growth ... 4
Application of integerated biofertilizer ,organic Material and Inorganic Fertilizer ... 5
Bioferilizer ... 5
Role of biofertilizerbacteria ... 7
Biofertilizer provides nutrient source ... 7
Bifertilizer a produce growth hormons ... 8
Biofertilizer for biocontrol of plant diseases. ... 9
Plant Growth Promoting Rizobacteria ... 10
Description of Microbial that use as biofertilizer ... 10
Technology of biofertilizer and storage ... 12
Use media carrier biofertilizer ... 12
Preservation of Microorganism by drying ... 13
MATERIAL AND METHOD ... 14
Time and place of research ... 14
Material and Equipment ... 14
Experimental Design ... 15 .
Preparation of biofertilizer ... 15
Observation viability of bacteria during storage ... 17
Preparation of soil and planting ... 17
Application of compost & NPK and biofertilizer ... 17
Parameter observaed ... 18
Statistical Analysis... 19
RESULT ... 20
Result of analysis soil ... 20
Result of analysis compost ……… ... 20
Viability of bacteria ... 20
Effect of biofertilizer on nutrient absorption at tomato ... 21
Effect of biofertilizer on nutrient absorption on potato ... 22
Plant growth of tomato plant ... 26
Effect of combination biofertilizer and nutrient source on Plant growth of potato ... 28
Effect of biofertilizer on production of tomato ... 29
Effect of biofertilizer on production of potato ... 31
Effect of Combination biofertilizer and nutrient source on tomato Plant production ... ..32
Effect of Combination biofertilizer and nutrient source on potato Plant production ... 33
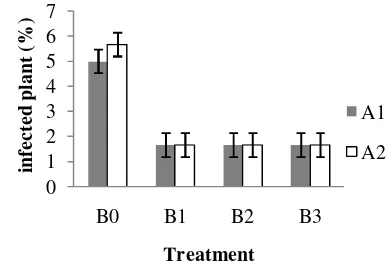
Effect of biofertilizer as disease resistance ... ...34
DISCUSSION ... 36
CONCLUSION... 41
SUGGESTION ... 42
REFERENCES ... 43
Number Page 1. Viability of bacteria during storage ... 20
Number Page 1. The first experiment design of tomato and potato. ... 15
2. The second Experiment design of tomato and potato ... 15 3. Stander curve of bacteria ... 16 4.Nutrient absorption macro and micro as response to different
biofertilizer in tomato plant. ... 22 5. Nutrient absorption macro and micro as response to different
biofertilizer in tomato plant ... .23 6. Plant growth as response to biofertilizer application in
tomato plant . ... ...24 7. Plant growth as response to biofertilizer application in
potato plant… ... ….26 8. Plant growth as response to combination biofertilizer and
nutrient source in plant growth at tomato plant ... .. 27 9. Plant growth as response to combination biofertilizer and
nutrient source in plant growth at potato plant. ... ...39 10. Plant production as response to biofertilizer application in
tomato plant….………… ... ...30 11. Plant production as response to biofertilizer application in
potato plant ... 31 12. Plant production as response to combination biofertilizer
and nutrient source in tomato plant production ... 32 13. Plant production as response to combination biofertilizer
and nutrient source in potato plant production ... …33 14. The effect of biofertilizer as disease resistance. ... 34 15. The effect of combination of biofertilizer and nutrient
Page
1 Chemical and Physical properties of soil Experiment ... 50
2. Table assessment criteria Soil Chemical properties ... 51
3. Chemical Properties of Compos that used in Research ... 52
4. Compost quality Standards (SNI 1970302004) ... 53
5.Result of data analysis for nutrient absorption at tomato ... 54
6.Result of data analysis for nutrient absorption at potato ... 57
7. Result of analysis vegetative growth in tomato plant with bifertilizer ... 60
8. Result of analysis vegetative growth of potato with biofertilizer ... 62
9. Result of analysis vegetative growth of tomato with combination biofertilizer with nutrient source………… … ... 64
10. Result of analysis of vegetative growth at potato with combination Biofertilizer with nutrient source ... 66
11. Result of analysis tomato production with biofertilizer ... .68
12. Result of analysis of potato production with biofertilizer. ... 70
13. Result of analysis tomato production at combination biofertilizer with nutrient source ... 72
Background
Lower soil fertility and crop production is major problem that has been facing by all farmers. The low soil fertility is caused by continues cropping and heavy application of inorganic fertilizer or chemical fertilizer. Chemical fertilizers were used intensively around the world to increase crop yield. However, they start depositing their harmful effects to the soil and environment causing reduction in soil quality and environmental degradation (Rodriguez et al. 2004; Setboonsarng and Gilman 2006). Moreover intensification of agriculture have negative impact to agriculture ecosystem that is including ossification of soil and ground, decrease of organic matter (Stoate et al. 2001).
To achieve high production value targets while maintaining preservation of sustainable agriculture resources needs effort and appropriate strategies including the utilization of biofertilizers and organic fertilizers. Lately, farmers have started to give greater attention to the application of biofertilizer. One of these driving force is the farmer awareness of the potential environmental pollution through the use of excessive chemical fertilizers (Simanungkalit 2001). According to Havlin et al. (2005), the use of inorganic fertilizer continuously will cause damage on physical, chemical and biological properties of soil, so that the soil fertility will be more decreased. Organic fertilizer is a substance that is important in improving the biological properties of the soil so as to create a better environment for plant roots. Utilization of organic materials and micro-organisms is useful to develop sustainable agriculture and to minimize the use of inorganic chemicals (Ghorbani et al. 2008). Therefore, the biofertilizers were introduced as alternative fertilizers to the farmers for reducing application of the chemical fertilizers and preserving the environment in the long run (Mezuan et al. 2004).
many of which have been called plant growth promoting Rizhobacteria (PGPR) that activity colonize the rhizophere, improve the plant growth and increase yield (Wu et al. 2005). Among of them are strain from genera such as Azotobacter, Azospirillium, Bacillus, Rhyzobium and Pseudomonas. According to Simanungkalit (2001), Azospirillum sp. and Azotobacter sp is an aerobic, free-living soil microbe which fixes nitrogen from the atmosphere. While,
Pseudomonas sp. and Bacillus sp. are bacteria that able to increase the availability of P and K in soil, enhanced N, P and K uptake (Han and Lee 2005).
Pseudomonas sp. and Azospirillum sp. also can produce hormone to enhance plant growth and biocontrol.
Biofertilizers has limitation in the storage. The efforts to keep the organism alive during storage are became very important. Biofertilizer in the liquid medium has limitation in the storage and the packaging. The efficiency of biofertilizer depends on the viability of bacteria that may be decrease under exposed in the external factor such as temperature, humidity and light. Therfour, the biofertilizer shoud be maintained effectively during storage to avoid the reduction of viability. In order to maintain viability, it is necessary to mixed the bacterium with a material as carrier that not only useful preventing the decrease of viability but also easy to apply. Hamim et al. (2007) have examined the effectiveness of some biofertilizers carrier media including rice flour, corn flour, sea weed flour and peat soil. They found that peat was the best formulation of solid biofertilizers consisting of plant growth promoting Rhizobacteria (PGPR). It is still necessary to evaluate the effect of peat, dry biofertilizer and it is period of storage on plant physiology and morphology as well as plant growth and production in the field still need to be studied more.
The Objective of The Research
Benefit of This Research
LITERATURE REVIEW
Nutrition and Plant Growth
Plant growth is not only controlled by the internal factors but also determined by external factors. Among the external factors influenced plant growth and development are essential elements. If these elements are not available or in lower concentration, the plant will show deficiency symptoms (Roy et al. 2006).
One factor that plays a role in plant growth is the availability of adequate amount of nutrition. Nutrients element in the soil are very limited and will be reduced because of some factors such as the monoculture farming system in a long time. To overcome this problem needs to be done whether the supply of nutrients in the form of organic or inorganic, or the use of microbes that play a role to help the plant in nutrient, absorption (Simanungkalit 2001). Based on the amount necessary for the plant, the nutrients differentiated in to macro and micro nutrients (Roy et al. 2006). Macro element consists of nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sulfur (S) and silicon (Si). Micro element consists of iron (Fe), zinc (Zn), copper (Cu), manganese (Mn), boron (B), molybdenum (Mo) and chlorine (Cl), natrium (Na) and nickel (Ni).
Nutrients element that can be absorbed by the plants can be obtained through the decomposition of organic compounds in the form of compost or manure, immediately or inorganic fertilizers such as urea SP36, KCI (NPK) or mutual association microbes and the plant that play a role in the provision or supply of certain nutrients. There are several studies reporting the importance of applying the fertilizer to improve soil fertility and increase yield of crops. Ghorbani et al. (2008) have studied in field experiments the effects of organic amendments productivity and storability of tomato (Lycopersicon esculentum
Mill.). They found, that application of sheep manure and chemical fertilizers lead to high total tomato yield. The compost made of manure, therefore appears to be a promising ecological alternative to classical fertilizers.
complex compounds with metal ions that are toxic to the plants such as Al, Fe and Mn. Organic manure is very beneficial for improving crop production quality and quantity, reduce environmental pollution, and improve the quality of sustainable land. The use of organic fertilizers in the long run can increase productivity and prevent land degradation. In addition the use of organic fertilizer is also useful for soil microbes. Several researchers previously reported that the application of organic materials other than as a source of nutrients for plants also serves as an energy source that soil microbes can increase the activity microbes in plant nutrient supply (Suriadikarta & Simanungkalit 2006).
Applications of Integrated Biofertilizer, Organic Materials and Inorganic Fertilizers
Organic manures are well established to be involved in fertilization of plants due to its beneficial effect on the physical, chemical and biological characteristics of the soil. Chemical fertilizers can not replace the dual benefits of organic materials, but can be added for expedite the process and make nutrients more available (Sutanto 2002). Integrated approach using combination biofertilizers, organic fertilizer, and chemical fertilizers is a good approach. Experiments Hamim et al. (2007) in a greenhouse by using a combination of biofertilizers and compost increase dry weight of corn when compared without biofertilizers and increased total weight grain in rice plant.
Biofertilizer
Biofertilizer is defined as a substance contain living microorganisms which, when applied to seed, plant surface or soil, colonize the rhizosphere or interior of the plant and promoting growth by increasing the supply of availability of primary nutrients to the host plant (Vessey 2003).
natural soil fertility (5) provide protection against drought and some soil borne diseases, and (6) activate the soil biologically (Yazdani et al. 2009).
Several investigations have studied the effect of biofertilizer to improve plant yield. Suwanbutr et al. (2006) studied the effect of EM (effective microorganisms) on yield and quality of tomatoes. They found that the addition of NPK fertilizer together with manure significantly increased vegetative growth (plant height, bush width and stem width) from the crop establishment stage to harvest. Every treatment combined with EM had the same effect as manure on growth. There was no effect on quality of tomato in terms of fruit weight and number per plant in all treatments. However the treatments EM, molasses and decomposed manure, molasses and manure and NPK fertilizer increased fruit weight, and fruit number.
Azospirillum, Azotobacter has been found are beneficial to a wide array of crops covering cereals, millets, vegetables, cotton and sugarcane. These microbes are free living and non-symbiotic nitrogen fixing organism that also produces certain substances that good for the growth of plants and antibodies that suppress many root pathogens (Simanungkalit 2001). This microorganism are potential to be used as biofertilizer to improve soil fertility .
Claudia et al. (2006) studied the effect of Azospirillum sp in root of tomato plants through a mechanism that involves ethylene. They found that Azospirilla
were localized on roots and within xylem tissue that able to increase in shoot and root fresh weight, main root hair length, and root surface indicated that inoculation with A. brasilense resulted in plant growth improvement. Similarly, Mezuan et al. (2004) studied Azotobacter sp, Aspergillus sp, and Penicllium sp at
Oryza sativa. They found that bacteria are able to increased the growth and production of rice. Sanhita et al. (2000) also isolated Rhizobacteria from the rhizosphere of tomato plants to inoculate seeds and roots, they found that the three bacteria, Azospirillum sp., Azotobacter chroococcum and Pseudomonas fluorescens, provided a significant (P= 0.05) increase in seedling emergence rate.
on lignite based biofertilizer under different storage conditions, she found, that optimum temperature for storage of the biofertilizer was about 280C.
Many attentions has also been paid for reservation method of biofertilizer. Silva et al. (2007) studied the reservation methods of Tolypothrix tenuis for use as a cyanobacterial fertilizer. They reported the suitability of the cyanobacterium
Tolypothrix tenuis as a potential biofertilizer and evaluated these microbes by measuring cell viability in samples preserved by various methods, including freezing at −β0°C, freeze-drying, desiccation as flakes and immobilization in alginate beads. The viability recovery and the retained viability index (RVI10)
were used as indicators of cell survival after 3 and 15 months storage. On the other hands, Ngampimol et al. (2008) observed effects of light on biofertilizer. They reported, liquid biofertilizer produced from vegetable waste contains high amount of viable microbial population after four months of storage. The two conditions of storage, with and without light. They found that there was no significant difference (p>0.05) upon viable microbial population, chemical and physical characteristics.
Role of Biofertilizer Bacteria a. Provides Nutrients
At the root region some bacteria are profitable because of it is role to improve plant growth through increasing nutrients absorption. The bacteria have role as a provider of nutrients because of it is ability to dissolve mineral form complex compounds so that it can be absorp by the root. Vessey (2003) reported that the Pseudomonas sp. and Bacillus sp. can dissolve P (phosphate) from the soil to become available for the plant.
and shoot growth of winter wheat, and increase N, P, and K contents of plant components. On the other hand, some bacteria have a role to fixation nitrogen (non-symbiosis) such as Azotobacter, Azospirillum sp, that are able to fixes atmospheric nitrogen in loose association with plant roots and provides the host plant by about 30-50% of nitrogen requirements ( Dalla et al. 2004).
Bacillus bacteria if combined with Bradyrhizibum japonicum, can be used to improve the root development and form nodules on roots, and increase the absorption of nutrients especially N in soybean plant (Bai et al. 2003). Similarly, the Phosphate Solubilizing Bacteria (PSB) Bacillus megaterium and Potassium Solubilizing Bacteria (KSB) Bacillus mucilaginosus, increased the availability of P and K in soil, enhanced N, P and K uptake, and promoted growth of eggplant (Han & Lee 2005).
Wu et al. (2005) carried out a study to evaluate the effects of four biofertilizer containing an arbuscular mycorrhizal fungus (Glomus mosseae or
Glomus intraradices) with or without N-fixer (Azotobacter chroococcum), P solubilizer (Bacillus megaterium) and K solubilizer (Bacillus mucilaginous) on soil properties and the growth of Zea mays. They found, that the biofertilizer significantly increased the growth of Zea mays. Microbial inoculums not only increased the nutritional assimilation of plant (total N, P and K), but also improved soil properties, such as organic matter content and total N in the soil. b. Produce Hormone
Many soil bacteria such as Azotobacter sp, Azospirillum sp and
Pseudomonas sp can be promote plant growth by production of phytohormon such as auxin, Cytokinin, Gibberellins and abisic acacid (Bottini et al. 2004; Safak & Nilfer. 2006) which can be beneficial to stimulate plant growth and increase plant production.
c. Biocontrol of Plant Diseases
Biologicalcontrol is being considered as an alternative or a supplemental way of reducing the use of chemicals in agriculture. There has been many studied to describing potentialuses of plant associated bacteria as agents stimulating plant growth and managing soil and plant health such as Pseudomonas spand Bacillus
PGPR group of bacteria as abiocontrol by directly or indirectly. Directly role by activity such as nutrition source, competition by colonization and competition with bacteria pathogens at the root area (Whipps 2001;Compant et al. 2005). While, mechanism indirectly with some PGPR to plant can also provide systemic resistance against a broad spectrum of plant pathogens. Diseases of fungal,bacterial, and viral origin, and in some instances even damagecaused by insects and nematodes, can be reduced after application of PGPR and product compound allelochemicals (Compant et al. 2005).
Many of group PGPR such as biocontrol have ability to produce antibiotic like phenazine-1-carboxyalic, 2,4-diacetyle phloroglunicol, pyoluteorin, pyrrolnitrin. Besides that system resistance plant also can be induced by bacteria PGPR such as Bacillus, Pseudomonas, Azospirillum and Rhizobium (Kloepper et al. 2004; Compant et al. 2005 and Fernando et al. 2005). Pseudomonasbacteria, such as Pseudomonas fluorescens, have been applied directly to soils and seeds to prevent the growth of crop pathogens, and increasing the plant production.
Although biocontrol activity of microorgansims involving synthesis of allelochemicals has been studied extensively with free-livingrhizobacteria, similar mechanisms apply to endophytic bacteria(Lodewyckx et al. 2002), since they can also synthesize metabolites with antagonistic activity toward plant pathogens (Chen et al. 2002). Moreover, Castillo et al. (2002) demonstrated that munumbicins, antibiotics producedby the endophytic bacterium Streptomyces sp. can inhibit in vitro growth of phytopathogenic fungi P. ultimum, and F. oxysporum. Subsequently, it has been reported that certain endophytic bacteria isolated from field-grown potato plants can reduce the in vitro growth of
PGPR (Plant Growth Promoting Rhizobacteria)
Group of bacteria that actively colonize plant roots and increase plant growth and yield known as PGPR (Wu et al. 2005). There are many species from bacteria such as Azotobacter sp., Azospirillium sp, Azoaracus sp, Bacillus sp, Clostridium sp, Entrobacter sp, Gluconoacetobacter sp, Pseudomonas sp and
Serratia sp (Somers et al. 2004). Plant growth promoting rhizobacteria (PGPR) can promote plant growth directly or indirectly. Directly by increasing plant growth are through phytohormones, such as auxin, cytokinin and gibberellins (Glick 1995). Indirect promotion of plant growth is through antibiotic type compounds, enhanced resistance to pathogenic diseases and/or abiotic stresses. The mechanisms by which PGPRs promote plant growth are not fully understood, but some probability include: the ability to produce phytohormons (Egamberdiyeva 2007), symbiotic N2 fixation (Mrkovacki and Milic 2001), and solubilization of mineral phosphates and other nutrients (Cattelan et al. 1999). Effect PGPR to enhance plant growth depends on ability bacteria to form area colonization in the root with high density, so that exists in the root region and becomes more steady to produce profitable product for the plant (Rokazadi et al.
2008; Ashrafuzaman et al. 2009).
Description of Microbial Which Used As a Biofertilizer Bacillus sp.
Cells and straight rod-shaped, measuring approximately 0.5-2.5 x 1.2-10µm, arranged in pairs or a chain with the tip rounded or square. Bacillus
including Gram-positive bacteria and movement with flagella. Endospora form oval, and sometimes rounded or cylindrical and highly resistant to many unfavorable conditions. These bacteria are aerobic or facultative anaerobic, has a wide diversity, sensitive to heat and salinity. Bacillus including chemoorganotrophy Organism, with spacious habitats, a small number of pathogen species are vertebrates or invertebrate (Holt et al. 1994).
used in this study proved capable of producing IAA of 67.2 ppm in the medium containing tryptophan and able to dissolve phosphate.
Pseudomonas sp
Pseudomonas has the straight form or crooked stems slender, but not twisting where size 0.5-1.0 x 1,5-5,0 µm. Most of these species to accumulate poly- -hydroxybutirat as carbon storage materials. These microbes are not covered by the capsules and do not have a break phase. Pseudomonas is a Gram-negative bacteria, moved by using one or more flagella, rarely non motil, aerobic, have a perfect type of system respiratory metabolism with oxygen as an alternative electron acceptor, in line with the growth of anaerobic conditions. Most of the species also does not require organic growth factors. Some chemotrophic facultative species can use the H2 or CO as energy source (Holt et al. 1994). Bacterium Pseudomonas sp isolates (PD13) used in this study proved capable of producing IIA in medium containing tryptophan and also able to dissolve phosphate (Ditjen PLA Deptan and LPPM IPB 2006).
Azospirllum sp
Azospirillum vibrioid shapes or straight rod 0.9-1.2 µm in length, is the group of Gram-negative bacteria, contain poly- -hydroxibutirat, characterisitic are motil with vibrating motion in liquid media with an average of single polar flagella. Some pigment produce bright pink or dark pink pigment on agar medium potato. Optimum growth temperature 34-37C, grow well at pH 7 or acid. This bacterial group is fixation nitrogen and growth depends on the condition of oxygen or grow slightly better on a lot of air containing N2 as ammonium salt.
Under oxygen limitation, there many strains can not change NO3 to NO2 or N2O
and N2. These bacteria grow well on organic acid salts, such as malate, suksinat,
Azotobacter sp
Azotobacter is a genus of usually motile, oval or spherical bacteria that form thick-walled cysts, and may produce large quantities of capsular slime, elongated 1.4-2.0 µm diameter and rod-shaped cells. These bacteria being single and also couple, irregular colony, and sometimes a long chain with a variable.
Azotobacter does not produce endospora, but form cyst. This chemoorganotrophy bacteria, Gram negative, motility using flagella, or are not motil, aerobic, but can also grow under low oxygen pressure. Azotobacter can be fixed N (non symbiotic) at least 10 mg N2 per gram of carbohydrate (usually in the form of glucose) is
consumed. In certain species, these bacteria use nitrate, ammonium salts and certain amino acids as nitrogen sources, and able to grow in the pH range 4.8-8.5. While the pH optimum for nitrogen fixation and growth is 7.0-7.5. In the soil and water, this species may be associated with the root of plant (Holt et al. 1994).
Technology of Biofertilizer and Storage a. Use Media Carrier Biofertilizer
In general there are 2 types of media that can be used in formulating bio-fertilizers, the liquid media and solid carriers. There are several alternative types of solid media that can be used as carrier medium biofertilizers, including: rice flour, corn flour, seaweed and peat soil. Peat is more effective as a binding media solid formulation of biofertilizers that contain bacteria Azotobcter sp., Azospirillum sp., Bacillus sp., and Pseudomonas sp. This media able to maintain microbe’s viability until 6 months (Hamim et al. 2007).
b. Preservation of Microorganisms by Drying
Palmfeldt et al. (2003) sutdy the survival of pseudomonas chlororaphis
MATERIALS AND METHODS
Time and Place for Research
The experiment was conducted, in Laboratory of Plant Physiology and Laboratory of Microbiology Department of Biology Bogor Agricultural University and in the Research Institute for Vegetables (Balitsa) in Lembang, West Java, from April- December 2009
Material and Equipment
The materials that were used in this experiment was tomato seed var. Martha provided by Ballista and potato tuber variety Granola obtained from professional seed grower in Lembang, compost, anorganic fertilizers (NPK) and biofertilizer containing isolate bacteria of Azotobacter 13, Azospirillium IDM3,
Pseudomonas PD13 and Bacillus TG1. The equipments including freezdryer, oven and centifuge were used to produce biofertilizer.
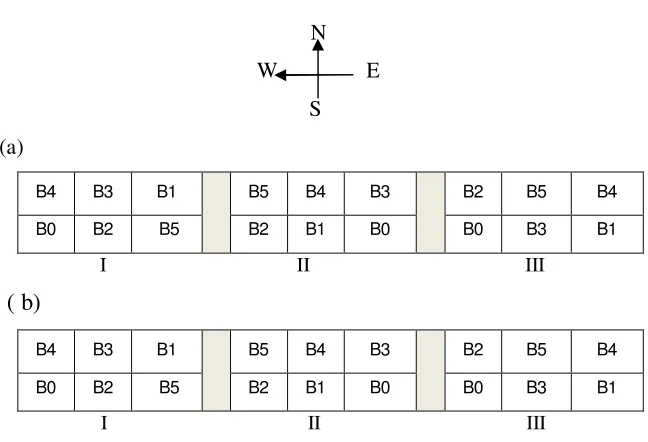
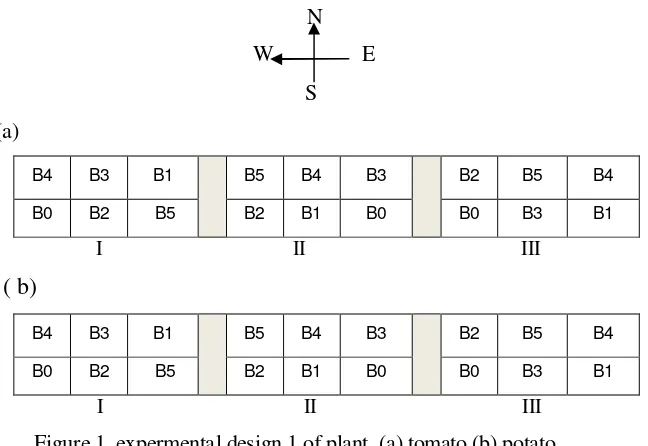
Experimental Design
N W E
S (a)
B4 B3 B1
B5 B4 B3
B2 B5 B4
B0 B2 B5 B2 B1 B0 B0 B3 B1
I II III
( b)
B4 B3 B1
B5 B4 B3
B2 B5 B4
B0 B2 B5 B2 B1 B0 B0 B3 B1
[image:32.595.161.485.78.299.2]I II III
Figure 1 expermental design 1 of plant (a) tomato (b) potato. Description: I, II, III = block/replication.
( a)
A1B0 A1B1 A1B0 A1B1 A1B1 A2B1
A1B3 A2B0 A2B1 A1B2 A2B2 A1B3
A2B2 A2B3 A2B2 A1B3 A1B0 A2B3
A1B2 A2B1 A2B0 A2B3 A1B2 A2B0
I II III
(b)
A1B0 A1B1 A1B0 A1B1 A1B1 A2B1
A1B4 A2B0 A2B1 A1B2 A2B2 A1B4
A2B2 A2B4 A2B2 A1B4 A1B0 A2B4
A1B2 A2B1 A2B0 A2B4 A1B2 A2B0
I II III Figure 2 expermental design 2 of plant (a) tomato (b) potato.
Description: I, II, III = block/replication.
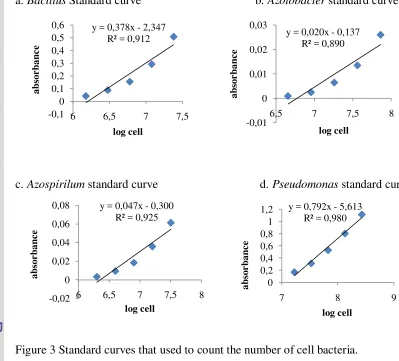
Preparation of Biofertilizer Rejuvenation of Bacteria
[image:32.595.152.484.368.581.2]and incubation for 48 hours, Azospirillium sp. in NFA solid medium and was incubated for 24 hours, Pseudomonas sp. in TSA medium and incubated for 24 hours, and Bacillus sp. in NA medium andwasincubated for 18-24 hours. Before mix liqiuid media with peat to produce biofertilizer for each isolate, firstily stander curve has been prepared to count number the cell population as in Figure 3. Secondly, prepared 1 lliter media from each bacteria, after that taken 1ml to measurement by spectrophotometer. Finaly, counting the number of cell by accordance to stander curve until 108 /ml.
a. Bacillus Standard curve b. Azotobacter standard curve
[image:33.595.98.497.260.621.2]
c. Azospirilum standard curve d. Pseudomonas standard curve
Figure 3 Standard curves that used to count the number of cell bacteria.
On the other side, peat was sterilized in the oven at 100C0 for 24 hours, cooled and stored in a suitable place. Cultur bacteria that have population 108/ml was mixed with peat using alternatively two methods: (1) with freeze dryer and (2) with concentrfugation mechanism. Before freeze dried, 1L of bacteria culture was mixed with 1kg of peat and were dried using freeze drier until moisture content
y = 0,378x - 2,347 R² = 0,912
-0,1 0 0,1 0,2 0,3 0,4 0,5 0,6
6 6,5 7 7,5
ab so r b an c e log cell
y = 0,020x - 0,137 R² = 0,890
-0,01 0 0,01 0,02 0,03
6,5 7 7,5 8
ab so r b an c e log cell
y = 0,047x - 0,300 R² = 0,925
-0,02 0 0,02 0,04 0,06 0,08
6 6,5 7 7,5 8
ab so r b an c e log cell
y = 0,792x - 5,613 R² = 0,980
0 0,2 0,4 0,6 0,8 1 1,2
7 8 9
was about 10% to produce biofertilizer. In the second methods, 1liter of bacteria was centrifuged at 10000 rpm for 5 minutes and the supernatant was thrown. After that the pellet were mixed with 1kg of peat to produce centrifuged biofertilizer. The products from both methods was packed into plastic bottles and stored in suitable conditions at room temperature.
Observation of Viability Bacteria During Storage
Observation of bacterium viability consisting biofertilizer were observed periodical during storage (0, 1, 2 and 3 month). Observation of viability was carried out by dissolved biofertilizer with 9 ml NACL 0.85% at various level starts from10-3 -10-6, and observed at petri dishes which were given each bacterium medium and incubated for calculation colonies. Acountining of cell population carried out according to methods petrii dish account (Hadioetomo 1993).
Preparations of Soil and Planting The Tomato Seed and Potato Tuber
The land was prepared and blocks were made with the size 3 x 3 m. Before planting the soil and compost were analyzed to understand the nutrient and acidity status. Seeds of tomato were germinated in the seed bed for 3 weeks. Tubers potato were planted directly to the plot with the distance of 50x30 cm2, while after 3 weeks, tomato seedling then were grown with the distance of 70x50 cm2.
Application of Compost & NPK and Biofertilizer
to each plant with the dosage of 40 mlper plant after two week of planting and with dosage 60 ml per plant after two week from the first application.
Parameters Observed The following measurements were recorded:
a. Analysis of nutrient content of soil and compost samples.
At the beginning of the experiment samples were taken from the soil that used at depth of 25-30 cm and nutrient content was analayzed to see availability of nutrients. Besides that, nutrient in the compost was also analyzed.
b. Plant growth including:
Parameter was recorded during vegetative stage included: plant growth such as plant height, leaf number, diameter of steam, and plant dry weight. The mesurment of plant growth of tomato and potato was conducted every 10 days after the 14-day-old plants.
c. Plant production:
The crop production was recorded during harvest time including number fruit /cluster, number of fruit/ tuber per plant, tuber/fruit weight per plant and fruit size (for tomato) and production tuber/fruit per per plot.
d. Analysis of plant nutrient absorption.
Nutrient absorption data was obtained by measuring the absorption pattern of macro (N, P, K, Ca, and Mg) and micro (Fe, Cu and Zn) elements of the whole plant to understand the effectiveness of biofertilizer to improve solubilization of those element as well as plant growth and productivity. Analysis of nutrient absorption was conducted in the early phase of generative plants (flowering) by took whole the plant sample and dried into oven at 700Cduring 3 days then analysed by Atomic Absorption Spectrophotometer (AAS) in Laboratory of the Department of Soil and Land Resources, Faculty of Agriculture IPB Bogor. While, N was analyzed by method of Kjeldahl and P by spectrophotometer.
Statistical Analysis
Statistical methods used to analyze data of this study is as follows: Yijk = + i + j+ k + ()ij + ijk
Where:
Yijk: Observation at the i th
biofertilizer to j th
anorganic fertilizer k block
: is the population mean;
i: is the treatment effect of the i th
biofertilzer;
j: is the treatment effect of the jthanorganic fertilizer;
()ij: is the interaction effect between the i th
bioferilizer and j th
anorganic fertilizer;
γk: is the bloc effect of the kth bioferilizer;
ijk: is the random error;
The data were statistically analyized using SPSS 16 for Windows. Duncan
test at probability level 0.05 was used to separate the means when the ANOVA
RESULT
Soil Analysis
Soil samples in this experiment were found to have chemical properties as follows: acid pH (5.80), low organic C content (2,31), N-total was low (0.20), P Brayl low (5.2 ppm), Ca Low (1.53 me/100g), low Mg (0,89 me/100g), high K (0.54 me/100g). Based on soil physical properties, the soil texture was dominated by sand 36.84%, dust 43.88% and clay 19.28% (Appendix 1).
Analysis Compost
Nutrient content of compost used in this study has well qualified in accordance with the National Standardization Agency. Value of C / N ratio is between the minimum value (10) and maximum (20) as in Appendix 2.
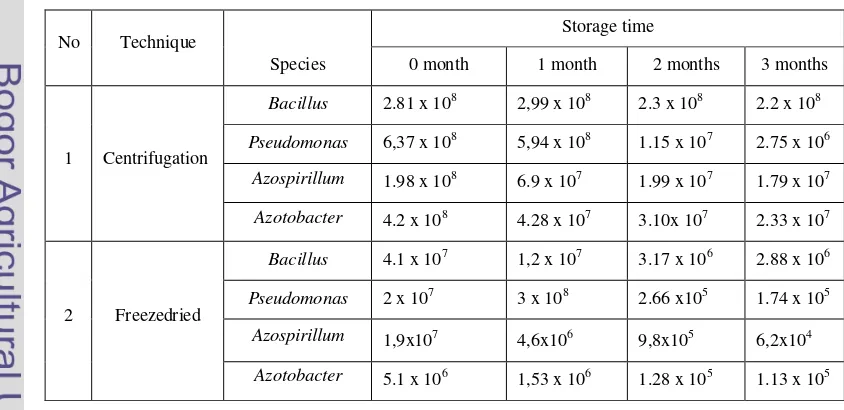
Viability of Bacteria
The result of viability test for bacteria during storage 0, 1, 2 and 3 months of storage presented in Table 1. Viability of bacterium declined slighting after freeze drying and centrifugation mechanism then it was stable until 2 month storage. After 2 months, the viability of each bacterium contained in biofertilizers was declined. Bacillus sp that produce by centrifugation showed did not decline in viability during storage. Pesodomonas bacteria also showed decline viability during storage. On the other hand, viability Azospirillum sp. and Azotobacter sp. also declined after 0 months storage in biofertilizer with both methods (Tabel 1).
Table 1 viability of bacteria that used as biofertilizer
No Technique
Species
Storage time
0 month 1 month 2 months 3 months
1 Centrifugation
Bacillus 2.81 x 108 2,99 x 108 2.3 x 108 2.2 x 108
Pseudomonas 6,37 x 108 5,94 x 108 1.15 x 107 2.75 x 106
Azospirillum 1.98 x 108
6.9 x 107 1.99 x 107 1.79 x 107
Azotobacter 4.2 x 108
4.28 x 107 3.10x 107 2.33 x 107
2 Freezedried
Bacillus 4.1 x 107 1,2 x 107 3.17 x 106 2.88 x 106
Pseudomonas 2 x 107 3 x 108 2.66 x105 1.74 x 105
Azospirillum 1,9x107
4,6x106 9,8x105 6,2x104
Azotobacter 5.1 x 106
[image:37.595.90.512.550.755.2]Generally, the viability of bacteria freezedried biofertilizer showed more decline rather than the bacterium viability of centrifugation mechanism bioferti-lizer.
Nutrient Absorption
A. Effect of Biofertlizer on Nutrient Absorption on Tomato Plant
[image:38.595.82.512.490.818.2]The results reported in Table 2 demonstrated clearly that application of biofertilizer at tomato plant increased significantly of nutrient absorption for the macro and micro as acompared to control. The highest increased of N absorption reached to 145.2 %, P 124.8%, K 98.9% that was obtained by application with centrifuged biofertilizer without storage (B4) that showed contains high amount of viable microbial population. While, absorption of Ca and Mg had maximum value by application of centrifuged biofertilizer with 3 months storage (B5) by about 447.3% and 317.9% as compared to control (B0). On the other hand, the lowest value of nutrient absorption macro showed by application with liquid biofertilizer (B1) at nutrient N, P, K, and Mg by approximately of 76.04 %, 45, 1%, 17% and 208.9% as a compared to control. For nutrient Ca, the lowest value indicated by freezedried biofertilizer without storage (B2).
Table 3 Means of nutrient absorption by application of biofertilizer in tomato.
treatment
Macro Micro
N(g) P(g) K(g) Ca(g) Mg(g) Fe (mg) Cu(mg) Zn(mg)
B0 1.7 a 0.3 a 3.3 a 0.1 a 0.3 a 19422.9 a 3668.3 a 10348.6 a
B1 3.1 b 0.4 ab 3.9 a 0.5 b 0.8 b 40220.3 bc 5514.6 ab 15524.7 abc
B2 3.3 b 0.5 bc 4.5ab 0.5 b 0.9 bc 34705.2 b 4871.9 a 14451.4 ab
B3 3.7 b 0.6 bc 4.6ab 0.6 b 0.9 bc 52971.9 c 5682.8 ab 16371.9 abc
B4 4.3 b 0.7c 6.6c 0.7 b 0.9 bc 43152.5 bc 7440.1 b 21850.4 c
B5 3.7 b 0.6 bc 5.9bc 0.7 b 1.2 c 41881.1 bc 5537.3 ab 18951.8 bc
Absorption micro nutrient siginifcantly influenced by biofertilizer application. The improvement was higher in Fe, with the maximum was showed by treatment of B3(172.2%). Cu and Zn also increased but not as high as Fe, and the maximam value showed by the treatment of B4 (102.8% and 111.1%) respectively (Table 2).
Biofertilizer improved total macro and micro nutrient uptake in tomato plant. The highest increased of total macro nutrient absorption obtained by application with centrifuged biofertilizer without storage (B4) by about 119.4% with mean 2.63 g per plant, and the highest increase of total micro nutrient absorption recorded by freezedried biofertilizer with storage 3 months (B3) followed by B4 about of 124.4% and 116.6% respectevily (Figure 4).
a.
Nutrient absorption macro b. Nutrient absorption micro
Figure 4 Nutrient absorption macro and micro as response to different biofertilizer application in tomato plant. B0: without biofertilizer, B1: liquid biofertilizer, B2: freezedried biofertilizer without storage, B3: freezedried biofertilizer with 3 months storage, B4: centrifuged biofertilizer without storage and B5: centrifuged biofertilizer with 3 months storage.
b.Effect of Biofertilizer on Nutrient Absorption in Potato Plant
Application of biofertilizer in potato plant not significant effect to nutrient absorption. There was atendency that application with liquid biofertilizer had the maximum in macro nutrient absorption (Table 3). On the other hand, the maximam absorption micro nutrient showed by application with B5.
0 2 4 6 8 10 12 14
B0 B1 B2 B3 B4 B5
n u tr ie n t ab so r p ti o n m ac r o (g) /p lan t biofertilizer Mg Ca K P N 0 10000 20000 30000 40000 50000 60000 70000 80000
B0 B1 B2 B3 B4 B5
Table 3 Means of nutrient absorption by application of biofertilizer in potato.
treatment
Macro Micro
N(g) P(g) K(g) Ca(g) Mg(g) Fe(mg) Cu(mg) Zn(mg)
B0 2.5 a 0.4 a 4.4 a 0.4 a 0.4 a 39141.7 a 4170.8a 13520.8a
B1 3.7 a 0.6 a 6.9a 0.6 a 0.7 a 75025.7 b 5885.8ab 21457.2a
B2 3.5 a 0.6 a 5.7a 0.6 a 0.5 a 61617.2ab 5287.1a 19777.6a
B3 2.9 a 0.5 a 4.4a 0.4 a 0.4 a 52558 ab 4215.3a 15047.1a
B4 2.5 a 0.4 a 4.3a 0.4 a 0.4 a 53622.2 a 5865.7ab 15728.5a
B5 3.3 a 0.6 a 6.4a 0.4 a 0.6 a 78176.6 b 8774.9b 21756.0a
Where: B0: without biofertilizer, B1: liquid biofertilizer, B2: freezedried biofertilizerwithout storage, B3: freezedried biofertilizer with 3 months storage, B4: centrifuged biofertilizer without storage and B5: centrifuged biofertilizer with 3 months storage. Means fowllowed by same litter in column are not significantly different at level 0, 05% by Duncan.
Total macro nutrient absorption tended to increase by application of biofertilizer except in B3 and B4. The highest increased of total macro nutrient were obtained by liquid biofertilizer (B1) by about of 53.9% with mean 2.51 g per plant as compared to control as in figure 4. In the total micro nutrient absorption, the highest increased was recorded by centrifuged biofertilizer with 3 months storage (B5) by about of 91.3% with mean 36235.8 mg per plant as compared to control (Figure 5).
a. Nutreint absorption macro b. Nutreint absorption micro
Figure 5 Nutrient absorption macro and micro as response to different biofertilizer application in potato plant. B0: without biofertilizer. B1: liquid biofertilizer, B2: freezedried biofertilizer without storage, B3: freezedried biofertilizer with 3 months storage, B4: centrifuged biofertilizer without storage and B5: centrifuged biofertilizer with 3 months storage. 0 2 4 6 8 10 12 14
B0 B1 B2 B3 B4 B5
n u tr ie n t ab so r p ti o n m ac r o (g) /p lan t biofertilizer Mg Ca K P N 0,0 20000,0 40000,0 60000,0 80000,0 100000,0 120000,0
B0 B1 B2 B3 B4 B5
[image:40.595.84.506.489.817.2]Plant Growth
A. Effect of Biofertilizer on Vegetative Growth of Tomato
The result showed that the application of biofertilizer increased vegetative growth indicated by plant height, steam diameter, leaf number and total dry weight (Figure 6).
a. Steam diameter (cm) b. leaf number
c. Plant height (cm)
d. Root dry weight
e. Total dry weight (g)
Figure 6 Tomato growth as response to different application biofertilizer. B0: without biofertilizer, B1: liquid biofertilizer, B2: freezedried biofertilizer without storage, B3: freezedried biofertilizer with 3 months storage, B4: centrifuged biofertilizer without storage and B5: centrifuged biofertilizer with 3 months storage. Stander error (SE) which show no different significant by Duncan test at 0.05%.
0 0,5 1 1,5 2
B0 B1 B2 B3 B4 B5
ste am d iam te r (m m ) biofertilizer 0 50 100 150
B0 B1 B2 B3 B4 B5
p lan t h igh (c m ) biofertilizer 0 50 100 150 200
B0 B1 B2 B3 B4 B5
to tal d r yw ie gh t biofertilizer 50 150 250 350 450
B0 B1 B2 B3 B4 B5
le af n u m b e r biofertilizer 0 2 4 6 8 10 12
B0 B1 B2 B3 B4 B5
Total dry weight and leaf number was significantly effected by application of either biofertilizer, meanwhile, plant height, steam diameter and root dry weight were only slightly affected (Figure 6). This caused significantly increased of total dry weight by the biofertilizer. The application of centrifugated biofertilizer (B4 and B5) that contains high amount of viable microbial population (Table 1) increased leaf number and total dry weight when compared with freezedried biofertilizer ( B2 and B3).
B. Effect of Biofertilizer on Vegetative Growth of Potato
a Plant heigh(cm) b. Leaf number
c.Steam diameter(cm) d. Root dry weight(g)
e. Total dry weight
Figure 7 Potato growth as response to different application biofertilizer. B0: without biofertilizer, B1: liquid biofertilizer, B2: freezedried biofertilizer without storage, B3: freezedried biofertilizer with 3 months storage, B4: centrifuged biofertilizer without storage and B5: centrifuged biofertilizer with 3 months storage. Stander error (SE) which show no different significant by Duncan test at 0.05%.
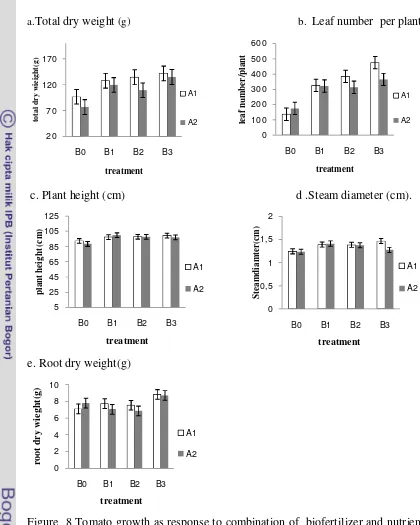
B. Effect of Combination Biofertilizer and Nutrition Sources on Plant Growth of Tomato
The result showed that the application of biofertilizer in combination with nutrition source caused increased vegetative growth of tomato plant when compared to control (A2B0) as presented in figure 8.
0,1 10,1 20,1 30,1 40,1 50,1
B0 B1 B2 B3 B4 B5
p lan t h igh (c m ) biofertilizer 0 0,2 0,4 0,6 0,8 1 1,2
B0 B1 B2 B3 B4 B5
ste am d ia m te r biofertilizer 0 50 100 150 200
B0 B1 B2 B3 B4 B5
to tal d r y w ie gh t(g) biofertilizer 50 150 250 350 450 550
B0 B1 B2 B3 B4 B5
le af n u m b e r biofertilizer 0 0,5 1 1,5 2 2,5 3
B0 B1 B2 B3 B4 B5
a.Total dry weight (g) b. Leaf number per plant
c. Plant height (cm) d .Steam diameter (cm).
e. Root dry weight(g) [image:44.595.86.506.94.619.2]
Figure 8 Tomato growth as response to combination of biofertilizer and nutrient source. B0: without biofertilizer, B1: liquid biofertilizer, B2: freezedried biofertilizer without storage , B3: freezddried biofertilizer with 3 months storage, A1:50% NPK, A2: 100% NPK. Stander error (SE) which show no different significant by Duncan test at 0.05%.
Leaf number was the parameter that mostly affected by application of either biofertilizer and anorganic fertilizer. On the other hand, plant height and steam diameter slightly affected (Figure 8). Consequently this caused significantly
20 70 120 170
B0 B1 B2 B3
to ta l d r y w ie ig h t(g ) treatment A1 A2 5 25 45 65 85 105 125
B0 B1 B2 B3
p lan t h e igh t (c m ) treatment A1 A2 0 2 4 6 8 10
B0 B1 B2 B3
r o o t d r y w ie gh t(g) treatment A1 A2 0 100 200 300 400 500 600
B0 B1 B2 B3
le af n u m b e r /p lan t treatment A1 A2 0 0,5 1 1,5 2
B0 B1 B2 B3
response of total dry weight by the treatment. Eventhough not significantly different, application with 50% of NPK tended to have higher response than 100% of NPK. Application with 100% NPK resulted in almost the same dry weight and plant height for combination with B1, B2 as well as B3.
C. Effect of Biofertilizer and Nutrition Source on Vegetative Growth of Potato
a. Leaf nuber b. Root dry weight (g)
c. Plant height(cm) d. Diameter of steam (cm)
c. Total dry weight (g)
[image:46.595.93.509.77.596.2]
Figure 9 Potato growth as response to combination of biofertilizer and nutrient source: B0 without biofertilizer, B1: liquid biofertilizer, B2: freezddried biofertilizer without storage, B3: centrifuged biofertilizer without storage, A1:50% NPK, A2: 100% NPK. Stander error (SE) which show no different significant by Duncan test at 0.05%.
Plant Production
A. Effect of Biofertilizer on Production of Tomato Plant
The result showed that the application of biofertilizer causes increased of average of crop production of tomato plant (Figure 10). There was no significant improvement in number fruit per plant and number fruit/cluster but the fruit weight per plant was increase as response to biofertilizer (Figure 10). The improvement was due to increase of fruit size indicated by higher fruit weight per
0 100 200 300 400 500 600
B0 B1 B2 B3
le af n u m b e r /p lan t treatment A1 A2 0 20 40 60
B0 B1 B2 B3
p lan t h igh (c m ) treatment A1 A2 0 50 100 150 200
B0 B1 B2 B3
to tal d r y w ie gh t(g) treatment A1 A2 0 0,5 1 1,5 2 2,5 3
B0 B1 B2 B3
r o o t d r y w ie gh t(g) treatment A1 A2 0 0,2 0,4 0,6 0,8 1 1,2
B0 B1 B2 B3
plant. In total plant, the production also increased significantly as response to biofertilizer. The higher increase of fruit weight per plant by application of B4 that contains high amount of viable microbial population by approximately 1,98x108 cell/mlmore than B3( 6,2x104 cell/ml), B5 and B2 (Table 1). Generally, application with centrifuged biofertilizer showed increased the fruit weight per plant rather than freezedried biofertilizer. In production per plot, the higher increase was by application with B2 (4,1x107cell/ml) and the lowest production by application of B3 (6.2x104 cell/ml).
a. Number Fruit /cluster b. Number of fruit per plant
c. Fruit weight(kg) per plant d. Plant production per plot
Figure 10 Tomato plant production as response to biofertilizer application. B0:
without biofertilizer, B1: liquid biofertilizer, B2: freezedried biofertilizer without storage, B3: freezedried biofertilizer with 3 months storage, B4: centrifuged biofertilizer with out storage and B5: centrifuged biofertilizer with 3 months storage. Stander error (SE) which show no different significant by Duncan test at 0.05%.
0 1 2 3 4 5 6 7 8
B0 B1 B2 B3 B4 B5
n u m b e r fr u it/ c lu ste r biofertilizer 0,50 1,00 1,50 2,00 2,50
B0 B1 B2 B3 B4 B5
fr u it w ie gh t( k g) /p lan t biofertilizer 0,5 5,5 10,5 15,5 20,5 25,5 30,5 35,5 40,5
B0 B1 B2 B3 B4 B5
n u m b e r fr u it/ p lan t biofertilizer 5 25 45 65 85 105
B0 B1 B2 B3 B4 B5
b. Effect of Biofertilizer on Production of Potato Plant
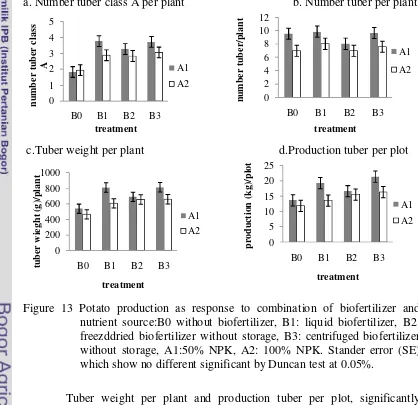
The result showed that the application of biofertilizer improve potato production (Figure 11). The number tuber per plant, tuber weight and number tuber class A per plant did not significantly affected by application of biofertilizer but it tended to increase. Production tuber per plot also significantly affected by treatment. The highest increased recorded by application with centrifuged biofertilizer with 3 months storage (B5) by about 47.3%, while, application with liquid biofertilizer (B1) showed the lowest value by about 14.1% as compared to control(B0) as in figure 11 .
a. Number tuber per plant b. Tuber weight (g) per plant
c. Number tuber class A per plant d. Production (kg) per plot
Figure 11 Potato plant production as response to biofertilizer application. B0: without biofertilizer, B1: liquid biofertilizer, B2: freezedried biofertilizer without storage, B3: freezedried biofertilizer with 3 months storage, B4: centrifuged biofertilizer with out storage and B5: centrifuged biofertilizer with 3 months storage. Stander error (SE) which show no different significant by Duncan test at 0.05%.
0 2 4 6 8 10
B0 B1 B2 B3 B4 B5
n u m b e r t u b e r / p lan t biofertilizer 0,0 0,5 1,0 1,5 2,0 2,5 3,