EFFECT OF TEMPEH SUPPLEMENTATION ON
GUT MICROBIOTA AND IMMUNOGLOBULIN A PROFILES
IN SPRAGUE DAWLEY RATS
SUSAN SOKA
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
DECLARATION OF THE SOURCE OF THIS DISSERTATION
I declare that this dissertation, entitled Effect of Tempeh Supplementation on Gut Microbiota and Immunoglobulin A Profiles in Sprague Dawley Rats is entirely my own work, assisted by a supervisory committee and has not been submitted in any form for another degree or diploma to any university or other tertiary institution of education. Where this dissertation draws on existing publications, those sources are cited in the text and listed in the references section.
RINGKASAN
SUSAN SOKA. Pengaruh Suplementasi Tempe terhadap Profil Mikrobiota Usus dan Imunoglobulin A pada Tikus Sprague Dawley. Dibimbing oleh ANTONIUS SUWANTO, DONDIN SAJUTHI dan IMAN RUSMANA.
Penelitian nutrigenomika saat ini telah menunjukkan bahwa interaksi gen dan makanan mempunyai peranan penting dalam menjaga kesehatan yang optimal. Selain itu, studi nutrigenomika juga telah menunjukkan bahwa triliunan bakteri yang ada pada saluran pencernaan manusia, atau yang lebih dikenal dengan mikrobiota usus, dapat mempengaruhi penyerapan nutrisi dan regulasi energi. Hal ini menunjukkan pentingnya peranan mikrobiota usus pada kesehatan manusia dalam perkembangan sistem kekebalan tubuh dan penggunaan energi.
Tempe merupakan makanan fermentasi tradisional Indonesia yang terbuat dari kacang kedelai (Glycine max) dan telah menjadi makanan yang populer di dunia terutama bagi kelompok vegetarian. Tempe dihasilkan melalui proses fermentasi kacang kedelai yang melibatkan sejumlah mikroorganisme seperti cendawan, khamir, bakteri asam laktat dan juga beberapa kelompok bakteri Gram negatif. Selama proses fermentasi, terjadi biosintesis vitamin, senyawa fitokimia dan komponen bioaktif lainnya yang dapat berpengaruh terhadap kesehatan. Selain itu, pada tempe masih dapat ditemukan komponen oligosakarida dan polisakarida yang bernilai positif terhadap kesehatan.
Mikroorganisme yang ada di dalam tempe akan turut masuk ke dalam saluran pencernaan jika tempe dimakan. Beberapa penelitian telah menunjukkan bahwa aktivitas probiotik dari mikroorganisme mati seringkali menunjukkan pengaruh yang sama dibandingkan dengan mikroorganisme hidup. Kandungan mikroorganisme yang ada di dalam tempe dapat menjadi salah satu alternatif suplemen diet yang mengandung probiotik dan memicu stimulan kekebalan tubuh pada manusia. Imunoglobulin A (IgA) merupakan imunoglobulin utama yang terdapat di saluran pencernaan dan mempunyai peranan dalam melindungi tubuh dari komponen dan juga mikrob asing.
Penelitian ini bertujuan menentukan dinamika populasi mikrobiota dan IgA dalam usus tikus Sprague-Dawley (SD). Sebanyak 30 ekor tikus SD betina diberi suplementasi pakan standar yang ditambah dengan kedelai yang belum diberi laru atau tempe (mentah atau masak) selama 28 hari. Teknik real-time polymerase chain reaction (RT-PCR) dengan primer spesifik terhadap sekuens gen penyandi 16S rRNA digunakan untuk mengkuantifikasi kelompok bakteri spesifik dari sampel feses tikus. Analisis ekspresi gen IgA dilakukan menggunakan metode quantitative RT-PCR, sedangkan protein IgA dianalisis dengan metode enzyme-linked immunosorbent assay dan pewarnaan imunohistokimia.
Hasil penelitian ini menunjukkan bahwa tempe dapat memodulasi komposisi mikrobiota usus, namun jenis tempe yang berbeda dapat memberikan perubahan komposisi yang berbeda pula. Hal ini disebabkan oleh keberadaan mikroorganisme yang unik pada setiap tempe.
menstimulasi sekresi protein IgA. Proses pemasakan tempe sangat dianjurkan dengan tujuan untuk menginaktivasi mikroorganisme patogen yang tidak diinginkan selama proses fermentasi.
SUMMARY
SUSAN SOKA. Effect of Tempeh Supplementation on Gut Microbiota and Immunoglobulin A Profiles in Sprague Dawley Rats. Supervised by ANTONIUS SUWANTO, DONDIN SAJUTHI and IMAN RUSMANA.
Recent nutrigenomics studies have showed that the interplay between genes and diet plays an important role in the development of certain diseases and in the maintenance of optimal metabolism. In addition, nutrigenomics showed that trillions of bacteria that normally reside within the human gastrointestinal tract, also referred as the gut microbiota, has been showed to affect nutrient acquisition and energy regulation. These findings showed the important role of gut microbiota in human health through its effect on the gut defense barrier, immune development, and nutrient utilization.
Tempeh is a well-known Indonesian fermented food made from soybean (Glycine max), and is now becoming popular in the world, especially in the diets of vegetarians. A diverse group of microorganisms including moulds, yeasts, lactic acid bacteria and different Gram-negative bacteria plays an important role during the fermentation process. Many studies have also reported that tempeh is a good source of protein, vitamin B12, phytochemicals and other bioactive
substances. Stachyose and raffinose are non-digestible-galactooligosaccharides in soybean which can still be found in tempeh. There are many health benefits associated with this dietary fiber component, not least the effect on satiety and fecal bulking.
Tempeh is consumed in the form of cooked tempeh and consuming cooked tempeh may further expose gastrointestinal tract to many non-viable microorganisms. Despite the common definition that probiotics are live microorganisms, various biological responses have been reported after administering dead, frequently heat-killed, probiotics to various mammalian and avian species. Direct interactions between the host and non-viable bacterial cells are based on the capacity of human cells to recognize specific bacterial components or products, giving rise to responses that commonly involve the mucosa-associated lymphoid tissue and, therefore, the immune system. Secretory immunoglobulin A (IgA), as the most abundant intestinal immunoglobulin on the surface of the mucosa, has further the combined task of protecting against foreign substances and microbes
The objective of this study was to evaluate the impact of Empang and Warung Jambu tempeh on gut microbiota composition and mucosal IgA in Sprague-Dawley rats. In this study, thirty female Sprague-Dawley (SD) rats were fed a standard diet, supplemented with either non-fermented soybeans or tempeh (raw or cooked), for 28 days. The specific bacterial groups in fecal samples were quantified using real-time PCR with 16S rRNA gene-targeted, group-specific primers. The gene expression of intestinal IgA was analyzed using the semi quantitative real-time PCR, and intestinal IgA was further quantified from the ileum wash using the ELISA and immunohistochemistry methods.
variable health benefits due to its unique microorganism composition that plays an important role during the fermentation process.
The result of IgA analysis indicated that both live and dead microorganisms can confer health benefits by stimulating IgA secretion in gut mucosal tissue. Consuming cooked tempeh may offer a further health benefit, as the cooking process inactivates pathogenic microorganisms that may occur in tempeh during the fermentation processes.
©Copyright Bogor Agricultural University, 2014
This copyright is protected by law
Any unauthorized quotation of part or this entire dissertation without referencing the author and institution is prohibited. Reproduction of this material is only permitted for research and education purposes and must not undermine the rights of Bogor Agricultural University
Dissertation
as one of the requirements of the PhD program in the Major Microbiology
EFFECT OF TEMPEH SUPPLEMENTATION ON
GUT MICROBIOTA AND IMMUNOGLOBULIN A PROFILES
IN SPRAGUE DAWLEY RATS
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
Examiners of Closed Examination : Prof. Dr. Lilis Nuraida, M.Sc. Raymond Tjandrawinata, Ph.D.
Title of Dissertation : Effect of Tempeh Supplementation on Gut Microbiota and Immunoglobulin A Profiles in Sprague Dawley Rats Name : Susan Soka
NIM : G36111041
Approved by Supervisory Committee
Prof. Dr. Antonius Suwanto, M.Sc Head of Committee
Prof. drh. Dondin Sajuthi, MST, Ph.D. Dr. Iman Rusmana, M.Sc.
Member of Committee Member of Committee
Authorized by
Head of Major Microbiology
Prof. Dr. Anja Meryandini
Dean of Graduate School
Dr Ir Dahrul Syah, M.Sc.Agr.
Date of Opened Examination: 27 November 2014
ACKNOWLEDGEMENTS
It would not have been possible to complete this dissertation without the support, patience and guidance of the following people. It is to them that I owe my deepest gratitude.
My promotor, Prof. Dr. Antonius Suwanto, M.Sc., for his endless support, enthusiasm, knowledge and patience during my graduate study at Bogor Agricultural University.
My co-promotor, Prof. drh. Dondin Sajuthi, MST, Ph.D. for his guidance and knowledge in Immunology and animal study.
My co-promotor, Dr. Iman Rusmana for his support, guidance and input throughout finalizing my dissertation.
Indonesian Minister of Education for the doctorate scholarship and research funding.
Dr. Ir. Gayuh Rahayu, MS and Prof. Dr. Anja Meryandini as former Head and Head of Graduate Programe Microbiology in Bogor Agricultural University for their support during my study, respectively.
Dean of Faculty of Biotechnology at Atma Jaya Catholic University of Indonesia, Dr. Diana E. Waturangi, M.Si., for giving me the opportunity to finish my doctorate study and also for providing the laboratory facilities.
Dr. Diah Iskandriati, MS for the provision of the animal laboratory facility in Primate Research Center at the Bogor Agricultural University.
My fellow postgraduate students and friends both at Bogor Agricultural University and PT. Bimana Indomedical in Bogor for their support and friendships.
Finally, and most importantly, I take this opportunity to express my gratitude to my family for their love, unfailing encouragement and support. I would like to thank my husband Erwin for his patience during the ups and downs of my study. I would like to thank my sons, Darrence and Jeremy, for their loves. I thank my parents for their love and faith in me. And also, I thank
Erwin’s parents, for providing me with unending encouragement and support.
CONTENTS
LIST OF TABLES xv
LIST OF FIGURES xv
INTRODUCTION 1
Background 1
Hypothesis 2
Objectives 2
Novelty 3
LITERATURE REVIEW 3
Tempeh 3
Carbohydrates in Soybean 4
Oligosaccharides in Soybean Tempeh 4
Gut Microbiota and Immune System 6
The Paraprobiotic Concept 8
Real Time-Polymerase Chain Reaction 8
MATERIALS AND METHODS 9
Tempeh Preparation 9
Protein Quantification in Tempeh 9
Animal Study 9
Total Fecal Bacterial DNA Extraction 10
Construction of Standard Curve 10
Quantification of Bacterial Specific-Group from Feces 11
Extraction of the Ileum and the Colon 11
ELISA IgA Quantification 11
Assesment of mRNA Gene Expression Using Real-Time Polymerase Chain
Reaction (PCR) 12
Immunohistochemistry of sIgA in the Gut 13
Statistical Analysis 13
RESULTS AND DISCUSSIONS 13
Empang- and Warung Jambu Tempeh 13
Impact of Tempeh Supplementation on Gut Microbiota Composition in
Sprague-Dawley Rats 15
Impact of Tempeh Supplementation on Mucosal Immunoglobulin A in
Sprague-Dawley Rats 21
CONCLUSION 32
REFERENCES 33
APPENDIX 40
LIST OF TABLES
1 16S rRNA gene-targeted, group specific primers 11
2 Primer sequences used for qRT-PCR 12
3 qRT-PCR conditions 13
4 The protein content and total added non-fermented soybean and tempeh
in animal feed. 15
5 Impact of Empang (EMP) tempeh on gut microbiota composition 17 6 Impact of Warung Jambu (WJB) tempeh on gut microbiota composition 18 7 The ratio of Firmicutes/Bacteroidetes in the three groups following
supplementation with Empang tempeh for 28 days 20 8 The ratio of Firmicutes/Bacteroidetes in the three groups following
supplementation with Warung Jambu tempeh for 28 days. 20
LIST OF FIGURES
1 The difference of total fecal bacteria before and after Empang tempeh
supplementation for 28 days 19
2 The difference of total fecal bacteria before and after Warung Jambu
tempeh supplementation for 28 days 19
3 Effect of tempeh supplementation on IgA gene expression 22 4 Effect of tempeh supplementation on IgA concentration in ileum
washes 23
5 Immunohistochemical detection of IgA in the ileum section of rats
supplemented with cooked Empang tempeh 25
6 Immunohistochemical detection of IgA in the ileum section of rats
supplemented with cooked Empang tempeh 26
7 Immunohistochemical detection of IgA in the ileum section of rats supplemented with non-fermented Empang soybean 27 8 Immunohistochemical detection of IgA in the ileum section of rats
supplemented with cooked Warung Jambu tempeh 28
9 Immunohistochemical detection of IgA in the ileum section of rats
supplemented with raw Warung Jambu tempeh 29
INTRODUCTION
Background
Recent nutrigenomics studies have showed that the interplay between genes and diet plays an important role in the development of certain diseases and in the maintenance of optimal metabolism. In addition, nutrigenomics showed that trillions of bacteria that normally reside within the human gastrointestinal tract (referred to as the gut microbiota) can affect nutrient acquisition and energy regulation. This suggests that obese and lean people have different gut microbiota (Ley et al. 2005). These findings showed the important role of gut microbiota in human health through their effect on the gut defense barrier, immune development, and nutrient utilization. Comparative studies using 16S rRNA sequencing have shown that the intestinal microbiota in mice and humans is very
similar in composition at the division level (i.e., ≥80% of both mouse and human
microbiota is dominated by two phyla, the Firmicutes and the Bacteroidetes) (Sekirov et al. 2010). However, composition of a microbial community is host
specific. It evolves throughout an individual’s lifetime and is susceptible to both
exogenous and endogenous modifications. The composition of human gut microbiota is mainly influenced by maternal colonization but is further influenced by diet, environmental exposures, and antimicrobial therapies. Diverse disorders, such as antibiotic-associated diarrhea, Crohn’s disease, ulcerative colitis, and obesity have been correlated with large-scale imbalances in the gastrointestinal microbiota, demonstrating the importance of commensal microorganisms in maintaining gastrointestinal balance.
Tempeh is a well-known Indonesian fermented food made from soybean (Glycine max). It is gaining popularity in the world, especially in the diets of vegetarians. A diverse group of microorganisms including molds, yeasts, lactic acid-producing bacteria, and some Gram-negative bacteria plays an important role during the fermentation process (Steinkraus et al. 1983). Many studies also have reported that tempeh is a good source of protein (Astuti et al. 2000), vitamin B12
2
(Samra and Anderson, 2007) and fecal bulking. Therefore, many countries are attempting to increase the recommended dietary fiber intake in an effort to prevent some of the diseases afflicting modern society.
Moreover, tempeh is consumed in the form of cooked tempeh and consuming cooked tempeh may further expose gastrointestinal tract (GIT) to many non-viable microorganisms. Most scientific studies define probiotics according to the FAO/WHO definition, which describes them as dietary supplements containing viable nonpathogenic microorganisms that, when administered in adequate amounts, confer a health benefit on the host. However, recent studies have shown that the different amounts of non-viable microorganisms found in probiotic products might contribute to the variation in response that is often seen with live probiotics (Kataria et al. 2009). Direct interactions between the host and non-viable bacterial cells are based on the capacity of human cells to recognize specific bacterial components or products, giving rise to responses that commonly involve the mucosa-associated lymphoid tissue (MALT) and, therefore, the immune system (Adams 2010). Various
microbial components such as cell homogenates, β-glucans, teichoic and lipoteichoic acids, peptidoglycans (PGNs), and lipopolysaccharides (LPSs) have been proven to have an immunomodulatory effect by stimulating innate immune systems (Adams 2010; Taverniti and Guglielmetti 2011). Secretory immunoglobulin A (sIgA) is the most abundant intestinal immunoglobulin on the surface of the mucosa, which has the combined task of protecting against foreign substances and microbes (Harris et al. 2006).
Hypothesis
Many microorganisms are involved during the making of tempeh. Whether tempeh is consumed in the cooked or raw form, these microorganisms may serve as non-viable probiotics for the host and therefore increasing the immune system. Moreover, non-soluble polysaccharides presented mainly by soybean and Rhizopus sp., can serve as prebiotics. Consuming tempeh that might play a role as probiotics and prebiotics might shift the bacterial composition of gut microbiota. The following microbial shifting is expected toward a healthier gut microbiota composition.
Objectives
3 Novelty
The impact of tempeh supplementation on gut microbiota composition and mucosal immunity in Sprague-Dawley rats has not been studied. This research will study how tempeh, which can be considered as a potential source of non-viable probiotics and prebiotics, may become a possible strategy to stimulate the immune system and manage the complex gut microbial community towards a health-promoting composition. Therefore, the result of this nutrigenomic studies will further give tempeh an added nutritional value.
LITERATURE REVIEW
Tempeh
Tempeh is a traditional Indonesian fermented food in which soybeans are hydrated and acidified, dehulled, cooked and fermented with Rhizopus spp. There is no standard process for tempeh making, therefore many variations can be found from one region and one producer from another. During the fermentation process of tempeh, there are many valuable changes not only in the increase of nutritional values of some nutrients in soybean, but also in the development of vitamins, phytochemicals and other bioactive compounds (Astuti et al. 2000). Enzymes produced during fermentation affect protein, fat and carbohydrates contents in soybean. Soybean oligosaccharides such as stachyose and raffinose, which cause flatulence, are broken down into digestible sugars. Moreover, the fungus produces the enzyme phytase that mobilize phytic acid improving bioavailability of minerals (Nout and Kiers 2005).
Most of microbiological studies on tempeh production have been focused on the fungi as starter, however, the association of bacteria and yeast during the fermentation process has been reported (Nout and Kiers 2005; Barus et al. 2008; Seumahu et al. 2012). Klebsiella pneumoniae and Citrobacter freundii found in fresh tempeh implicated their roles in the production of vitamin B12 during the
4
faecium and for producing malic acid is L. casei (Mulyowidarso et al. 1991a). The presence of bacteria producing organic acids during the soaking process showed their important roles in the inhibition of various pathogenic and spoilage microorganisms in tempeh (Nout and Kiers 2005).
Carbohydrates in Soybean
Soybean (Glycine max) contains ~35% carbohydrates which consist mainly of non-starch polysaccharides and oligosaccharides (Dixit et al. 2011). Polysaccharides are composed mainly of insoluble dietary fiber, such as cellulose and pectins. Soybean oligosaccharide is a group of soluble low molecular weight oligosaccharides in soybean seeds, which include sucrose (1%), stachyose (4%) and raffinose (1.1%) (Choct et al. 2010). Stachyose is a tetraose with a structure
of α-D-galactopyranosyl-[1→6] – α – D – galactopyranosyl – [1→ 6] – α – D – glucopyranosyl –[1 → 2] –β–D–fructofuranoside, while raffinose is a triose with
a structure of α–D–galactopyranosyl–[1→6]–α–D-glucopyranosyl–[1→2]–β–D– fructofuranoside. Stachyose and raffinose are defined as non-digestible galactooligosaccharides (GOS). GOS content of soybean has been shown to vary with the degree of maturity. Immature green seeds contain fewer amounts of GOS than fully matured yellow soybean (Espinosa-Martos and Rupérez 2006).
Humans do not posses α-galactosidase necessary for hydrolyzing the linkage present in oligosaccharides, so that they cannot be digested when consumed. When these intact oligosacharides reach the colon, they will be preferentially fermented by colonic bacteria that posses the enzyme (Liu 1999). Anaerobic bacterial fermentation of these substrates results in the production of gases which cause uncomfortable flatulence. Many studies have been carried out to reduce the oligosaccharides content in soybean products by processing techniques such as soaking, cooking, fermentation and enzyme treatment (Egounlety and Aworh 2003; Gote et al. 2004). However, many clinical researches have suggested that oligosaccharides, with approximately 30 to 50% caloric value of sucrose, are interesting because of their prebiotic activity and associated health benefit (Roberfroid and Slavin 2000). Along with the progress of oligosaccharides researches, it was found that soybean oligosaccharides are not the direct causes of flatulence (Cui 2001).
Oligosaccharides in Soybean Tempeh
Stachyose and raffinose are non-digestible-galactooligosaccharides in soybean which can still be found in tempeh although it has been reported that the concentrations of oligosaccharides in soybeans are reduced during the fermentation process. However, there are considerable differences in the extent of the reductions reported.
5 65% and 50% respectively after soaking at 30°C for 24h. Meanwhile, when a mixture of antibiotics was added in the soak-water in order to completely inhibit the growth of microorganisms, the decrease of stachyose concentrations was reduced. Thus, at the end of 24h soaking, the soybean contained approximately 1.6% stachyose, compared with approximately 1% stachyose for soaking in the absence of antibiotics (Mulyowidarso et al. 1991b). The decreased concentration of raffinose was approximately the same as that which occurred during soaking in the absence of antibiotics.
The changes of these oligosaccharide concentrations within the beans during soaking could be attributed to endogenous enzyme metabolism, diffusion into the external environment of the soak-water and microorganisms activities that grow in the soak-water (Mulyowidarso et al. 1991b). Total microbial populations of 1010 CFU mL-1were developed during the first 18h of soaking at 30°C with the
main species are L. casei, S. epidermidis, S. faecium and Streptococcus dysgalactiae (Mulyowidarso et al. 1989). α-galactosidases produced by these microbial species in the soak water could diffuse into the soybean and accelerate the hydrolysis of soybean oligosaccharides. Moreover, microbial fermentation of glucose and reduction in pH of the soak-water and soybean to 5.0 could affect the
activity of soybean α-galactosidase. Since the pH optimum of this enzyme has been reported to be 5.0 (Harpaz et al. 1977), microbial fermentation is likely to stimulate the enzyme activity. Recent studies have also reported the role of some bacteria during the fermentation process in tempeh production (Barus et al. 2008; Seumahu et al. 2012). In conclusion, the decrease of the oligosaccharides concentration within the soybean will depend on the microbial species that develop and predominate in the soak-water (Mulyowidarso et al. 1991b).
Furthermore, Ruiz-Terán and Owens (1999) studied the fate of oligosaccharides, stachyose and raffinose, during production of soybean tempeh. Stachyose and raffinose were monitored during the preparation of bacteria-free tempeh made with R. oligosporus NRRL 2710. The essential losses of oligosaccharides were due to leaching during hydration, washing and cooking of the soybean. Meanwhile, the concentrations of these oligosaccharides did not change during the fermentation. The result of this study was in agreement with observations that, in vitro, tempeh moulds did not use the oligosaccharides as sole sources of carbon and energy (Graffham et al. 1995). Therefore, it is possible to control the concentration of oligosaccharides by making choice of conditions for hydrating and cooking the beans. This control might be facilitated by the fact that the oligosaccharides are not utilized in tempeh by the moulds.
On the other hand, a recent study on the effect of fermentation with Rhizopus oligosporus on the oligosaccharides of soybean, cowpea (Vigna unguiculata) and groundbean (Macrotyloma geocarpa) was done by Egounlety and Aworh (2003). Stachyose and raffinose decreased during 48h fermentation with a reduction of 83.9% and 55.4%; 91.5% and 53.8%, and 85.5% and 54.0%
respectively for soybean, cowpea and groundbean. This study concluded that α-D
6
The conflicting reports in the literature on the production of oligosaccharidases and the utilization of oligosaccharides during tempeh fermentation are possibly a consequence of the presence of bacteria, or, in some cases, could be due to the use of different mould strains (Ruiz-Terán and Owens 1999). However, it is still possible to control the concentration of oligosaccharides by making choice of conditions for hydrating and cooking the beans and selecting the microorganisms used during fermentation. Therefore, the possible prebiotic effect of soybean can still be found in tempeh.
Gut Microbiota and Immune System
Recent studies suggested that the trillions of bacteria that normally reside within the human gastrointestinal tract, also referred as the gut microbiota, affect nutrient acquisition and energy regulation; it suggests further that obese and lean people have different gut microbiota. The composition of this microbial community is host specific, evolving throughout an individual's lifetime and susceptible to both exogenous and endogenous modifications (Sekirov et al. 2010). Firmicutes and Bacteroidetes are closely related to body fat in humans and mice, which are the two dominant bacterial divisions of gut microbiota in mammals. Comparisons of the distal gut microbiota of obese and lean mice, and those of obese and lean humans revealed that a statistically significant reduction in the relative abundance of Bacteriodetes and a significantly greater proportion of Firmicutes were in obese animals than in lean controls (Ley et al. 2005).
In healthy adults, 80% of the identified fecal microbiota can be classified into three dominant phyla: Bacteroidetes, Firmicutes, and Actinobacteria (Lay et al. 2005). The Firmicutes and Bacteroidetes ratio is regarded to be of significant relevance in human gut microbiota composition. However, the fecal microbiota is a highly complex and diverse bacterial ecosystem. Within this ecosystems exists a hierarchy of dominant (>109 CFU g-1) anaerobic bacteria, represented by the genera Bacteroides, Eubacterium, Bifidobacterium, Peptostreptococcus, Ruminococcus, Clostridium and Propionibacterium, and sub-dominant (<109 CFUg-1), bacteria of the Enterobacteriaceae family, especially Escherichia coli,
and the genera Streptococcus, Enterococcus, Lactobacillus, Fusobacterium, Desulfovibrio, and Methanobrevibacter (Harmsen et al. 2002).
7 induce the formation of antimicrobial and formation a potent physico-chemical barrier, which is access and the growth of pathogens such as Clostridia, Salmonella, and Shigella (DiBaise et al. 2008; Bensussan and Routhiau 2010). In addition, the gut microbiota is also implicated in gastrointestinal motility and various metabolic functions such as breaking down of toxin and carcinogen compounds, synthesizing micronutrients, synthesis of micronutrients, and assisting in the absorption of certain electrolytes and trace minerals (DiBaise et al. 2008).
Mucosal surfaces consist of various lymphoid structures collectively referred to as mucosa-associated lymphoid tissue (MALT) (Macpherson et al. 2008). This secondary lymphoid organ can be further divided in functionally connected subregion, including the gut-associated lymphoid tissue (GALT), nasopharynx-associated lymphoid tissue (NALT), and bronchus-associated lymphoid tissue (BALT) (Kiyono and Fukuyama 2004). The development of
GALT, such as Peyer’s patches (PPs) of distal ileum, isolated lymphoid follicles (ILFs) and mesenteric lymph nodes (MLNs), is initiated prenataly in the sterile environment of the fetus through induction by lymphoid tissue inducer (LTi) cells (Maynard et al. 2012). During fetal development, LTi cells disseminate to the MLN and PPs anlagen, stimulating the development of these structures, and the recruitment of B and T cells into B-cell follicles and T-cell zones that characterize secondary lymphoid tissues (Cherrier and Eberl 2012). Meanwhile, ILFs develop from cryptopatches only after colonization by the intestinal microbiota.
Secretory immunoglobulin A (sIgA) is the most abundant intestine immunoglobulin on the surface of the mucosa and has the combined task of protecting against foreign substances and microbes (Harris et al. 2006). sIgA present in milk is transferred to the offspring and the specificities of this IgA have been shaped by the maternal microbiota (Maynard et al. 2012). The inner mucous layer of small intestine provides a relatively impermeable barrier against the
microbiota. This characteristic is a result of the layer’s compact structure and its
function as reservoir for antimicrobial products, including sIgA and antimicrobial peptides. The polymeric IgA (pIgA) secreted by plasma cells diffuses through the stroma and binds to the polymeric immunoglobulin receptor (pIgR) on the basolateral surface of the epithelial cells to form the pIgA-pIgR complex, which in turn is translocated to the apical surface of the epithelial cell where it is cleaved and secreted into lumen as sIgA (Kaetzel and Mostov 2005).
8
The Paraprobiotic Concept
Probiotics are usually defined as dietary supplements, containing viable non-pathogenic microorganisms, which are considered to confer health benefits to the host through their interactions with gastrointestinal microbiota and directly with the immune system. Despite the general definition that probiotics are live microorganisms, a variety of biological responses have been reported from administering dead, frequently heat-killed, probiotics to various mammalian and avian species. The preparations of dead cells have also been fractionated and various cellular components shown to produce a range of biological responses (Adams 2010). Several mechanistic studies have demonstrated that specific chemical compounds isolated from bacteria can induce specific immune responses (Taverniti and Guglielmetti 2011). These investigations provide the scientific basis for a molecular explanation of the immunological effects observed in vivo after administration of inactivated probiotic bacteria or probiotic cell extracts. Excluding extracellular bacterial products, a major role in immunomodulatory activity should be mediated by the structural components of the cell, particularly the cell envelope, the outermost structure that immune system cells come into contact with first, which includes cell wall constituents or, if they are present, S-layer proteins, capsule and pellicle (Chapot-Chartier et al. 2010).
Real Time-Polymerase Chain Reaction
Real-time PCR (RT-PCR) amplifies a specific target sequence in a sample then monitors the amplification progress using fluorescent technology (Valasek and Repa 2005). Enough amplified product accumulates to yield a detectable fluorescent signal. Cycle time (CT) is the cycle number when fluorescent is
detectable. If a large amount of templates present at the start of the amplification reaction, the reaction will have low CT value, and just the opposite. In this study,
SYBR green, which is an intercalating agent, will be used for detection (Bustin et al. 2005). SYBR green is a nonspecific dye. It binds to the minor groove of dsDNA, emitting 1,000-fold greater fluorescence than when it is free in solution.
9 Group-specific primers developed by Guo et al. (2008) and Matsuki et al. (2004) were used in this study to quantify Firmicutes, C. leptum, Bacteroidetes and Bacteroides fragilis from fecal samples. Guo et al. (2008) developed RT-PCR assays that allowed rapid and convenient quantification of Firmicutes and Bacteroidetes in fecal samples. On the other hand, Matsuki et al. (2004) developed RT-PCR method to investigate the composition of human intestinal flora by using DNA extracted from fecal samples. The advantages of this method are higher sensitivity, easy sample handling, and simple procedures. Therefore, the group-specific PCR method showed promise as an effective tool for investigating the effects of prebiotics or probiotics.
Many studies have further confirmed that RT-PCR is a powerful technique in studying the diverse and complex fecal microbiota. Mariat et al. (2009) used this technique to determine the Firmicutes/Bacteroidetes ratio which evolves throughout life, from early childhood to old age. This technique was also used to study the impact of coffee consumption on the human gut microbiota (Jaquet et al. 2009) and in vitro modulation of the human gastrointestinal microbial community by plant-derived polysaccharide-rich dietary supplements (Marzorati et al. 2010).
MATERIALS AND METHODS
Tempeh Preparation
Tempeh was collected from two different locations in Bogor, Indonesia. Empang (EMP) and Warung Jambu (WJB) tempeh collected in April 2013 were used for this study. These tempeh samples have been previously explored by Barus et al. (2008) and Seumahu et al. (2012). Cooked samples of tempeh were prepared by steaming tempeh for 10 min in a steamer. The raw and cooked samples of soybean tempeh were further sliced, freeze-dried, and powdered. Soybean tempeh powder was then mixed with the animal feed. Non-fermented soybean from EMP and WJB tempeh were added to the feed for the control group.
Protein Quantification in Tempeh
The protein content of non-fermented soybean and tempeh was quantified using Kjeldahl method (AOAC 1990).
Animal Study
10
into six groups of five rats. The first and second groups were supplemented with cooked and raw EMP tempeh. The third group was the control EMP tempeh group, which was supplemented with non-fermented EMP soybean. The fourth and fifth groups were supplemented with cooked and raw WJB tempeh. The sixth group was a control WJB tempeh group, which was supplemented with non-fermented WJB soybean. Tempeh added 10% protein to the standard basal diet containing 18% protein, 3% crude fat, and 18% crude fiber. Fecal samples were collected before and after 28 days of treatment. All animal procedures were approved by Ethical Commission of Primate Research Center, Bogor Agricultural University, Bogor, Indonesia (ACUC Nr. R.09-13-IR)
Total Fecal Bacterial DNA Extraction
Total fecal bacterial DNA was extracted using QIAamp® DNA Stool Minikit (Qiagen, Hilden, Germany) with a 1-min bead-beating modification (Furet et al. 2009). To verify genomic DNA, electrophoresis was performed with 5 µL DNA in a 1% agarose gel run at 60 V for 90 min, stained with ethidium bromide and visualized using UV light.
Construction of Standard Curve
The polymerase chain reaction (PCR) master mix contained 1 µL of DNA template, 12.5 µL GoTaq Green® Master Mix (Promega, Madison, WI, USA), 1 µL of each primer (10 pmol µL-1) (Table 1) (Matsuki et al. 2004; Guo et al.
2008), and nuclease-free water for a total volume of 25 µL. The PCR reaction conditions for amplification of DNA were 94 °C for 5 min; 30 cycles of 94 °C for 30 s, 50 °C or 60 °C for 30s (Table 1), and 72 °C for 1 min; and final extension at 72 °C for 10 min. PCR was performed with Applied Biosystems 2720 thermal cycler (Applied Biosystems, Foster City, CA, USA).
The amplified products were purified using the QIAquick® PCR Purification Kit (Qiagen, Valencia, CA, USA), cloned into pGEM-T Easy vectors
(Promega, Madison, WI, USA), and transformed into DH5α competent
Escherichia coli cells. The plasmid containing 16S rRNA gene sequences of the targeted group was extracted using the Wizard®Plus SV Minipreps DNA
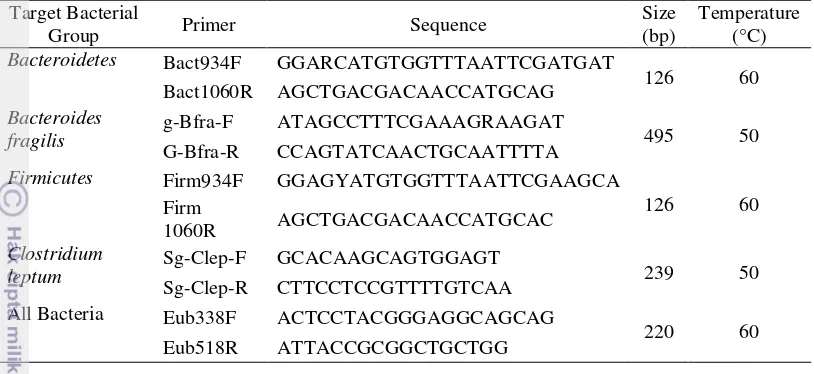
11 Table 1 16S rRNA gene-targeted, group-specific primers
Target Bacterial
leptum Sg-Clep-F GCACAAGCAGTGGAGT 239 50
Sg-Clep-R CTTCCTCCGTTTTGTCAA All Bacteria Eub338F ACTCCTACGGGAGGCAGCAG
220 60
Eub518R ATTACCGCGGCTGCTGG
Quantification of Bacterial Specific-Group from Feces
Fecal samples were collected before and after 28 days of tempeh supplementation. RT-PCR amplification and detection of bacterial specific-group from fecal samples were performed with the iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad, Palo Alto, CA, USA). A triplicate PCR reaction was performed on a 20 µL (total volume) mixture of 10 µL SsoFast™ EvaGreen® Supermix (Bio-Rad, Hercules, CA, USA), 1 µL each of the specific primers at a concentration of 10 pmol µL-1, 1 µL template DNA, and nuclease-free water. The amplification reaction conditions were 94 °C for 5 min; 40 cycles of 94 °C for 20 s, 50 or 60 °C for 20 s (Table 1); and 72 °C for 50 s (Matsuki et al. 2004; Guo et al. 2008).
Extraction of the Ileum and the Colon
A maximum of 30 mg of tissue was obtained from each animal, corresponding to the distal ileum and the proximal colon. Specimens were frozen in liquid N2 and stored immediately at -80 °C until processing. The distal portion
of small intestine and proximal colon were weighed, longitudinally opened, and incubated with phosphate-buffered saline (PBS) in a shaker for 20 min at 37 °C. The obtained suspension was centrifuged and the supernatant (intestinal wash) was stored at -20 °C until IgA quantification using the enzyme-linked immunosorbent assay (ELISA) technique.
ELISA IgA Quantification
12
to PBS-Tween-5% skimmed milk for 1 h at 37 °C, and then the plates were washed four times with PBS-Tween 0.01%. The plates were incubated for 1 h at 37 °C with goat anti-mouse IgA horseradish peroxide (HRP) (Santa Cruz Biotechnology, Texas, USA) at a 1:1000 dilution. Subsequently, IgA was detected by adding the substrate (3,3',5,5'-tetramethylbenzidine), which was diluted in phosphate citrate buffer (Sigma, USA). The enzymatic reaction was stopped using H2SO4 2N, and absorbance was measured at 450 nm. Data were interpolated into
the IgA standard curve, and IgA concentrations were expressed as nanogram per milligram gut weight (ng mg-1).
Assessment of mRNA Gene Expression Using Real-Time Polymerase Chain Reaction (PCR)
Total RNA was extracted from ileum and colon tissue samples using
RNeasy Mini Kit (Qiagen, USA), according to the manufacturer’s instructions.
The obtained RNA was quantified by a NanoDrop 2000 (Thermo Fisher Scientific, USA) instrument.
Primer sequences, specific for IgA- and β-actin-encoding genes, were designed using the NetPrimer program (Table 2). mRNA sequence encoding using the Rattus norvegicus Fc fragment of the IgA gene was used as a template to
design the primer, and the housekeeping gene, β-actin, was used as the reference gene. Full sequences of the IgA and β-actin genes were taken from the GenBank database (http://www.ncbi.nlm.nih.gov).
Table 2 Primer sequences used for qRT-PCR
Target gene Accession No. Primers IgA NM_201992 F:
Quantitative real-time PCR (qRT-PCR) was performed in an iQ5 real-time detection system (BioRad, USA) using a KAPA SYBR FAST one-step qRT-PCR kit (Kapa Biosystem, South Africa). The master mix for qRT-PCR consisted of 10 µL 2x KAPA SYBR FAST qPCR master mix, 1 µ L forward primer (10 pmol µL
-1), 1 µ L reverse primer (10 pmol µL-1), 0.4 µ L dUTP (10 mM), 0.4 µ L KAPA RT
mix, 6.2 µL nuclease-free water, and 1 µ L RNA template (100 ng µ L-1). The
conditions for qRT-PCR are described in Table 3.
Gene expression levels were given by 2-ΔΔCt, where Ct is the cycle number at which the fluorescence signal of the PCR product crosses an arbitrary threshold
set within the exponential phase of the PCR and ∆∆Ct = [(Ct (unknown sample) – Ct (β -actin, unknown sample)) – (Ct (control) – Ct (β-actin, control))]. The results are expressed as the
13 Table 3 qRT-PCR conditions
Steps Temperature (°C) Time Repeat(s)
cDNA synthesis (min)
Immunohistochemistry of sIgA in the Gut
Monoclonal IgA antibody (Santa Cruz Biotehnology, USA) was used as the primary antibody. Paraffin-embedded section of ileum was deparaffinized in xylene, rehydrated in a graded alcohol series, and stained immunohistochemically using a OneStep polymer HRP anti-rabbit detection system (GeneTex, Taiwan, ROC), according to the manufacturer’s instructions. The sections were incubated with the DAB chromogen reagent and counterstained with hematoxylin. Then, the sections were dehydrated, cleared, and mounted as normal, which were subsequently observed by an Olympus microscope and photographed.
Statistical Analysis
Statistically significant differences of gut microbiota composition before and after intervention in each group were analyzed using paired-samples t-tests (p<0.05) using SPSS Statistics 20.0 software (SPSS Inc., Chicago, IL, USA). Significant differences among ratios of Firmicutes/Bacteroidetes, the control group, and the groups fed with tempeh were calculated by one way analysis of variance (ANOVA) followed by the least significant difference test.
Significant differences of IgA analysis between the control group and the groups fed with tempeh were calculated using non-parametric Mann-Whitney U and Wilcoxon tests for independent and related samples. Effects were considered statistically significant at p<0.05.
RESULTS AND DISCUSSIONS
Empang- and Warung Jambu Tempeh
14
compact cake (Okada 1989). Tempeh in Indonesia is fermented using a mixed culture of molds, yeasts, lactic acid bacteria, and different Gram-negative bacteria. Rhizopus oligosporus is the most dominant fungus in tempeh although some other molds, such as Rhizopus oryzae or Mucor spp. During the fermentation process, the mixed culture or microorganisms will digest the soybean, creating a valuable changes not only the increase of nutrition value but also the development of vitamins, minerals, and antioxidants (Babu et al. 2009; Dixit et al. 2011). Klebsiella pneumoniae and Citrobacter freundii were previously reported as bacteria producing vitamin B12 in tempeh (Keuth and Bisping 1994). Bacteria
belong to phylum of Proteobacteria and Firmicutes were also recently reported as bacteria producingvitaminB12intempeh (Seumahu et al. 2012). Further study on
vitamin B12 producing bacteria showed that K. pneumoniae found in tempeh has
different genetics profile compared with pathogenic isolates (Ayu et al. 2014). As the most popular traditional fermentative food in Indonesia, tempeh plays an important role in supporting the dietary nutrition. The solubility of macromolecules in soybean such as carbohydrates, proteins, and lipids will be increased due to the action of digestive enzymes from the molds, making them more digestible for human consumption. Microbial activity during fermentation also increases the availability of trace minerals such as iron and calcium that work as an enzymatic cofactor. The fermentation also produces a great number of isoflavones that are structurally similar to mammalian estradiol that can bind to an estrogen receptor, thus called phytoestrogen. Although the isoflavones are not an essential nutrient for life, they also play an important role for maintaining healthy lifestyle by reducing cancer risk and improve digestive tract function (Dixit et al. 2011; Astuti et al. 2000).
In the present study, Empang (EMP) and Warung Jambu (WJB) tempeh were obtained from local sources in the Bogor area and used as feed supplement for Sprague-Dawley rats. EMP- and WJB tempeh have different procedures in soaking, dehulling and cooking methods. EMP tempeh was produced without second cooking and leads further a higher number of bacterial cells and higher bacterial biodiversity than WJB tempeh. Acetobacter indonesiensis, K. pneumoniae, Bacillus subtilis, and Flavobacterium sp. were the dominant bacteria found in EMP tempeh; whilst Klebsiella sp. and Pseudomonas putida were the dominant bacteria found in WJB tempeh (Barus et al. 2008). Moreno et al. (2002) and Nout and Kiers (2005) showed previously that soybean cooking process reduced microbial populations in large numbers. Recent study confirmed that the populations of yeast and lactic acid bacteria (LAB) were reduced during the second cooking process after the end of soybean soaking process (Efriwati et al. 2013). These differences in bacteria communities between both tempeh will further influence the nutrients in tempeh.
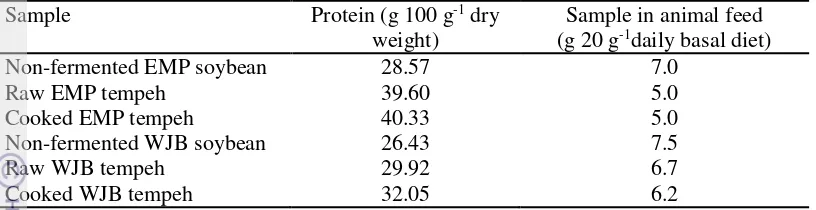
15 Table 4 The protein content and total added non-fermented soybean and tempeh
in animal feed.
Sample Protein (g 100 g-1 dry
weight)
Sample in animal feed (g 20 g-1daily basal diet)
Non-fermented EMP soybean 28.57 7.0
Raw EMP tempeh 39.60 5.0
Cooked EMP tempeh 40.33 5.0
Non-fermented WJB soybean 26.43 7.5
Raw WJB tempeh 29.92 6.7
Cooked WJB tempeh 32.05 6.2
The result of this study showed an increased protein content in tempeh compared to the non-fermented soybean (Table 4). Increased soluble protein content was due to the action of protease enzyme producedby mould during fermentation.
Impact of Tempeh Supplementation on Gut Microbiota Composition in Sprague-Dawley Rats
In this study, Real-time PCR (RT-PCR) of gene-targeted, group-specific 16S rRNA was done to quantify and analyze Bacteroidetes, Bacteroides fragilis, Firmicutes, Clostridium leptum, and all bacteria. This method had been applied to microbiota analysis as the most sensitive and rapid method (Matsuki et al. 2004). The specificity of each primer was previously assayed by Matsuki et al. (2004) and Guo et al. (2008). All of the RT-PCR assays showed a linear relationship between Ct value and the log of plasmid DNA copy number (R2>0.98) in all cases,
which allowed for determination of the concentration of the unknown sample based on their Ct values (Appendix 1).
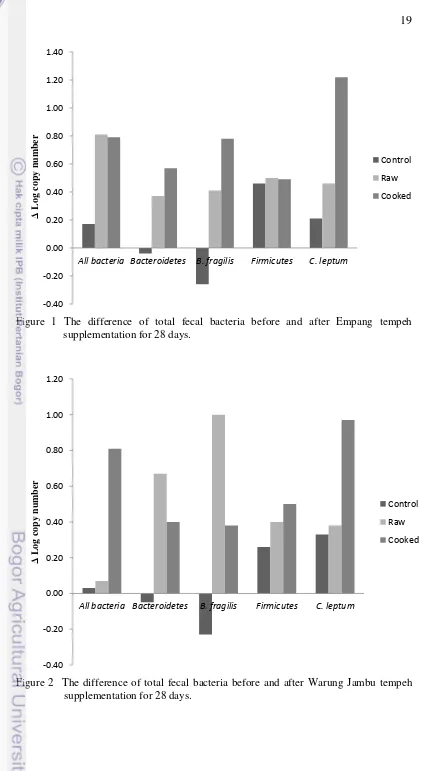
The results of this study showed that in the control group supplemented with EMP and WJB soybean, the population of all bacteria, Firmicutes, and C. leptum increased slightly, while the population of Bacteroidetes and B. fragilis decreased (Table 5 and 6).
Firmicute sand C. leptum populations increased significantly after rats were supplemented with WJB soybean for 28 days; only C. leptum increased significantly in the group supplemented with EMP soybean. The populations of all bacteria, Bacteroidetes, Firmicutes, B. fragilis and C. leptum increased after rats were supplemented with EMP or WJB tempeh for 28 days (Table 5 and 6).
16
Many recent studies showed that gut microbiota play an important role in maintaining human health. Gut microbiota can affect host immunity and be a physical barrier from other pathogens. Furthermore, gut microbiota composition can be manipulated through diet. Tempeh is an Indonesian fermented food that contains some resistant carbohydrates, such as stachyose, raffinose, and galactooligosaccharides (Wang et al. 2007; Fujisawa et al. 2011). Resistant carbohydrates are defined as carbohydrate that cannot be hydrolyzed by endogenous enzymes in the small intestine of humans. However, these carbohydrates play an important role in modulating gut microbiota composition, which results in positive impacts on human health (Scott et al. 2008).
Changes in the composition of gut microbiota in response to EMP and WJB tempeh supplementation might occur because both tempeh have different bacterial composition. The supplementation of raw EMP and WJB tempeh increased significantly the copy number of Firmicutes and Bacteroidetes respectively (Table 5 and 6). A higher LAB population, which belongs to Firmicutes phylum, in EMP tempeh might increase significantly the copy number of Firmicutes in the group supplemented with raw EMP tempeh compared to the group supplemented with raw WJB tempeh. A significant increased population of Clostridium leptum was found in the group supplemented with raw and cooked WJB tempeh (Table 6). The differences of bacterial copy numbers before and after EMP and WJB tempeh supplementation are shown in Figure 1 and 2, respectively.
The presence of carbohydrates in soybean might further play an important role in modulating gut microbiota composition. Carbohydrates in soybeans comprise mainly cell wall polysaccharides and the small sugars fructose, raffinose and stachyose. The concentration of these small sugars is reduced during soaking, cooking and fermentation of the soybeans (Mulyowidarso et al. 1991b; Egounlety and Aworh 2003). The insoluble cell wall polysaccharides, such as pectin, cellulose and hemicellulose are (partly) degraded during fermentation by the enzymes of the mould which leads to their enhanced water-solubility (Kiers et al. 2000). The major monosaccharide constituents in soybean cell walls are galactose, glucose, arabinose and galacturonic acid (Huisman et al. 1998).
17
Table 5 Impact of Empang (EMP) tempeh on gut microbiota composition. Total all bacteria, Bacteroidetes, Bacteroides fragilis,
Firmicutes, and Clostridium leptum fecal microbiota in Sprague-Dawley rats before (T0) and after (T28) supplemented with:
non-fermented EMP soybean, raw EMP tempeh, and cooked EMP tempeh. Values are in log10 copies per gram of wet weight of feces ± SEM (n=5/group).
EMP
log10 copies/g feces ± SEM
Non-fermented soybean (control) Raw Cooked
T0 T28 T0 T28 T0 T28
All bacteria 9.11 ± 0.47 9.28 ± 0.12 9.04 ± 0.32 9.85 ± 0.10 8.72 ± 0.42 9.51 ± 0.33
p value (T0/T28) 0.38 0.004* 0.09
Bacteroidetes 8.94 ± 0.36 8.90 ± 0.42 8.98 ± 0.16 9.35 ± 0.19 8.29 ± 0.05 8.86 ± 0.35
p value (T0/T28) 0.66 0.051 0.01*
B. fragilis 6.67 ± 0.44 6.41 ± 0.37 7.02 ± 0.48 7.43 ± 0.29 6.09 ± 0.05 6.87 ± 0.44
p value (T0/T28) 0.32 0.23 0.045*
Firmicutes 7.48 ± 0.41 7.94 ± 0.44 7.76 ± 0.16 8.26 ± 0.23 7.23 ± 0.59 7.72 ± 0.37
p value (T0/T28) 0.26 0.003* 0.22
C. leptum 6.73 ± 0.37 6.94 ± 0.31 6.99 ± 0.33 7.45 ± 0.24 6.25 ± 0.77 7.47 ± 0.11
p value (T0/T28) 0.07 0.053 0.03*
*Significant differences: p<0.05 vs group after intervention.
18
Table 6 Impact of Warung Jambu (WJB) tempeh on gut microbiota composition. Total all bacteria, Bacteroidetes, Bacteroides fragilis,
Firmicutes, and Clostridium leptum fecal microbiota in Sprague-Dawley rats before (T0) and after (T28) supplemented with:
non-fermented WJB soybean, raw WJB tempeh, and cooked WJB tempeh. Values are in log10 copies per gram of wet weight of feces±SEM (n=5/group).
WJB
log10 copies/g feces ± SEM
Non-fermented soybean (control) Raw Cooked
T0 T28 T0 T28 T0 T28
All bacteria 9.50 ± 0.48 9.53 ± 0.33 9.23 ± 0.74 9.30 ± 0.39 9.01 ± 0.52 9.82 ± 0.51
p value (T0/T28) 0.91 0.76 0.04*
Bacteroidetes 9.11 ± 0.34 9.06 ± 0.40 8.64 ± 0.39 9.31 ± 0.31 8.73 ± 0.20 9.13 ± 0.37
p value (T0/T28) 0.6 0.01* 0.17
B. fragilis 6.85 ± 0.44 6.62 ± 0.13 6.06 ± 0.71 7.06 ± 0.14 6.64 ± 0.36 7.02 ± 0.21
p value (T0/T28) 0.29 0.29 0.13
Firmicutes 7.50 ± 0.11 7.76 ± 0.12 7.59 ± 0.26 7.99 ± 0.35 7.49 ±0.25 7.99 ± 0.41
p value (T0/T28) 0.01* 0.16 0.09
C. leptum 7.02 ± 0.33 7.35 ± 0.28 7.20 ± 0.26 7.58 ± 0.27 6.56 ±0.37 7.53 ± 0.49
p value (T0/T28) 0.03* 0.03* 0.049*
*Significant differences: p<0.05 vs group after intervention.
19
All bacteria Bacteroidetes B. fragilis Firmicutes C. leptum
Δ
All bacteria Bacteroidetes B. fragilis Firmicutes C. leptum
20
The ratio of Firmicutes/Bacteroidetes in the groups supplemented with either EMP or WJB tempeh is not significantly different compared to the control group (Table 7 and 8). The presence of a higher Firmicutes group in EMP tempeh caused a lower ratio of Firmicutes/Bacteroidetes after 28-days EMP tempeh supplementation (Table 7).
Table 7 The ratio of Firmicutes/Bacteroidetes in the three groups following supplementation with Empang tempeh for 28 days.
Group Firmicutes/Bacteroidetes ratio
EMP soybean 0.90±0.09
Raw EMP tempeh 0.88±0.03
Cooked EMP tempeh 0.87±0.07
p value* 0.871
*Based on one-way analysis of variance test (α0.05)
Table 8 The ratio of Firmicutes/Bacteroidetes in the three groups following supplementation with Warung Jambu tempeh for 28 days.
*Based on one-way analysis of variance test (α0.05)
A lower ratio of Firmicutes/Bacteroidetes after EMP tempeh supplementation might further indicate that tempeh can be a new strategy for a low-diet plan and positively impact human health (Mariat et al. 2009). The result of this study showed that population of Bacteroidetes and Bacteroides fragilis increased significantly in the group supplemented with cooked EMP tempeh (Table 5). Comparison of gut microbiota of obese and lean mice and obese and lean humans showed a statistically significant reduction in the relative abundance of Bacteroidetes and a significantly greater proportion of Firmicutes in obese mice and humans (Ley et al. 2005; Turnbaugh et al. 2009). Moreover, the abundance of Bacteroidetes will impact other factors, such as altering host gastrointestinal tract (GIT), which leads to morphological and functional changes. Bacteroidetes can stimulate the activation of T-cell-mediated responses and limit GIT colonization by pathogenic bacteria (Thomas et al. 2012). The results of this study showed further that different types of tempeh may offer variable health benefits due to its unique microorganism composition that plays an important role during the fermentation process.
Moreover, previous studies have also shown the beneficial effect of tempeh diet on fecal microflora. It has been reported that the presence of raffinose and soybean oligosaccharide in tempeh brings about an increase of bifidobacteria in human feces. Bifidobacteria are one of the predominant microorganisms in humans that contribute to the physiological well-being of the individual
Group Firmicutes/Bacteroidetes ratio
WJB soybean 0.86±0.03
Raw WJB tempeh 0.86±0.04
Cooked WJB tempeh 0.88±0.03
21 (Hayakawa et al. 1990). Similar beneficial effect was also found with the intake of natto miso soup. Natto is an original and traditional Japanese food, and consists of soybean products fermented by B. subtilis (Sumi et al. 1990). The effect of consumption of natto miso soup over 14 days on the bacterial flora of healthy volunteers showed that the numbers of bacilli and bifidobacteria were significantly increased, whereas numbers of clostridia tended to decrease (Fujisawa et al. 2006)
Impact of Tempeh Supplementation on Mucosal Immunoglobulin A in Sprague-Dawley Rats
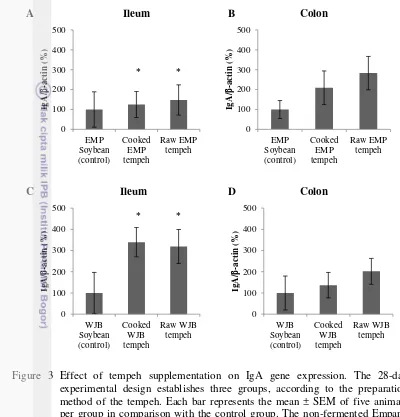
22 experimental design establishes three groups, according to the preparation method of the tempeh. Each bar represents the mean ± SEM of five animals per group in comparison with the control group. The non-fermented Empang (EMP)- and Warung Jambu (WJB) soybean- control groups represent 100% gene expression. The percentage of IgA gene expression in the animal groups supplemented with EMP tempeh in the ileum (A) and the colon (B) are compared to the associated control group. The percentage of IgA gene expression in the animal groups supplemented with WJB tempeh in the ileum (C) and the colon (D) are also compared to the associated control group. Statistical differences, where p<0.05 vs. the control group, are indicated by *.
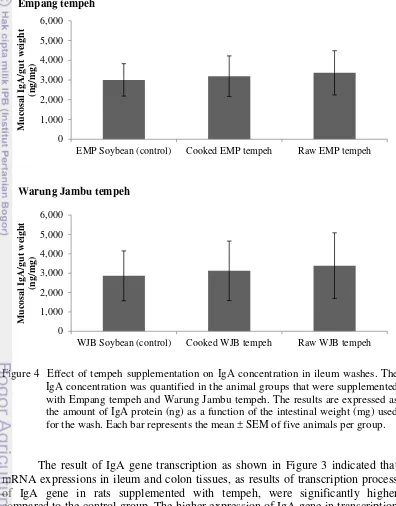
23 cooked- and raw WJB tempeh, mucosal IgA concentration were also higher, 3122 and 3391 ng/mg ileum tissue respectively, compared to the control group which was 2860 ng/mg ileum tissue (Figure 4). Ileum IgA gene expression in animals receiving tempeh for 28 days followed the same up-regulated pattern. These results demonstrated that tempeh supplementation increased the expression of the IgA gene and protein, thereby enhancing the development of the rat’s IgA defense system.
Figure 4 Effect of tempeh supplementation on IgA concentration in ileum washes. The IgA concentration was quantified in the animal groups that were supplemented with Empang tempeh and Warung Jambu tempeh. The results are expressed as the amount of IgA protein (ng) as a function of the intestinal weight (mg) used for the wash. Each bar represents the mean ± SEM of five animals per group.
The result of IgA gene transcription as shown in Figure 3 indicated that mRNA expressions in ileum and colon tissues, as results of transcription process of IgA gene in rats supplemented with tempeh, were significantly higher compared to the control group. The higher expression of IgA gene in transcription level was also followed by the higher secretion of IgA protein as a result of
EMP Soybean (control) Cooked EMP tempeh Raw EMP tempeh
M
WJB Soybean (control) Cooked WJB tempeh Raw WJB tempeh
24
concentration, quantified from the ileum wash, showed no significant difference between the animal groups supplemented with soybeans and those supplemented with cooked or raw tempeh (Figure 4).
Jessica (2014) showed previously that the mRNA expression as a result of transcription process of IgA gene in rats supplemented with raw and cooked tempeh were higher compared to the group fed with the standard diet. IgA protein in rats supplemented with tempeh were also higher, 3815 and 3225 ng/mg ileum tissue in rat supplemented with raw and cooked tempeh, compared to the control group which was 1500 ng/mg ileum tissue. Moreover, IgA protein secretion in rat small intestine was previously reported around 1500-1600 ng/mg ileum tissue (Cladera et al. 2012).Control group in this study was supplemented with non-fermented soybeans. Soybean feeding has been previously reported to trigger humoral and cellular immune responses resulting into formation of protein specific IgG, IgA and IgM (Dréau et al. 1994, Lallès et al. 1995).
Further immunohistological analysis showed that higher concentration of IgA protein in the groups supplemented with tempeh was detected in the
intracellular section of Peyer’s patch cells (Figure 5-6 and 8-9). Therefore, the concentration of IgA protein in ileum wash did not follow the significant up-regulated pattern as shown in the result of IgA gene expression analysis. Figure 5
showed an abundance of IgA in Peyer’s patches cells was detected from rat with low concentration of IgA.
The increase of intestinal IgA after tempeh supplementation was also reported by Utama et al. (2013). This study investigated the effect of dietary tempeh on the colonic environment of rats fed high-fat (HF, 30% fat) or low-fat (LF, 6%) diets. With both the HF and LF diets, dietary tempeh consumption markedly elevated fecal IgA, but caused no influence on serum IgA. The increase in intestinal IgA is suggested to be involved in the suppression of colon cancer (Perdigón et al. 2002). Other soybean products such as soy sauce (shoyu) increased also intestinal IgA in mice (Matsushita et al. 2008).
Mucosal surfaces are colonized by large communities of commensal bacteria, and represent the primary site of entry for pathogenic agents (Cerutti et al. 2011). The three phyla Bacteroidetes (Gram-negative), Firmicutes (Gram-positive), and Actinobacteria (Gram-positive) are the most abundant in the intestine (den Besten et al. 2013). The gut microbiota plays an important role in the proper development of the immune system, as indicated by the fact that germ-free (GF) mice have poorly developed lymphoid tissues (Kosiewicz et al. 2011). GF mice have spleens with few germinal centers and poorly formed T and B cell
zones, hypoplastic Peyer’s patches, lower number of lamina propria cells and
25
A
B
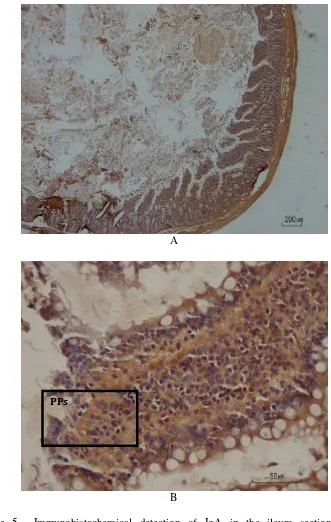
Figure 5 Immunohistochemical detection of IgA in the ileum section of rats supplemented with cooked Empang tempeh. Ileum section from rats was stained by using monoclonal IgA antibody (brown staining) as described in Materials and Methods. Original magnification, x40 (A) and x400 (B). Representative photomicrograph from rat with 1567 ng IgA/ mg tissue. PPs: Peyer’s patches.
26
A
B
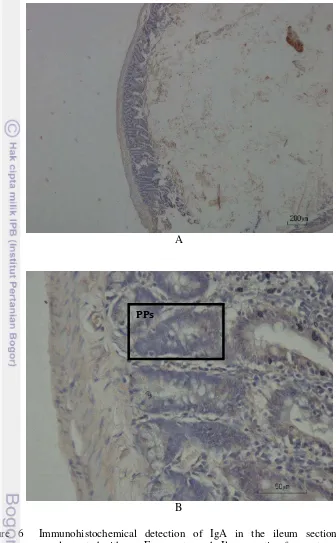
Figure 6 Immunohistochemical detection of IgA in the ileum section of rats supplemented with raw Empang tempeh. Ileum section from rats was stained by using monoclonal IgA antibody (brown staining) as described in Materials and Methods. Original magnification, x40 (A) and x400 (B). Representative photomicrographs from 5 rats/group. PPs: Peyer’s patches.
27
A
B
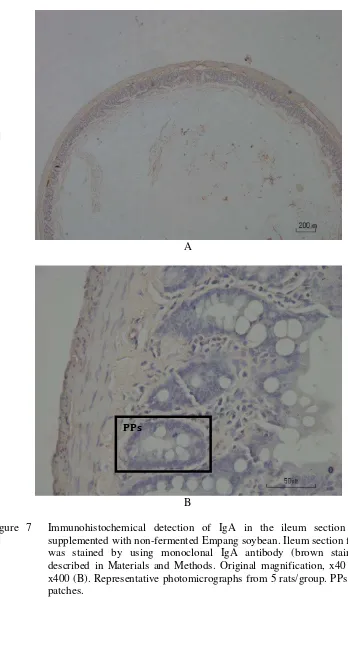
Figure 7 Immunohistochemical detection of IgA in the ileum section of rats supplemented with non-fermented Empang soybean. Ileum section from rats was stained by using monoclonal IgA antibody (brown staining) as described in Materials and Methods. Original magnification, x40 (A) and x400 (B). Representative photomicrographs from 5 rats/group. PPs: Peyer’s patches.
28
A
B
Figure 8 Immunohistochemical detection of IgA in the ileum section of rats supplemented with cooked Warung Jambu tempeh. Ileum section from rats was stained by using monoclonal IgA antibody (brown staining) as described in Materials and Methods. Original magnification, x40 (A) and x400 (B). Representative photomicrographs from 5 rats/group. PPs: Peyer’s patches.
29
A
B
Figure 9 Immunohistochemical detection of IgA in the ileum section of rats supplemented with raw Warung Jambu tempeh. Ileum section from rats was stained by using monoclonal IgA antibody (brown staining) as described in Materials and Methods. Original magnification, x40 (A) and x400 (B). Representative photomicrographs from 5 rats/group. PPs: Peyer’s patches.
30
A
B
Figure 10 Immunohistochemical detection of IgA in the ileum section of rats supplemented with non-fermented Warung Jambu soybean. Ileum section from rats was stained by using monoclonal IgA antibody (brown staining) as described in Materials and Methods. Original magnification, x40 (A) and x400 (B). Representative photomicrographs from 5 rats/group. PPs: Peyer’s patches.