www.elsevier.nlrlocateraqua-online
ž
/
The effect of dietary arachidonic acid 20:4 n
y
6
on growth, survival and resistance to handling stress
ž
/
in gilthead seabream Sparus aurata larvae
W. Koven
a,), Y. Barr
a, S. Lutzky
a, I. Ben-Atia
a, R. Weiss
a,
M. Harel
b, P. Behrens
c, A. Tandler
aa
Israel Oceanographic and Limnological Research, The National Center for Mariculture, P.O. Box 1212, Eilat 88112, Israel
b
Center of Marine Biotechnology, 701 East Pratt Street, Baltimore, MD 21202, USA
c
Martek Biosciences Corporation, 6480 Dobbin Road, Columbia, MD 21045, USA
Received 25 February 2000; received in revised form 7 July 2000; accepted 7 July 2000
Abstract
Ž .
The effects of high dietary docosahexaenoic acid 22:6 ny3, DHA and varying arachidonic
Ž .
acid 20:4 ny6, AA were tested on growth, survival and resistance to handling stress in 5–35 day old gilthead seabream larvae. Three enrichment treatments differing in their DHArAA ratios
Ž . Ž
were fed to rotifers Brachionus rotundiformis and Artemia nauplii. The high DHA 35.9%
. Ž .
TFA enrichment treatment DHA-PL contained no AA and included lipid from the heterotrophi-cally grown DHA-rich dinoflagellate Crypthecodinium sp. A second enrichment treatment
ŽAADHA , selected from an earlier screening study, supplemented the high DHA enrichment.
Ž .
treatment with an AA-rich lipid 52% TFA from the heterotrophically grown fungus Mortierella
Ž .
alpina. A third enrichment treatment ALGA was the commercial product Algamac 2000, which
Ž
is devoid of AA, but includes approximately 12.9% of TFA as docosapentaenoic acid DPA,
.
22:5ny6 . Rotifers fed the DHA-PL, AADHA and ALGA treatments demonstrated a range of
Ž . Ž
DHArAA ratios 20.9, 5.6 and 10.1, respectively as did the Artemia nauplii 25.8, 3.7 and 4.6,
.
respectively .
The enriched rotifers were fed to larvae reared in 400 l V-tanks from day 5 to day 19 post-hatching. Following this period, larvae were exposed to controlled handling stress during transfer to 27 l aquaria, where they were then fed the enriched nauplii from day 20 to day 35
)Corresponding author. Tel.:q972-7-6361443; fax:q972-7-6375761.
Ž .
E-mail address: [email protected] W. Koven .
0044-8486r01r$ - see front matterq2001 Elsevier Science B.V. All rights reserved. Ž .
post-hatching. Although larval fatty acid profiles reflected the enrichment treatments, there were
Ž .
no marked differences P)0.05 in survival and growth in 5–19 day old larvae at the end of rotifer feeding. However, the larvae fed the AA enriched rotifers prior to the handling stress of
Ž .
transfer to the aquaria demonstrated daily and significantly P-0.05 lower accumulated
Ž .
mortality after transfer and during Artemia feeding than larvae fed the AA-deficient DHA-PL and ALGA-enriched rotifers. As larvae fed the ALGA, rotifers partially retroconverted DPA to
Ž . Ž .
AA in their tissues, the final survival 31.0% in these larvae was markedly better P-0.05 than
Ž . Ž .
larvae fed the DHA rotifers 17.5% , but significantly P-0.05 lower than larvae ingesting
Ž .
AADHA rotifers 42.9% . Conversely, the high-accumulated mortality in larvae fed the AA-defi-cient rotifers could not be corrected during the post-handling phase by feeding AA supplemented
Artemia. The results suggest that dietary AA fed prior to handling stress improved survival more
effectively than when fed following handling stress. These findings imply, as well, the importance of early larval nutrition on later larval and juvenile survival during crowding, grading and other handling stressors.q2001 Elsevier Science B.V. All rights reserved.
Keywords: Stress resistance; Essential fatty acid; Arachidonic acid; Docosahexaenoic acid; Marine fish larvae; Eicosanoids; Survival
1. Introduction
The essential dietary requirement in many marine teleosts for the long chain
Ž . Ž .
polyunsaturated fatty acids PUFA , docosahexaenoic acid DHA, 22:6 ny3 and
Ž . Ž
eicosapentaenoic acid EPA, 20:5ny3 has long been established Kitajima et al., 1980a,b; Watanabe et al., 1983a,b; Kanazawa, 1985; Izquierdo et al., 1989, Koven et al.,
.
1989, 1990, 1992a; Izquierdo, 1996 . These fatty acids, as components of phospholipids, function as critical structural and physiological components of the cell membranes of
Ž .
most tissues Gurr and Harwood, 1991; Sargent et al., 1993a . DHA, in particular, has high biological value during larval development and is selectively incorporated into the neural tissues contributing to pigmentation, visual acuity and presumably prey hunting
Ž
success Koven et al., 1992b; Kanazawa, 1993; Sargent et al., 1993b; Watanabe, 1993; .
Bell et al., 1995 . A dietary deficiency in DHA in larvae of farmed marine teleosts has been correlated with poor growth, high mortality and susceptibility to stress and disease ŽWaagboe et al., 1993; Kiron et al., 1995; Lingenfelser et al., 1995 . In general, and. unlike their freshwater counterparts, marine fish larvae lack D5 desaturase rendering
them unable to synthesize DHA and EPA from the shorter PUFA precursor linolenic
Ž .
acid 18:3ny3 . As a consequence, commercial preparations routinely used to enrich
Ž .
the live food rotifers and Artemia nauplii of the larval stages of cultured marine teleosts, contain abundant amounts of DHA, together with relatively lower levels of EPA.
However, little attention has been given to the PUFA of the ny6 series, despite broad hints in the literature attesting to its potential importance. Quantitatively, these fatty acids are much less represented in the tissues of marine fish where lipids can
Ž .
typically have an ny3rny6 ratio of 10–15:1 Ackman, 1980 . Despite this discrep-ancy in cellular membrane levels, studies have suggested that the long chain ny6
Ž .
ŽOstrowski and Divakaran, 1990; Castell et al., 1994; Bessonart et al., 1999 . Workers. have reported that AA was preferentially retained in various species together with DHA during starvation, suggesting a metabolic priority for conservation of certain fatty acids ŽOstrowski and Divakaran, 1990; Rainuzzo et al., 1994; Izquierdo, 1996 . In turbot,.
Ž dietary deficiencies in AA have resulted in high mortality and obvious pathology Bell
. Ž .
et al., 1985a , while Castell et al. 1994 reported a positive effect of AA on survival Ž .
from levels ranging from 0.5–1.0%. Bessonart et al. 1999 found that AA was more effective in improving survival of gilthead seabream larvae if provided in the presence of a high dietary DHArEPA ratio. On the other hand, AA supplementation may also have adverse affects. Other studies on turbot and halibut found an increased incidence of malpigmentation correlated with brain AA, whereas higher EPArAA dietary levels
Ž .
improved pigmentation McEvoy et al., 1998 .
The above findings suggest that AA contributes to survival in larvae of a variety of marine teleosts. However, the question remains whether AA is equally effective throughout larval development. Moreover, due to the possible effect of AA-based eicosanoids on cortisol production during stress, the dietary AA effect on survival may be related to stress resistance. Consequently, the aim of the present study on the larvae of the gilthead seabream was twofold. Firstly, to test the effect of dietary AA on growth and survival in early and late larval development. Secondly, to determine the influence of dietary AA on larval growth and survival, prior to andror following handling stress.
2. Methods and materials
2.1. Screening study to determine optimal dietary AA leÕel during early larÕal rearing
The determination of an optimal dietary AA level in early seabream larvae was carried out in a screening study. In this experiment, five rotifer enrichment treatments,
Ž varying in their DHArAA ratios, were fed to 5–19 day old gilthead seabream Sparus
.
aurata larvae in conical V-tanks. Following the handling stress of transfer to glass aquaria, all the larvae, from day 19 to day 35 post-hatching, were fed Artemia nauplii
Ž
enriched on the commercial preparation AlgaMac 2000 Biomarine, Hawthorne, Califor-.
nia, USA . During this period, growth and accumulated mortality of larvae fed the different rotifer treatments before transfer were monitored.
The control treatment rotifers were fed a spray dried, lipid extracted algal meal mixed
Ž . Ž .
with a phospholipid containing 35.9% of total fatty acids TFA as DHA DHA-PL . These ingredients were residual products from an industrial oil extraction process of the
Ž
heterotrophically grown dinoflagellate Crypthecodinium sp. Martek Biosciences, .
Columbia, Maryland, USA . The other rotifer treatments consisted of replacing 12.5%,
Ž .
25% and 50% of the DHA-rich meal with a meal mixed with an AA-rich 52.5% TFA Ž .
phospholipid AA-PL . This phospholipid and meal were residual products remaining from an industrial oil extraction process of the heterotrophically grown fungus
Ž .
Ž
was a commercial enrichment preparation, AlgaMac 2000 Bio-Marine, Hawthorne,
. Ž .
California, USA . The component and selected fatty acid composition % TFA of the enrichment preparations and of the rotifers that consumed the different treatments are shown in Tables 1 and 2, respectively.
Ž .
The experimental system consisted of 25 conical V-tanks 400 l supplied with Ž .
continuously filtered seawater 25‰ at a flow rate of one tank exchange per day, where the temperature was gradually raised from 198C to 218C. The tanks were stocked with
Ž y1.
2-day-old gilthead seabream larvae 100 larvae l , originating from spawns of locally maintained broodstock. The five rotifer treatments were tested in five tank replicates per
Ž
treatment, where larvae from day 3 to day 19 were fed twice daily rotifers 7.5 rotifers
y1 y1.
ml tank that were previously enriched for 18 h with Nannochloropsis sp. Ž30=106 cells mly1., followed by their respective enrichment treatments. Based on
Ž .
routine rearing procedures for gilthead seabream Tandler et al., 1987 , Nannochloropsis sp. was also added to the larval rearing tanks to reach a tank concentration of 0.5=106
cells mly1
.
Rotifer enrichments were carried out in V-tanks containing 600 l of well-aerated Ž .
seawater 25‰ thermostatically controlled at 288C and stocked with approximately
300–500 rotifers mly1. Each of the five rotifer-enrichment tanks received a 100 mg ly1 feeding of enrichment preparation at the start of feeding and after 4 h during the 8 h enrichment period. At the end of rotifer enrichment, the rotifers were washed well with clean filtered seawater, dead rotifers removed and the surviving zooplankters counted before feeding to the larvae. Larvae were fed according to this protocol until they reached 19 days post-hatching and then sampled for dry weight determination.
The second stage of the study commenced with the transfer and stocking of 20-day-old larvae from each of the DHArAA treatments into five 27 l glass aquaria
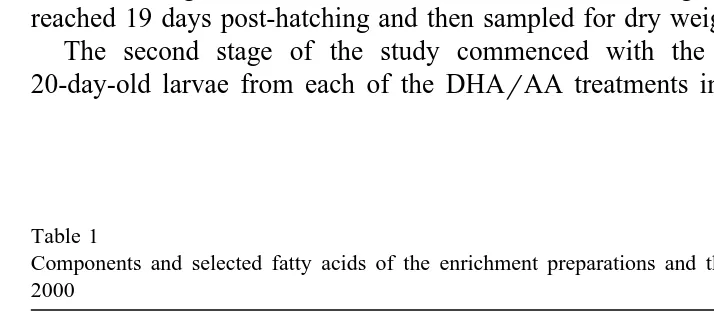
Table 1
Components and selected fatty acids of the enrichment preparations and the commercial product AlgaMac 2000
Components DHA-PL ARA-PL AlgaMac
qalgal meal qalgal meal 2000
Proximate analysis
Ž .
Total fat % dry wt 48.2 29.4 32.0
Ž .
Total carbohydrate % dry wt 27.5 40.0 13.0
Ž .
Selected Fatty acids % of TFA
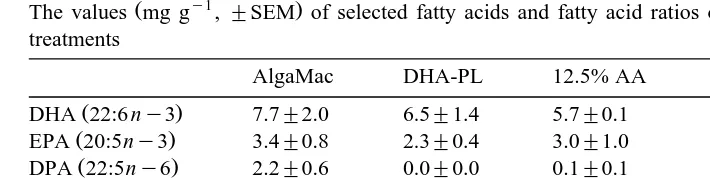
Table 2
Ž y1 .
The values mg g ,"SEM of selected fatty acids and fatty acid ratios of the rotifers fed the enrichment treatments
AlgaMac DHA-PL 12.5% AA 25% AA 50% AA
Ž .
200 larvae aquarium . The aquaria were supplied with filtered seawater whose temperature was gradually increased from 218C to 248C during the feeding trial. From
day 20 to day 34, the larvae from the 25 glass aquaria were fed Artemia nauplii twice Ž y1.
daily 1 nauplii ml that were enriched on AlgaMac 2000. Dead larvae from all the aquaria were collected each day and recorded for the first 7 days following transfer.
2.2. The dietary AA effect on growth and surÕiÕal in early and late larÕal seabream deÕelopment
Following the determination of the optimal level of AA for early larval performance, a study was conducted to determine the effect of dietary AA on growth and survival in early and late larval seabream development. In this experiment, the treatment that gave the best larval performance in the screening trial, the 12.5% AA-PL supplement ŽAADHA , was compared with the high DHA but AA-deficient treatment DHA-PL and.
Ž .
the commercial product AlgaMac 2000 ALGA . The experimental tank system was identical and similarly stocked with larvae as described in the screening study. The three rotifer treatments were prepared, as detailed earlier, and tested on 3–19 day old larvae in eight tank replicates per treatment. At the end of the experiment, 19-day-old larvae were sampled for fatty acid analysis and dry weight determination.
The following day, 20-day-old larvae from each of the DHA-PL and AADHA Ž
treatments were transferred and stocked into sets of 12 glass aquaria 27 l, 300 larvae
y1.
aquarium supplied with filtered and temperature controlled seawater, as outlined in the screening study. Each set of 12 aquaria per treatment was subdivided into three
Ž
groups of four so that the three Artemia enrichment treatments DHA-PL, AADHA and .
ALGA could be tested in replicates of four on larvae that were previously fed DHA-PL or AADHA enriched rotifers. The commercial control larvae from the ALGA treatment
Ž y1.
were stocked 300 larvae aquarium in three 27 l glass aquaria and were fed ALGA enriched Artemia.
The Artemia nauplii enrichments were carried out in 600 l V-tanks supplied with Ž .
well-aerated filtered seawater 25‰ thermostatically controlled at 288C and stocked
Ž y1.
preparation 300 mg l at the onset of feeding and after 8 h during the 16 h enrichment period. At the end of enrichment, the Artemia were washed well with clean seawater, dead nauplii removed and the remaining Artemia counted. From day 20 to day 34, the larvae from the 27 glass aquaria were fed their respective Artemia
Ž y1.
treatments twice daily 1 nauplii ml . Dead larvae from all the aquaria were collected each day and recorded.
During the course of the experiment, Artemia and rotifer samples were taken on three separate occasions for lipid and fatty acid analyses. At the completion of the experiment, the larvae from each of the aquaria were counted and samples were taken for dry weight determination and for lipid and fatty acid analyses. The average larval dry weight per treatment was determined by washing a sample from each of the aquaria, first with fresh water, and then, distilled water. This was followed by averaging 50
Ž .
individually weighed larvae Sartorius BP 210S "0.1 mg that had previously been oven dried for 48 h at 608C.
The rotifer, Artemia and larval samples were lyophilized and then lipid extracted ŽFolch et al. 1957 . Total lipid levels were determined gravimetrically Sartorius BP. Ž
.
210S "0.1 mg . The lipid samples were transmethylated to their corresponding fatty Ž .
acid methyl esters FAME by acidified methylation overnight at 508C in 1% H SO in
2 4 Ž .
methanol vrv . After purification on 20=20 cm TLC plates pre-coated with silica gel
Ž . Ž
G60 E. Merk, Darmstadt, FRG using the solvent system hexanerdiethyl ether 1:1,
. Ž y1 .
vrv , the resulting FAME were concentrated in hexane 2 mg FAME ml hexane . The samples were injected into an on-column Chrompack CP9001 gas chromatograph equipped with a Chrompack WCOT fused silica 30 M=0.32 mm capillary column that used hydrogen as a carrier gas. FAME were identified by known purified standards and
Ž .
quantified using a response factor to an internal standard heptadecanoic acid, 19:0 . Following testing for homogeneity of variance, the growth, cumulative mortality and survival data were analyzed using the statistical software package SPSS for Windows
Ž .
Version 6.1.3 SPSS, Chicago, IL, USA to perform one-way analysis of variance ŽANOVA and the Duncan’s multiple range test..
3. Results
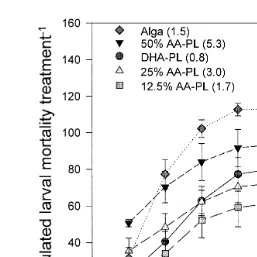
Growth of 5–19 day old seabream larvae at the end of the screening study was
Ž .
independent of dietary AA level and not P)0.05 different among the rotifer
Ž .
treatments data not shown . However, the results indicated that larvae fed rotifers
Ž y1 .
enriched on the 12.5% AA-PL 1.8 mg AA g DW replacement of the DHA-PL
Ž .
consistently gave the lowest, although non-significant P)0.05 , accumulated mortality throughout the 7 days, following transfer than larvae fed the 0% AA-PL replacement or
Ž y1 . Ž y1 . Ž
DHA-PL 0.8 mg AA g DW , 25% AA-PL 3.0 mg AA g DW , 50% AA-PL 5.3
y1 . Ž y1 . Ž .
mg AA g DW and the ALGA 1.5 mg AA g DW rotifers Fig. 1 .
Ž . In general, the selected fatty acids and fatty acid classes in rotifers Table 3 and
Ž .
Artemia Table 4 from the main study on early and late larval development reflected the Ž .
Ž y1 .
Fig. 1. Accumulated larval mortality, as a function of rotifer treatment mg AA g DW , in the screening study after transfer to aquaria and start of Artemia feeding.
Ž y1 .
AA levels were highest in the AADHA 2.7 mg g DW , compared to the DHA-PL
Ž y1 .
and ALGA treatments 0.8 and 1.3 mg g DW, respectively , while DHA levels did
Table 3
Ž y1 .
Selected fatty acids and fatty acid groups mg g DW found in rotifers Rotifer treatments
Fatty acids AADHA DHA-PL ALGA
Saturates 10.9"0.3 11.4"0.3 16.7"3.2
Monounsaturates 25.1"3.2 25.3"0.2 9.4"0.1
ny3 Polyunsaturates 19.1"1.7 22.3"1.3 17.3"0.6 ny6 Polyunsaturates 7.4"0.8 5.2"0.1 6.6"0.3 ny3rny6 Polyunsaturates 2.6"0.5 4.3"0.2 2.6"0.1
Ž .
DHA 22:6 ny3 14.7"1.6 16.0"1.6 13.4"0.7
Ž .
EPA 20:5ny3 2.9"0.1 4.2"0.6 2.5"0.1
Ž .
AA 20:4 ny6 2.7"0.3 0.8"0.1 1.3"0.0
Ž .
DPA 22:5ny6 0.6"0.6 0.1"0.1 4.4"0.3
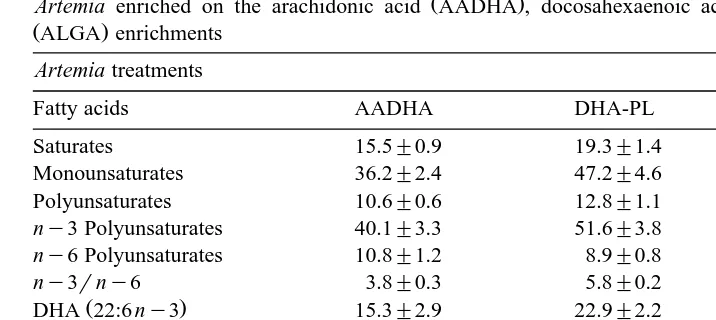
Table 4
Ž . Ž .
Artemia enriched on the arachidonic acid AADHA , docosahexaenoic acid DHA-PL and the AlgaMac
ŽALGA enrichments.
Artemia treatments
Fatty acids AADHA DHA-PL ALGA
Saturates 15.5"0.9 19.3"1.4 20.2"0.8
Monounsaturates 36.2"2.4 47.2"4.6 20.1"0.6 Polyunsaturates 10.6"0.6 12.8"1.1 7.7"0.2 ny3 Polyunsaturates 40.1"3.3 51.6"3.8 30.8"1.1 ny6 Polyunsaturates 10.8"1.2 8.9"0.8 7.7"0.3
not vary markedly. This resulted in the AADHA treatment rotifers having significantly ŽP-0.05 lower DHA. rAA ratios 5.6 than the DHA-PL 20.9 and ALGA 10.1Ž . Ž . Ž .
Ž y1 .
treatments. Noteworthy is the elevated level of 22:5ny6 4.4 mg g DW in the ALGA rotifers, reflecting the relatively high levels of this fatty acid in this enrichment
Ž . Ž .
preparation Table 1 . Similarly, Artemia nauplii Table 4 fed the AADHA treatment Ž y1 .
demonstrated the highest levels of AA 4.6 mg g DW compared to the DHA and
Ž y1 .
ALGA nauplii 0.9 and 1.9 mg g DW, respectively . On the other hand, the DHA
Ž y1 .
levels in the AADHA and DHA-PL nauplii 15.3 and 22.9 mg g DW, respectively Ž y1 .
were considerably higher than the ALGA nauplii 8.9 mg g DW . This resulted in the Ž .
DHArAA ratios in the AADHA nauplii 3.7 being lower but similar to the ALGA Ž .
nauplii 4.6 , while both these treatment nauplii were much smaller than the ratio in the Ž .
DHA-PL nauplii 25.8 . As in rotifers, the level of 22:5ny6 in the ALGA nauplii was Ž y1 .
notably higher 2.9 mg g DW than the trace levels in the AADHA and DHA-PL
Ž y1 .
nauplii 0.0 and 0.1 mg g DW, respectively .
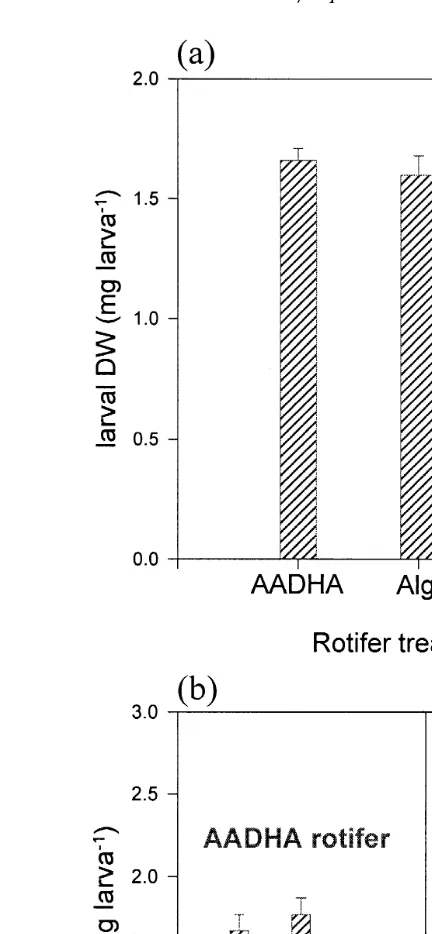
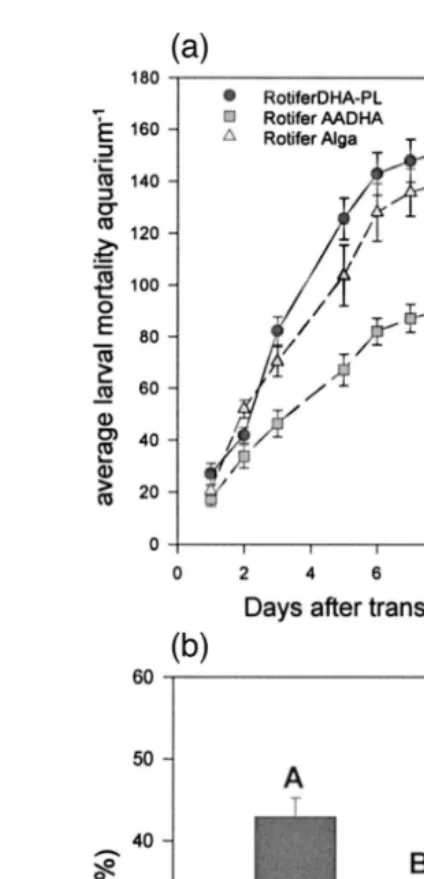
The absence or presence of dietary AA in rotifers or Artemia had no discernible Ž .
effect on larval growth Fig. 2 . On the other hand, an effect of dietary AA fed prior to handling stress on accumulated mortality following handling stress was very clear ŽP-0.05 . Larvae fed the AADHA rotifers in the V-tanks prior to transfer to the. aquaria showed a markedly and consistently reduced daily accumulated mortality after
Ž .
transfer than the DHA-PL and ALGA larvae Fig. 3a . This trend was verified by the
Ž . Ž . Ž .
higher P-0.05 survival Fig. 3b at the end of the experiment 35 days post-hatching Ž .
in larvae fed the AADHA rotifers 42.9% compared to larvae fed the ALGA rotifers Ž31% . The ALGA rotifer fed larvae, in turn, survived better P. Ž -0.05 than larvae fed.
Ž .
Ž .
Fig. 2. a The effect of the AADHA, DHA-PL and ALGA rotifer treatments on larval dry weight prior
Ž .
transfer to aquaria and b the effect of AADHA, DHA-PL and ALGA Artemia treatments on larval dry weight at end of feeding trial in the aquaria.
Ž . Ž .
Ž .
Fig. 3. a Larval accumulated mortality occurring in the aquaria as a function of the rotifer treatments fed
Ž . Ž .
prior to transfer and b larval survival % at the end of Artemia feeding in the aquaria. Survival values
Ž .
having the same letters were not significantly P-0.05 different.
The selected fatty acids and fatty acid classes of day 19 and day 35 larvae are shown Ž .
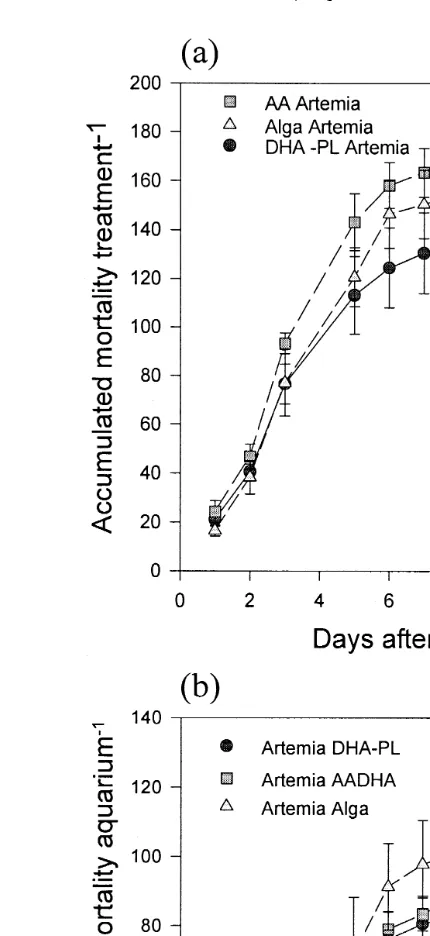
Fig. 4. Accumulated mortality occurring in the aquaria as a function of the Artemia treatments in larvae fed
Ž . Ž .
the a AADHA or b DHA-PL rotifers prior transfer to the aquaria.
levels of DHA, while those fed the AADHA rotifers demonstrated the highest levels of Ž y1 .
Table 5
Selected fatty acids and fatty acid groups in day 19 larvae fed the DHA-PL, AADHA and ALGA rotifer treatments
Rotifer treatments
Fatty acids AADHA DHA-PL ALGA
Saturates 15.2"1.5 17.4"2.6 14.0"0.9
Monounsaturates 10.5"0.9 13.9"1.5 8.7"0.7
Polyunsaturates 5.7"1.2 5.2"0.5 3.5"0.3
ny3 Polyunsaturates 17.1"0.8 20.3"1.5 17.3"1.6 ny6 Polyunsaturates 5.0"0.2 4.3"0.4 5.8"0.5
rotifers 1.8 and 2.1 mg g DW, respectively . The DHArAA ratios in day 19 Table
. Ž .
5 and day 35 larvae Tables 6 and 7 consistently followed the treatment pattern of DHA-PL)ALGA)AADHA, which agreed with the pattern of decreasing accumula-tive mortality. On the other hand, the AA levels of ALGA rotifer and ALGA Artemia fed larvae were always higher than expected considering that AA was absent in both the
Ž .
DHA-PL and ALGA enrichment preparations Tables 5, 6 and 7 . In fact, the AA levels of larvae fed first the AA rotifers and then the ALGA Artemia were virtually identical
Table 6
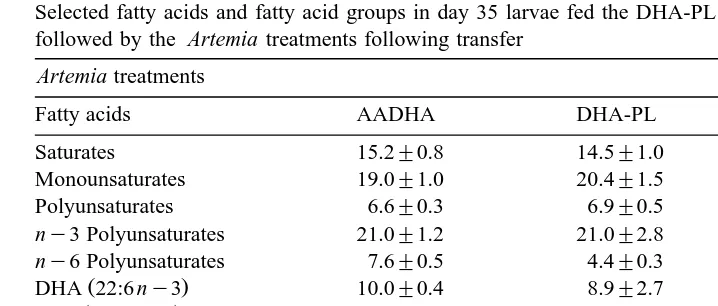
Selected fatty acids and fatty acid groups in day 35 larvae fed the AADHA rotifer treatment prior to transfer followed by the Artemia treatments following transfer
Artemia treatments
Fatty acids AADHA DHA-PL ALGA
Saturates 10.7"1.6 12.7"0.8 15.9"1.9
Monounsaturates 13.0"1.8 16.0"1.1 14.5"1.8
Polyunsaturates 4.8"0.7 5.4"0.4 6.0"0.7
Table 7
Selected fatty acids and fatty acid groups in day 35 larvae fed the DHA-PL rotifer treatment prior to transfer followed by the Artemia treatments following transfer
Artemia treatments
Fatty acids AADHA DHA-PL ALGA
Saturates 15.2"0.8 14.5"1.0 16.7"1.7
Monounsaturates 19.0"1.0 20.4"1.5 16.1"1.6
Polyunsaturates 6.6"0.3 6.9"0.5 6.7"0.6
ny3 Polyunsaturates 21.0"1.2 21.0"2.8 20.0"1.1 ny6 Polyunsaturates 7.6"0.5 4.4"0.3 7.0"0.4
to those of larvae fed both AADHA rotifers and Artemia 2.7 and 2.8 mg g DW, .
respectively .
4. Discussion
At the end of the present study and regardless of Artemia fatty acid content, larvae
Ž .
that were previously fed rotifers containing AA 20:4 ny6 survived significantly better
Ž .
than larvae consuming rotifers having DPA 22:5ny6 . Moreover, both of these treatments demonstrated markedly better survival than larvae consuming rotifers defi-cient in these two ny6 PUFA. It is noteworthy that the retroconversion of DPA into AA in 35-day-old ALGA larvae suggests why the larval survival in this treatment was intermediate between the AA and AA deficient treatments.
grading. In support of this, dietary AA supplementation in purified diets was found to Ž
improve survival in juvenile turbot more effectively than DHA Bell et al., 1985a; .
Castell et al., 1994 .
The complex relationship between dietary AA, stress and larval survival is likely derived from AA involvement in eicosanoid synthesis. Eicosanoid is a collective term referring to prostaglandins, thromboxanes and leukotrienes. These metabolites are involved in various areas of cellular regulation including control of fluid and electrolyte
Ž fluxes, the cardiovascular system, reproductive function and the neural system Mustafa
.
and Srivastava, 1989 . It has been suggested that the source for the biosynthesis of Ž . eicosanoids in fish, such as turbot, is the tissue phospholipid phosphatidylinositol PI ,
Ž .
which is generally rich in AA Bell et al., 1983, 1985b; Linares and Henderson, 1991 . EPA is also a precursor for eicosanoid generation and is much more prevalent in fish
Ž .
tissues than AA Ashton et al., 1994 . However, AA has been reported to be the
Ž .
preferred substrate for prostaglandin synthesis in plaice Tocher and Sargent, 1987 and
Ž .
in tissue homogenates of turbot Henderson et al., 1985 . Furthermore, EPA-derived eicosanoids showed lower biological activity than the AA homologues in mammalian
Ž .
tissue Lands, 1989 .
It is worthwhile to note that gilthead seabream and European seabass from natural fish populations have shown higher levels of tissue AA compared to those that were
Ž .
domesticated Alexis, 1997 . On the other hand, AA is likely required in relatively small quantities, due to the nature of its physiological role in regulation, which is in stark contrast to the relatively high levels of DHA and EPA that are necessary for membrane function and structure. Moreover, the contribution of AA to growth and survival may be masked if other essential fatty acid levels are sub-optimal. Consequently, larval growth and survival may reflect an ny3 PUFA deficiency and not the effect of AA
supplemen-Ž y1 .
tation. The level of DHA in the enriched rotifers 13.4–16.0 mg g DW and Artemia Ž8.9–23.0 mg gy1.in the present study were markedly high and the DHArEPA ratios
Ž .
well above the recommended levels for gilthead seabream Izquierdo, 1996 . The necessity of high dietary DHArEPA ratios for the full expression of AA influence was
Ž .
also reported by Bessonart et al. 1999 who found better survival in gilthead seabream
Ž .
feeding on AA supplemented 1.8% DW microdiets that also contained high ny3 PUFA levels.
In summary, the results suggest that dietary AA fed prior to stress improved survival more effectively than when fed following stress. This has significant implications in the aquaculture industry, where the larvae and juvenile stages are exposed to a range of acute and chronic stressors during rearing. Moreover, these findings underscore the important influence of early larval nutrition on later larval and juvenile survival and biomass production. However, the physiological mechanisms underlying the effect of dietary AA on fish larvae remain unclear and need to be elucidated.
Acknowledgements
References
Ž .
Ackman, R.G., 1980. Fish lipids, part 1. In: Connell, J.J. Ed. , Advances in Fish Science and Technology. Fishing News Books, Farnham, UK, pp. 86–103.
Alexis, M.N., 1997. Fish meal and fish oil replacers in Mediterranean marine fish diets. In: Tacon, A.,
Ž .
Basurco, B. Eds. , Feeding Tomorrow’s Fish. Proceedings from a Workshop of the CIHEAM Network on Technology of Aquaculture in the Mediterranean held June 24–26, 1996, Mazarron, Spain, vol. 22, pp. 183–204.
Ashton, I., Clements, K., Barrow, S.E., Secombes, C.J., Rowley, A.F., 1994. Effects of dietary fatty acids on
Ž
eicosanoid-generating capacity, fatty acid composition and chemotactic activity of rainbow trout
Oncor-.
hynchus mykiss leucocytes. Biochim. Biophys. Acta 1214, 253–262.
Ž . Ž .
Bell, M.V., Simpson, C.M.F., Sargent, J.R., 1983. ny3 and ny6 polyunsaturated fatty acids in the phosphoglycerides of salt-secreting epithelia from two marine fish species. Lipids 18, 720–726. Bell, M.V., Henderson, R.J., Pirie, B.J.S., Sargent, J.R., 1985a. Effects of dietary polyunsaturated fatty acid
Ž .
deficiencies on mortality, growth and gill structure in the turbot Scophthalmus maximus, Linnaeus . J. Fish Biol. 26, 181–191.
Ž
Bell, M.V., Henderson, R.J., Sargent, J.R., 1985b. Changes in the fatty acid composition of turbot
Scopthal-.
mus maximus in relation to dietary polyunsaturated fatty acid deficiencies. Comp. Biochem. Physiol. 81B, 193–198.
Bell, M.V., Batty, R.S., Dick, J.R., Fretwell, K., Navarro, J.C., Sargent, J.R., 1995. Dietary deficiency of
Ž .
docosahexaenoic acid impairs vision at low light intensities in juvenile herring Clupea harengus L. . Lipids 30, 443–449.
Bessonart, M., Izquierdo, M.S., Salhi, M., Hernandez-Cruz, C.M., Gonzalez, M.M., Fernandez-Palacios, H.,´ ´ ´
Ž
1999. Effect of dietary arachidonic acid levels on growth and survival of gilthead sea bream Sparus
.
aurata L. larvae. Aquaculture 179, 265–275.
Castell, J.D., Bell, J.G., Tocher, D.R., Sargent, J.R., 1994. Effects of purified diets containing different combinations of arachidonic and docosahexaenoic acid on survival, growth and fatty acid composition of
Ž .
juvenile turbot Scophthalmus maximus . Aquaculture 128, 315–333.
Folch, J., Lees, M., Stanley, G.H.S., 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226, 497–509.
Gurr, M.I., Harwood, J.L., 1991. Lipid Biochemistry. Chapman & Hall, London, 406 pp.
Henderson, R.J., Bell, M.V., Sargent, J.R., 1985. The conversion of polyunsaturated fatty acids to prosta-glandins by tissue homogenates of the turbot, Scophthalmus maximus, L. J. Exp. Mar. Biol. Ecol. 85, 93–99.
Izquierdo, M.S., 1996. Essential fatty acid requirements of cultured marine fish larvae. Aquacult. Nutr. 2, 183–191.
Izquierdo, M.S., Watanabe, T., Takeuchi, T., Arakawa, T., Kitajima, C., 1989. Requirement of larval red seabream, Pagrus major, for essential fatty acids. Nippon Suisan Gakkaishi 55, 859–867.
Kanazawa, A., 1985. Essential fatty acid and lipid requirements of fish. In: Cowey, C.B., Mackie, A.M., Bell,
Ž .
J.G. Eds. , Nutrition and Feeding in Fish. Academic Press, London, UK, pp. 281–298.
Kanazawa, A., 1993. Nutritional mechanisms involved in the occurrence of abnormal pigmentation in hatchery-reared flatfish. J. World Aquacult. Soc. 24, 162–166.
Kiron, V., Fukuda, H., Takeuchi, T., Watanabe, T., 1995. Essential fatty acid nutrition and defense mechanisms in rainbow trout Oncorhynchus mykiss. Comp. Biochem. Physiol. 111A, 361–367. Kitajima, C., Arakawa, T., Oowa, G., Fujita, S., Imada, O., Watanabe, T., Yone, Y., 1980a. Dietary value for
red seabream of rotifer, Brachionus plicatilis, cultured with a new type of yeast. Bull. Jpn. Soc. Sci. Fish. 46, 43–46.
Kitajima, C., Yoshida, M., Watanabe, T., 1980b. Dietary value for ayu, Plecoglossus altiÕelis, of rotifer, Brachionus plicatilis, cultured with baker’s yeast, Saccaromyces cereÕisiae, supplemented with cuttlefish liver oil. Bull. Jpn. Soc. Sci. Fish. 46, 47–50.
Koven, W.M., Kissil, G.Wm., Tandler, A., 1989. Lipid and ny3 requirement of Sparus aurata larvae during starvation and feeding. Aquaculture 79, 185–191.
ny3 highly unsaturated fatty acids on growth, survival and swim bladder development in Sparus aurata larvae. Aquaculture 91, 131–141.
Koven, W.M., Tandler, A., Kissil, G.Wm., Sklan, D., 1992a. The importance of ny3 highly unsaturated fatty acids for growth in larval Sparus aurata and their effect on survival, lipid composition and size distribution. Aquaculture 104, 91–104.
Koven, W.M., Tandler, A., Sklan, D., Kissil, G.Wm., 1992b. The association of eicosapentaenoic and docosahexaenoic acids in the phospholipids of different age Sparus aurata larvae with growth. Aquacul-ture 116, 71–82.
Lands, W.E.M., 1989. Differences in ny3 and ny6 eicosanoid precursors. In: Samuelsson, B., Wong,
Ž .
P.Y.K. Eds. , Advances in Prostaglandin, Thromboxane and Leukotriene Research, vol. 19. Raven Press, New York, USA, pp. 602–605.
Linares, F., Henderson, R.J., 1991. Incorporation of14C-labelled polyunsaturated fatty acids by juvenile turbot,
Ž .
Scophthalmus maximus L. in vivo. J. Fish. Biol. 38, 335–347.
Lingenfelser, J.T., Blazer, V.S., Gay, J., 1995. Influence of fish oils in production catfish feeds on selected disease resistance factors. J. Appl. Aquacult. 5, 37–48.
McEvoy, L., Estevez, A., Bell, J.G., Shields, R.J., Gara, B., Sargent, J.R., 1998. Influence of dietary levels of
Ž .
eicosapentaenoic and arachidonic acids on the pigmentation success of turbot Scophthalmus maximus L.
Ž Ž .
and halibut Hippoglossus hippoglossus L. In: Hendry, C. Ed. , Proceedings of the Live Feeds Session, Aquaculture Canada ’98. St. Andrews, NB, Canada, No. 98-4. pp. 17–20.
Ž .
Mustafa, T., Srivastava, K.C., 1989. Prostaglandins eicosanoids and their role in ectothermic organisms. Adv. Comp. Environ. Physiol. 5, 157–207.
Ostrowski, A.C., Divakaran, S., 1990. Survival and bioconversion of ny3 fatty acids during early
develop-Ž .
ment of dolphin Coryphaena hipparus larvae fed oil enriched rotifers. Aquaculture 89, 273–285. Rainuzzo, J.R., Reitan, K.I., Jorgensen, L., Olsen, Y., 1994. Lipid composition in turbot larvae fed live feed
cultured by emulsions of different lipid classes. Comp. Biochem. Physiol. 107, 699–710.
Sargent, J.R., Bell, J.G., Bell, M.V., Henderson, R.J., Tocher, D.R., 1993a. The metabolism of phospholipids
Ž .
and polyunsaturated fatty acids in fish. In: Lahlou, B., Vitello, P. Eds. , Aquaculture: Fundamental and Applied Research, Coastal and Estuarine studies 43. American Geophysical Union, Washington DC, USA, pp. 103–124.
Sargent, J.R., Bell, M.V., Tocher, D.R., 1993b. Docosahexaenoic acid and the development of brain and retina
Ž .
in marine fish. In: Drevon, C.A., Baksaas, I., Krokan, H.E. Eds. , Omega-3 fatty acids: Metabolism and Biological Effects. Birkhauser Verlag, Basel, Switzerland, pp. 139–149.¨
Tandler, A., Harel, M., Wilks, M., Levinson, A., Brickell, L., Christie, S., Avital, E., Barr, Y., 1987. Effect of environmental temperature on survival, growth and population structure in the mass rearing of the gilthead seabream, Sparus aurata. Aquaculture 78, 277–284.
Tocher, D.R., Sargent, J.R., 1987. The effect of calcium ionophore A23187 on the metabolism of arachidonic
Ž .
and eicosapentaenoic acids in neutrophils from a marine teleost fish rich in ny3 polyunsaturated fatty acids. Comp. Biochem. Physiol. 67B, 733–739.
Waagboe, R., Sandnes, K., Joergensen, J., Engstad, R., Glette, J., Lie, O., 1993. Health aspects of dietary lipid
Ž .
sources and vitamin E in Atlantic salmon Salmo salar 2: spleen and erythrocyte phospholipid fatty acid composition, non-specific immunity and disease resistance. Fiskeridir. Skr., Ser. Ernaer. 6, 63–74. Watanabe, T., Tamiya, T., Oka, A., Hirata, M., Kitajima, C., Fujita, S., 1983a. Improvement of dietary value
of live foods for fish larvae by feeding them onv3 highly unsaturated fatty acids and fat-soluble vitamins. Bull. Jpn. Soc. Sci. Fish. 49, 471–479.
Watanabe, T., Kitajima, C., Fujita, S., 1983b. Nutritonal values of live organism review. Aquaculture 34, 115–143.