Effects of molluscicide baits on field populations of house mice
G.J. Mutze
a,∗, D.J. Hubbard
baAnimal and Plant Control Commission, GPO Box 1671, Adelaide, SA 5001, Australia bPirsa Rural Solutions, PO Box 469, Murray Bridge, SA 5253, Australia
Received 7 June 1999; received in revised form 5 January 2000; accepted 25 January 2000
Abstract
Two trials were carried out to test the effects of field applications of snail baits on populations of house mice (Mus domesticus) inhabiting cereal-cropping fields during a mouse plague in South Australia. In the first trial, grain based pellets containing 2% w/w active ingredient methiocarb, were laid in trails across fields before crops were sown. The second tested the effect of whole wheat grains treated with 1% w/w methiocarb when broadcast across maturing barley crops. Treatment effects were estimated by comparison with untreated control plots. In the methiocarb-treated wheat trial, comparison was also made with 0.3% w/w strychnine-treated wheat treatments. Methiocarb baiting reduced estimated mouse numbers by 0–46%. Surviving mice did not accept the excess bait material available. In contrast, strychnine-treated wheat reduced estimated mouse numbers by 86–94%. These trials indicate that methiocarb is not likely to be a useful field rodenticide. Furthermore, the consumption of bait by mice is not likely to jeopardise snail control operations. However, methiocarb baits could cause sufficient mortality to pose a threat to rare or endangered granivorous rodents inhabiting agricultural fields. © 2000 Elsevier Science B.V. All rights reserved.
Keywords: Methiocarb; Molluscicide; Mus domesticus; Rodenticide; Strychnine; South Australia
1. Introduction
The use of snail baits for control of the introduced European snails Cernuella virgata, Theba pisana and
Cochlicella acuta in cereal crops has become
increas-ingly prevalent in some parts of South Australia during recent years. Treatment is carried out by field appli-cation of commercial grain-based pellets containing, as active ingredient, metaldehyde or methiocarb. Snail baiting usually commences in winter when the snails become active and move into emerging crops.
Methiocarb pellets are also known to be toxic to house mice (Mus domesticus) which inhabit the
∗Corresponding author. Tel.:+61-8-83039505; fax:+61-8-83039559.
E-mail address: [email protected] (G.J. Mutze)
same fields. Mice would need to eat only 0.04 g of methiocarb pellets in order to obtain an LD50 dose (Mesurol, 2% w/w active ingredient, Bayer, Germany; LD50≈55 mg/kg, Peter Hamblin, Bayer Australia, personal communication). Field application of me-thiocarb pellets is known to cause mortality of wood mice, Apodemus sylvaticus, in English wheatfields (Johnson et al., 1991, 1992). Anecdotal reports have been received of snail baits being used for mouse control around farm buildings in South Australia dur-ing mouse plagues when rodenticides have been in short supply. Several farmers also reported that they had achieved reasonable control of mice by using grain baits mixed with methiocarb at concentrations of 0.5–2% w/w active ingredient.
In winter 1993, house mice were present in plague numbers in many of the districts where snail baiting is
common practice. There was some concern that con-sumption of snail bait by mice may reduce the efficacy of snail control, or increase the amount of bait needed for control. In addition, it seemed likely that snail bait-ing may have some effect on mice, and could possibly reduce mouse numbers and damage cereal crops.
Mice began to damage cereal crops immediately af-ter sowing. When the vast extent of damage became apparent, a highly effective strychnine-baiting cam-paign was used to reduce the number of mice and to control crop damage (Mutze, 1998). Nevertheless, the interaction between snail baiting and mice remained of considerable interest because of the likely future use of snail bait in fields carrying high density mouse populations. The opportunity to study the effect of snail bait on an abundant field-dwelling pest rodent also offered a chance to increase understanding of the likely effect of snail bait on uncommon or endangered granivorous rodents inhabiting agricultural fields.
2. Materials and methods
Two whole-field trials were specifically conducted to test the effect of the snail baits on mice. The first trial examined the use of baiting with methiocarb pel-lets across the field before the crop was sown. The second trial determined whether methiocarb was more readily accepted by mice on a whole grain bait than in the commercial pellet formulation. The availabil-ity and use of strychnine made it possible to compare the effect of snail bait treatments on the abundance of mice with the effects of the very effective strychnine treatment (Mutze, 1989, 1993, 1998) as well as the nil treatment experimental control.
2.1. Trial 1, methiocarb pellet trails across the field
Trial 1 was conducted between 16 and 25th June 1993 in a severely mouse infested 24 ha field at Kulpara, South Australia (34◦04′S, 138◦02′E). The previous year’s wheat crop had been storm damaged so that many heads were knocked down and missed at harvest. The fallen heads were still obvious at the time of the trial despite the field having been cul-tivated once, but the grain in them had germinated or been eaten. Large mouse holes occurred at about 7 m intervals across the field. The field was divided
into four treatment plots. A 5×5 grid, with 10 m be-tween grid points, was measured in the centre of each plot. One Elliott and one Longworth trap were baited with loose rolled oats and placed at each grid point. Both trap types are single live-capture, aluminium box traps. The distance between the trap grids and the edge of the treatment plots was 80–100 m. Mice were trapped, marked and released alive at the site of capture for two nights before treatment. The traps were removed and two diagonally opposite plots were treated with methiocarb pellets laid in parallel trails at 20 m intervals. The rate of application was 2 kg/km which provided a treatment rate of 1 kg pellets/ha or approximately 20,000 LD50 doses/ha. The other two plots were left untreated. On the morning after treat-ment, mouse carcasses were picked up from the trap grids and surrounds in each plot at first light. The traps were replaced 3 days after treatment and mice were trapped, marked and released for further two nights. A crop was sown 1 week after completion of the trial.
2.2. Trial 2, broadcast methiocarb-treated wheat and strychnine-treated wheat
treatment (methiocarb, strychnine or no treatment). A trap grid was established in the centre of each plot as in Trial 1. Trap grids in baited areas were a minimum of 100 m from the boundary of adjacent treated plots and 200 m from unbaited control plots. Mice were trapped, individually marked and released, for three nights. Traps were removed and the crop was baited by the landholder, using a fertiliser spreader to broadcast the treated grain evenly through the crop. However, the spreader was poorly calibrated and the application rate was closer to 1.5 kg/ha than the intended 1.0 kg/ha. Trap grids were searched for dead mice at dawn on the day following treatment. Traps were replaced four nights after treatment and mice were trapped for a fur-ther three consecutive nights.
Following treatment, excess poison bait was avail-able throughout both experiments.
2.3. Data analysis
Three estimates of mouse abundance were made for each mouse population trapped. These were: total number captured per 100 trap nights; number of dif-ferent individuals captured per 100 trap nights; and a Petersen estimate of total population size. Treatment effects were estimated from the change in abundance on treated grids (T ) relative to the change on untreated controls (C ) according to the following formula.
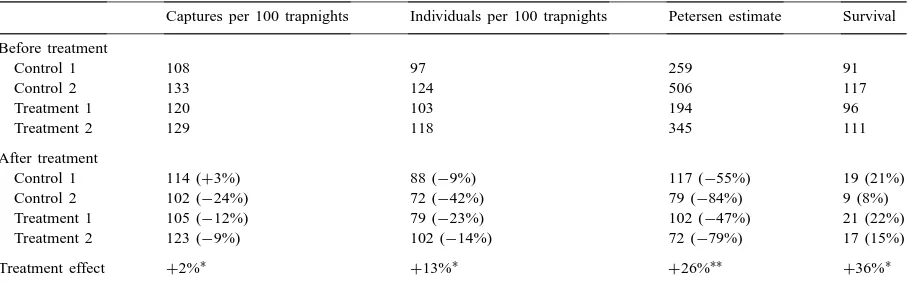
Table 1
Changes in abundance of mice following treatment of a field with trails of methiocarb pelletsa
Captures per 100 trapnights Individuals per 100 trapnights Petersen estimate Survival Before treatment
Control 1 108 97 259 91
Control 2 133 124 506 117
Treatment 1 120 103 194 96
Treatment 2 129 118 345 111
After treatment
Control 1 114 (+3%) 88 (−9%) 117 (−55%) 19 (21%)
Control 2 102 (−24%) 72 (−42%) 79 (−84%) 9 (8%)
Treatment 1 105 (−12%) 79 (−23%) 102 (−47%) 21 (22%)
Treatment 2 123 (−9%) 102 (−14%) 72 (−79%) 17 (15%)
Treatment effect +2%∗
+13%∗
+26%∗∗
+36%∗
aData shown for three population estimates before and after treatment, with the percentage change for each plot in parentheses. Data for
survival show the number of animals marked and released before treatment, and the number and proportion of marked animals recaptured in each plot after treatment. The treatment effect is the mean change in all treatments relative to controls.
∗p>0.15. ∗∗p≤0.01.
% change in treatment (T ) relative to controls
(C)=100×(T final×C initial/C final)−T initial T initial
In addition, the survival of marked individuals in treated areas was compared with that in untreated controls.
The significance of the treatment effects was esti-mated from a X2statistic, calculated using the GLIM program with Poisson errors and the log-link function (Payne, 1987; Crawley, 1993). For the second trial, the effect of treatment on final population numbers was estimated from a generalised linear model with the initial population estimates as a covariate, and with field and treatment as factors with two and three lev-els, respectively. The model was fitted again with the strychnine treatment data aliased to determine the sig-nificance of population changes due to the methiocarb treatments alone. The effect on survival was estimated from a logistic model assuming binomial errors.
3. Results
3.1. Trial 1, methiocarb pellet trails across the field
Multiple captures were common in the supposedly single-capture traps and on one occasion 11 mice were captured in a Longworth trap, with five more found huddled together under the trap. There was some evi-dence from the number of individuals captured of an overall population decline during the experimental pe-riod, but it is possible that the minor changes could be attributed to normal variability in daily trap success. None of the population indices showed a significant reduction in mouse numbers because of treatment. In fact, the Petersen estimate indicated a significant in-crease in mouse numbers on treated plots relative to controls (X21=6.93, p≤0.01).
The level of survival, measured as the proportion of marked animals recaptured, did not differ signif-icantly with treatment (X12=1.86, p≥0.17, Table 1). However, a reasonably high level of mortality occurred amongst marked mice; 48 of the 207 mice marked in the treatment plots were found dead on the ground on the morning after treatment. Of these 48 marked mice, 46 were found inside the two treatment trap grids or within 10 m of them. In those same areas a further 97 unmarked mice were found dead on the surface. Only two of the 71 dead mice picked up between 10–50 m from the trap grids were marked animals from those grids, and none of the 40 mice checked beyond 50 m were marked. From these observations it can be in-ferred that:
• The effective trapping area of 40 m×40 m grid was restricted largely to the area within 10 m of the traps, that is approximately 60 m×60 m=0.36 ha
• The activity range of most of the mice marked and released from the traps was primarily within the 0.36 ha. The number of these ‘resident’ mice marked and released
46
48×207=198
• Density of the trappable population within grids
198×((97+46)/46)
0.36×2 =857 mice/ha
The amount of bait laid was more than adequate for the mice in the area. Mice continued to be trapped in every trap placed within a metre of the remaining bait trails. No further dead mice were found after the first day, but the area was not checked again until trapping recommenced, and was not checked on the
two final days of trapping until an hour or two after first light. A flock of approximately 30 black kites (Milvus
nigrans) probably removed any further carcasses. The
kites were seen gorging on mouse carcasses within an hour of first light on the day after treatment. The kites were resident in the field throughout the experiment but none were seen to display symptoms of secondary poisoning from consuming mouse carcasses.
3.2. Trial 2, broadcast methiocarb-treated wheat and strychnine-treated wheat
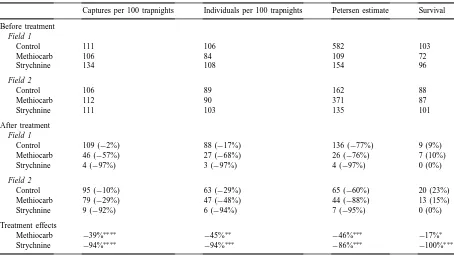
Broadcast methiocarb-treated wheat was much less effective for reducing numbers of mice than strychnine-treated wheat. The three population in-dices indicated that strychnine-treated wheat reduced the population estimates by 86–94%, but methiocarb caused a reduction of only 39–46% (Table 2). Conse-quently, the number of mice that survived treatment was 5- to 10-fold higher in the methiocarb-treated plots than those treated with strychnine. Estimates of population change differed between the two fields only for the number of individuals captured (X12=3.9,
p<0.05). Differences between plots in final mouse numbers were related to differences in initial numbers; all covariate terms in the analyses were significant (allX12>7.5, p<0.01).
None of the mice marked before poisoning on strychnine-treated grids were recaptured after poi-soning (Table 2). In contrast to that result, the recapture rate of marked mice (i.e., survival) on methiocarb-treated areas was not significantly lower than on the untreated controls (X21=0.88, p>0.25). Survival rates differed between the two fields by about 10% (X12=7.37, p≤0.01) but there was no difference in the effect of treatment between fields (X22=0.91,
p>0.5).
Table 2
Changes in abundance of mice following treatment of fields with broadcast 1% methiocarb-treated wheat and 0.3% strychnine-treated wheata
Captures per 100 trapnights Individuals per 100 trapnights Petersen estimate Survival Before treatment
Field 1
Control 111 106 582 103
Methiocarb 106 84 109 72
Strychnine 134 108 154 96
Field 2
Control 106 89 162 88
Methiocarb 112 90 371 87
Strychnine 111 103 135 101
After treatment
Field 1
Control 109 (−2%) 88 (−17%) 136 (−77%) 9 (9%)
Methiocarb 46 (−57%) 27 (−68%) 26 (−76%) 7 (10%)
Strychnine 4 (−97%) 3 (−97%) 4 (−97%) 0 (0%)
Field 2
Control 95 (−10%) 63 (−29%) 65 (−60%) 20 (23%)
Methiocarb 79 (−29%) 47 (−48%) 44 (−88%) 13 (15%)
Strychnine 9 (−92%) 6 (−94%) 7 (−95%) 0 (0%)
Treatment effects
Methiocarb −39%∗∗∗∗
−45%∗∗
−46%∗∗∗
−17%∗ Strychnine −94%∗∗∗∗
−94%∗∗∗
−86%∗∗∗
−100%∗∗∗
aData shown for three population estimates before and after treatment, with the percentage change for each plot in parentheses. Data for
survival show the number of animals marked and released before treatment, and the number and proportion of marked animals recaptured in each plot after treatment. The treatment effect is the mean change in all treatments relative to controls.
∗ p>0.3. ∗∗
p≤0.05. ∗∗∗
p≤0.01. ∗∗∗∗
p≤0.001.
4. Discussion
Treatment with methiocarb pellets did not signifi-cantly reduce the number of mice trapped in the first trial at Kulpara because the extremely high numbers of mice in the field completely swamped the trap grids used. Trap success remained above 100% even when a substantial proportion of the population was killed in the treatment plots. The 8 ha treatment areas also may have been inadequate to cope with the re-invasion of treated areas from adjacent untreated areas. This high-lights the difficulty of accurately determining changes in population density during mouse plagues, when the requirements for large treatment areas, high trapping intensity and adequate replication are often in conflict (Singleton et al., 1995).
In contrast to the evidence from the population estimates, known mortality of marked mice exceeded
20% and large numbers of unmarked mice were also killed. As well as the mice found dead on the surface, there were probably some that died underground. Previous work testing strychnine for mouse control in similar bare fields indicated that, even with a fast act-ing poison such as strychnine, less than 50% of mice die above ground (Mutze, 1998). Methiocarb acts less quickly, so it would seem likely that the 23% of marked animals recovered dead on the surface at Kul-para represents only a proportion of marked animals killed. Nevertheless, the survival of a high proportion of mice in the presence of abundant bait indicates that there was either strong aversion to the active ingredi-ent or poor acceptance of the pellet bait formulation.
strychnine-treated grain. This result for methiocarb is consistent with the recorded mortality of marked mice in the first experiment. The contrasting high level of population reduction achieved with strychnine indi-cates that the conditions under which the experiment was conducted were conducive to bait uptake because strychnine is generally considered to have low palata-bility (Gratz, 1973; Prakash and Mathur, 1992) and is not effective on mice where alternative palatable food is readily available (Mutze, 1998). The different results obtained for the two active ingredients suggest that poor acceptance of methiocarb bait was because of aversion to the active ingredient rather than neo-phobia or poor acceptance of the bait material used (pellet or whole grain). Furthermore, the level of mor-talities observed from methiocarb baits were consis-tent with anecdotal reports of good poisoning success by farmers who measured success by the number of mortalities (carcasses observed) but had no reliable measure of the proportion of mice surviving.
Methiocarb, when presented in food baits, is an ef-fective molluscicide (Kelly and Martin, 1989) and in-secticide (Wilton et al., 1973). Birds, on the other hand, often develop an aversive response before in-gesting a lethal dose and, consequently, it has been widely used as a bird repellent (Guarino, 1972; Bailey and Smith, 1979; Avery et al., 1995). The response of mice when exposed to bait containing an acute dose is intermediate between that commonly reported in birds and snails. A high level of mortality occurs but it is not sufficiently high for effective population regula-tion. Many mice appear to develop an aversion to the active ingredient before ingesting a lethal dose. The evidence from these trials is sufficient to reasonably discount consideration of methiocarb as a field roden-ticide. Furthermore, it is highly unlikely that consump-tion of bait by mice could affect snail control in fields. The results from house mice are consistent with studies of methiocarb toxicity in English wood mice,
A. sylvaticus. Three groups of wood mice offered
me-thiocarb pellets in laboratory cages averaged 38% mor-tality (range 25–50%) (Tarrant and Westlake, 1988). Field experiments with methiocarb pellets have all been somewhat limited by the number of wood mice in the sample and have given varied results. In one ex-periment, survival was significantly reduced but pop-ulation levels were restored by immigration after 7 days (Johnson et al., 1991, 1992). No information was
presented about persistence of pellets in the fields dur-ing the period of population recovery. In a second ex-periment reported in that study, with slightly bigger sample sizes, survival of marked individuals was 60% lower in the treated field than in the untreated, but the decline in total numbers of wood mice did not differ significantly between treatments. A third study (Shore et al., 1997) recorded significant declines in numbers of wood mice during autumn, 78%, and spring, 33%, following application of methiocarb pellets. The de-clines were unequivocally attributed by Shore et al. (1997) to the effect of methiocarb treatment but, dur-ing autumn, that study had no untreated experimen-tal control and, during spring, an inadequate control in which wood mice declined proportionately more than in the treated area, so the effect of other popu-lation processes cannot be discounted. Nevertheless, taken together the English studies suggest that me-thiocarb baiting in fields does present some threat to wood mice.
molluscicide commonly used for field application. It is used in molluscicide pellet baits in Australia at a concentration similar to that of methiocarb (2% w/w active ingredient) but its mammalian toxicity is ap-proximately 10-fold lower than that of methiocarb (rat LD50≈630 mg metaldehyde/kg; rat LD50≈13–135 mg methiocarb/kg, Budavari et al., 1996; Hayes and Laws, 1991).
Acknowledgements
The authors would like to thank the landholders that participated in this trial work; Malcolm Meier and Anthony, Leith and Roly Gregurke; for access to their properties and for their willing assistance with various stages of the work. Peter Hamblin and Bill Bloodworth of Bayer Australia were most helpful and provided Mesurol® for the methiocarb baiting trials. Tim Reynolds provided able assistance in the field and, along with Peter Allen, made useful comments on the manuscript. This project was made possible through funding from GRDC Project Number DAS 166B. We would particularly like to acknowledge GRDC’s rapid response to this project proposal when it became apparent that suitably high populations of mice were available for field trials.
References
Avery, M.L., Pavelka, M.L., Bergman, D.L., Decker, D.G., Knittle, C.E., Linz, G.M., 1995. Aversive conditioning to reduce raven predation on least tern eggs. Colonial Waterbirds 18, 131–138. Bailey, P.T., Smith, G., 1979. Methiocarb as a bird repellent on wine grapes. Aust. J. Exp. Agric. Anim. Husb. 19, 247–250. Budavari, S., Maryadele, J.O., Smith, A., Heckelman, P.E.,
Kinneary, J.F. (Eds.), 1996. The Merck Index, 12th Edition. Merck & Co., New Jersey.
Crawley, M.J., 1993. GLIM for Ecologists. Blackwell Scientific Publications, Oxford.
Gratz, N.G., 1973. A critical review of currently used single-dose rodenticides. Bull. World Health Org. 48, 469–477.
Guarino, J.L., 1972. Methiocarb, a chemical bird repellent: a review of its effectiveness on crops. Proceedings of the 5th Vert. Pest Conf., Fresno, California, pp. 108–111.
Hayes, W.J., Laws, E.R. (Eds.), 1991. Handbook of Pesticide Toxicology. Academic Press, San Diego, 1576 pp.
Johnson, I.P., Flowerdew, J.R., Hare, R., 1991. Effects of broadcasting and of drilling methiocarb molluscicide pellets on field populations of wood mice, Apodemus sylvaticus. Bull. Environ. Contam. Toxicol. 46, 84–91.
Johnson, I.P., Flowerdew, J.R., Hare, R., 1992. Populations and diet of small rodents and shrews in relation to pesticide usage. In: Greig-Smith, P.W., Frampton, G., Hardy, A. (Eds.), Pesticides, Cereal Farming and the Environment: The Boxworth Project. HMSO, London, pp. 144–156.
Kelly, J.R., Martin, T.J., 1989. Twenty-one years experience with methiocarb baits. In: Henderson, I. (Ed.), Slugs and Snails in World Agriculture. Monograph 41, British Crop Protection Council, Guildford, pp. 131–145.
Mutze, G.J., 1989. Effectiveness of strychnine bait trails for poisoning mice in cereal crops. Aust. Wildl. Res. 16, 459–465. Mutze, G.J., 1993. Cost-effectiveness of poison bait trails for control of house mice in mallee cereal crops. Wildl. Res. 20, 445–456.
Mutze, G.J., 1998. The 1993 strychnine baiting program for mouse control in South Australian grain crops. Wildl. Res. 25, 533– 546.
Payne, C.D. (Ed.), 1987. The GLIM System Release 3.77 Manual, 2nd Edition. Royal Statistical Society, Oxford.
Prakash, I., Mathur, R.P., 1992. Acute rodenticides. In: Prakash, I., Ghosh, P.K. (Eds.), Rodents in Indian Agriculture, Vol. 1. Scientific Publishers, Jodhpur, pp. 497–515.
Shore, R.F., Feber, R.E., Firbank, L.G., Fishwick, S.K., Macdonald, D.W., Nørum, U., 1997. The impacts of molluscicide pellets on spring and autumn populations of wood mice Apodemus
sylvaticus. Agric. Ecosyst. Environ. 64, 211–217.
Singleton, G.R., Chambers, L.K., Spratt, D.M., 1995. An experimental field study to examine whether Capillaria hepatica (Nematoda) can limit house mouse populations in eastern Australia. Wildl. Res. 22, 31–53.
Tarrant, K.A., Westlake, G.E., 1988. Laboratory evaluation of the hazard to wood mice, Apodemus sylvaticus, from the agricultural use of methiocarb molluscicide pellets. Bull. Environ. Contam. Toxicol. 40, 147–152.