www.elsevier.com / locate / bres
Research report
Effects of threonine injections in the lateral hypothalamus on intake of
amino acid imbalanced diets in rats
J. Ernie Blevins, Kimberly D. Dixon, Edward J. Hernandez, Jennifer A. Barrett,
*
Dorothy W. Gietzen
Department of Anatomy, Physiology, and Cell Biology, School of Veterinary Medicine, and Food Intake Laboratory, University of California, Davis,
CA95616, USA Accepted 18 July 2000
Abstract
Previous work from this laboratory suggests that animals decrease their intake of an amino acid imbalanced diet (IMB), due in part to a drop in the concentration of the dietary limiting amino (DLAA) in the anterior piriform cortex (APC). Administration of the DLAA, but not of a non-limiting amino acid into the APC, blocks the anorectic response to IMB. To our knowledge, the effects of DLAA injections on intake of a diet devoid of the DLAA (DEV), have not been examined in areas outside the APC. We hypothesized that the LH is a potential chemosensory area for DLAA. Our objectives were: (1) to determine whether injections of the DLAA threonine into the lateral hypothalamus (LH) alter intake of a threonine-devoid diet (DEV); and (2) to examine the dose–response effects of threonine injections into the LH on intake of threonine-corrected diet (COR). Administration of threonine into the LH stimulated DEV intake during the first 6 h at the 0.25 and 1-nmol doses by approximately 26 and 24%, respectively. Threonine (0.25, 2.5 nmol) did not alter COR intake at any time during the first 12 h. Our results suggest that: (1) the LH, along with the APC, likely acts as a chemosensory brain area for indispensable amino acids; and (2) both the APC and LH are part of a circuit that is involved in the short term anorectic response to amino acid imbalanced diets. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Appetite regulation; Amino acid imbalance; Hypothalamus; Rat
1. Introduction merely involved in processing, little effect should be observed.
Rats have the ability to detect a deficiency of dietary An amino acid imbalanced (IMB) or devoid diet (DEV) limiting amino acids (DLAA) within minutes after inges- results in a reduced concentration of the DLAA in the tion of a meal [16]. Evidence suggests this recognition brain. In response to IMB, the DLAA concentration is occurs in the central nervous system, specifically in the reduced much more in the brain relative to plasma anterior piriform cortex (APC). Ingestion of diet with [16,20,37,38,48,49]. A drop in tissue levels in the brain imbalanced DLAA (IMB) results in anorexia and if may initiate mechanisms which signal deficiency while prolonged, may result in increased susceptibility to epi- normal levels appear to signal balanced intake. Studies leptigenesis [14]. However, the APC may not be the only have shown that the DLAA transport into brain slices brain area involved in detection of amino acid levels. [32,47] is inhibited following introduction of the IMB Experiments show that the lateral hypothalamus (LH) may diets. IMB consumption returns to control levels if the also be involved in detection and processing of information dietary limiting amino acid (DLAA) is infused into the about DLAA levels. We hypothesized that if the LH acts as carotid artery, whereas infusion of the same concentration a chemosensory area, injection of DLAA into this region of the DLAA into the jugular vein does not prevent should block or diminish IMB anorexia. If the LH is anorexia [28,50].
The anterior piriform cortex (APC) serves an integral role in the recognition of amino acid deficiencies. Rats with bilateral lesions of the APC fail to reduce their food
*Corresponding author. Tel.: 11-530-752-9211; fax: 1
1-530-752-intake when fed IMB while lesions of many other brain
7690.
E-mail address: [email protected] (D.W. Gietzen). areas involved in food intake do not produce this effect
(reviewed in Refs. [13,30,33]). The APC also appears to specific area of the LH sampled. Microinjection of bal-have a direct role in the detection of amino acid de- anced amino acid solutions directly into the perifornical ficiencies. Rats fed IMB have reduced threonine levels in hypothalamus depressed eating [18], suggesting that an the APC [15], anterior cingulate cortex, locus ceruleus and area adjacent to the medial / dorsal LH is sensitive to the nucleus tractus solitarius (NTS), but not in the paraven- effects of amino acids on feeding behavior. Tract tracing tricular nucleus (PVN), lateral hypothalamus (LH), or studies from the area of the APC that we have found ventromedial hypothalamus (VMH) [17], areas implicated sensitive to threonine injections show projections to the in the control of food intake [2,21,27]. This suggests that LH, medial and reticular thalamic nuclei [1], suggesting ingestion of a diet deficient in the DLAA will result in a that repletion of DLAA is recognized by a pathway that low DLAA concentration in specific regions of the brain includes the APC and the LH.
involved with DLAA chemoreception. Furthermore, in- Sufficient evidence supports the hypothesis that the LH jection of threonine, but not other essential AA (iso- may have a role in the detection of DLAA. If the LH acts leucine), into the APC stimulates intake of a threonine as a chemosensory area for DLAA, then injections of IMB but not of a low protein diet [5]. Likewise, threonine threonine into the lateral hypothalamus should increase injections into the APC stimulate intake of DEV, but not of intake of a threonine devoid diet, while injections into the a standard diet [34]. Expression of the immediate-early LH should have no effect on COR intake.
gene, c-fos, also provides evidence that neurons in the APC are involved in DLAA chemoreception. Increased
Fos-like immunoreactivity is seen in the APC 1 h after 2. Methods
introduction of an IMB. By 2 h this Fos-like
immuno-reactivity is significantly greater than for rats fed an amino 2.1. Subjects acid corrected diet (COR) [53].
The medial and lateral hypothalamic nuclei, along with Adult, male Sprague Dawley rats from Simonsen Lab-the APC, are also involved in mediating Lab-the anorexic oratories, Gilroy, CA, USA weighing between 196 and 244 response to IMB or DEV diets. Electrolytic lesions of the g were used. Animal care was according to the National DMH increase 3-h IMB intake [4]. VMH lesioned rats eat Institutes of Health guidelines. Animal protocols were more of a mild ILE-IMB [29] and a histidine IMB diet approved by the University of California, Davis Animal [35] but not of a severe ILE IMB [29] or of a threonine Use and Care Committee. The animals were housed IMB diet [40]. Levels of the DLAA are decreased in the individually in hanging wire-mesh cages in a temperature-DMH in response to IMB [15]. Onset of c-fos expression controlled room (22628C) under a 12 / 12 h light–dark in the DMH, infralimbic cortex, claustrum, and dorsal cycle (lights off 1200 h) with deionized water and food endopiriform nucleus (deep piriform cortex [39]) was freely available.
similar to the APC suggesting that these areas may also be
involved in the early recognition of AA deficiency [53]. 2.2. Implantation of guide cannulas into the LH Evidence suggests that the LH, along with the APC,
[penicillin (Aquacillin, Vedco, St. Joseph, MO, USA Systems, West lafayette, IN, USA). Bilateral injections (0.3 300 000 units / ml); 60 000 units IM] was administered at ml per side) of either saline orL-threonine (Fluka,
Ronkon-the completion of surgery. komoa, NY, USA) were delivered simultaneously at a constant rate of 0.06ml / min within 2 h prior to the start of 2.3. Diet composition the dark cycle. The volume of injectate was verified by forward movement of an air bubble to a pre-calibrated Experimental diets, which have been described previ- distance (3 mm), corresponding to the appropriate volume. ously [6] (Table 1), and water were available ad libitum. Injection needles were left in place for approximately 60 s These diets included free L-AAs as the protein source, after the injection was completed before being removed with cornstarch and sucrose (2:1) as the carbohydrate slowly. Following each injection, obturators were replaced source, 5% corn oil as the fat source, 1% vitamin mix, and and each animal was returned to its cage. Food bowls were 5% salt mix. The threonine basal diet was a low protein returned to the respective cages at the completion of all diet, providing approximately 12% crude protein equiva- injections.
lent, with threonine as the growth-limiting AA, present at
about 40% of its requirement for the growing rat [23]; all 2.4.1. Trial 1: Effects of threonine injections into the other essential amino acids were added to account for ventral LH in rats fed a DEV diet
approximately 70% of their requirement for the growing Rats (n524, 210–244 g) fed moistened DEV diet (20% rat. The threonine corrected diet contained a balanced H O v:w) received bilateral injections of threonine (0,2
pattern of amino acids at approximately 100% of the 0.25, 0.5, 1, 2, 4 nmol) in saline into the LH. Food intake requirement. The DEV contained an excess of essential was measured at 3, 6, 9, 12, and 24 h post-injection. AAs other than threonine and was completely lacking in
threonine. For the addition of the AAs, a proportionate 2.4.2. Trial 2: Effects of threonine injections into the amount of the carbohydrate fraction was removed. Ani- dorsal LH in rats fed a DEV diet
mals were placed on the threonine basal diet for 7–16 days Due to the ineffectiveness following THR injections into post surgery. cannulas positioned within the ventral LH, in a separate Following implantation of the hypothalamic cannulas, group of animals (n520, 200–217 g) threonine was rats were adapted to handling and a 2-h fast prior to the administered in an identical manner via cannulas start of the dark cycle. Food intake was determined by positioned 0.1 mm dorsal and intake of dry powdered DEV weighing food bowls to the nearest 0.1 g and correcting for diet was measured. No differences in meal patterns have
spillage. been observed between moistened and powdered IMB
diets (unpublished data). Therefore, both diets were ex-2.4. Injection procedure amined in this study. Although evaporative loss from the moistened diet was negligible, the powdered diet was used Injections were made by lowering the 33-gauge injector in the trial 2 and in the COR diet study due to the with its tip extending 1 mm beyond the guide cannula into possibility of error associated with animals getting the diet the brain site of interest. Each injection needle was on their fur over time.
connected via PE-20 tubing (O.D. (0.0430 I.D. (0.0150)
Plastics One, Roanoke, VA, USA) to a 10-ml syringe fitted 2.5. Effects of threonine injections into the dorsal LH in into a microinjection pump (CMA / 100, Bioanalytical rats fed a COR diet
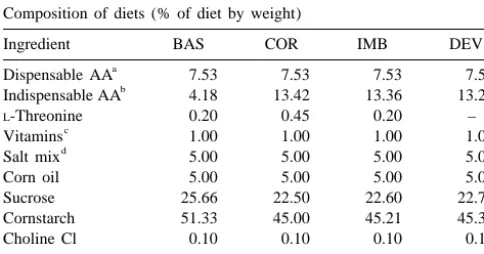
Table 1 Rats (n519, 196–212 g) fed a COR diet received
Composition of diets (% of diet by weight) bilateral injections of threonine (0, 0.25, 2.5 nmol) in
saline into the dorsal LH, the same site where THR
Ingredient BAS COR IMB DEV
a stimulated intake in both moistened and powdered DEV.
Dispensable AA 7.53 7.53 7.53 7.53
b These doses covered the range of effective doses that
Indispensable AA 4.18 13.42 13.36 13.28
L-Threonine 0.20 0.45 0.20 – stimulated intake of DEV (0.25, 2 nmol) and tested
c
Vitamins 1.00 1.00 1.00 1.00 whether the stimulatory effect of THR on DEV was
d
Salt mix 5.00 5.00 5.00 5.00 specific to a DEV situation. Food intake was measured as
Corn oil 5.00 5.00 5.00 5.00
described above.
Sucrose 25.66 22.50 22.60 22.70
Cornstarch 51.33 45.00 45.21 45.39
Choline Cl 0.10 0.10 0.10 0.10 2.6. Histological verification of injection sites
Total 100.00 100.00 100.00 100.00
Upon completion of the experiments, animals were
a
Dispensible Amino Acid Mix (Ajinomoto, USA).
b sacrificed by ether inhalation and immediately decapitated.
Basal Indispensible (Essential) Amino Acid Mix (Ajinomoto).
c This was followed by subsequent removal of the guide
Total Vitamin Supplement (United States Biochemical).
d
were stored in 10% formalin for 24 h followed by 10% 3. Results
sucrose for at least 48 h prior to freezing and sectioning.
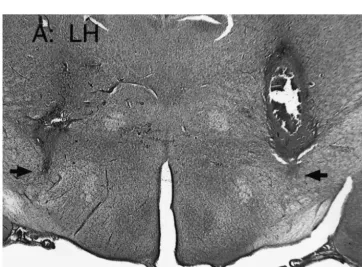
Coronal sections (40 mm) were taken through the most 3.1. Effects of threonine injections into the ventral LH ventral portion of the cannula track. Sections were on intake of DEV diet
mounted on microscope slides and allowed to dry for 24 h
prior to staining with Cresyl violet. A projection micro- Threonine injections into animals (n58) that had can-scope was used to project the image of the brain section nulas placed within the ventral LH as described in Section showing the most ventral portion of the injectate (Fig. 1), 2 did not affect intake of the moistened DEV diet at any defined as the injection site, onto the corresponding section time measured (data not shown).
from the rat brain atlas of Paxinos and Watson [36]. In trial 1, data were grouped based on cannula placements either
3.1.1. Trial 1. Moistened DEV diet (dorsal LH) symmetrically within the ventral LH or animals having one
Threonine injections into cannulas placed stimulated injection site within the ventral LH and the other at the
moistened DEV intake (n54) at 0.25 nmol through the level of the fornix. Similarly, in trial 2, data were grouped
first 9 (P50.056, trend) and 12 h (P50.046) by 35 and based on symmetrical injection sites within the dorsal LH
30%, respectively (data not shown). A similar response or had one injection site within the dorsal LH and the other
was seen in an animal whose placement was slightly at the level of the fornix.
ventral to the fornix on one side and dorsal to the fornix, ventral to the internal capsule on the other, 121 and 39% stimulation at 6 h and 29 and 62% stimulation at 9 h at 2.7. Statistical analyses
0.25 and 1 nmol respectively. Food intake over 24 h following saline injections was 12.6461.18 g, which was Data are presented as means6S.E.M. Results from each
not significantly different from intake at this time follow-study were analyzed separately by one-way ANOVA.
ing threonine injections at any dose. Results were considered significant if P,0.05. Each
animal served as its own control in a crossover design with
the exception of the effects of threonine into the dorsal LH 3.1.2. Trial 2. Powdered DEV diet (dorsal LH)
on intake of corrected diet. All analyses were conducted In a separate group of animals, threonine administered using version 7.0 of PC-SAS (SAS, Cary, NC, USA). into sites dorsal to the fornix (Fig. 2) tended to stimulate
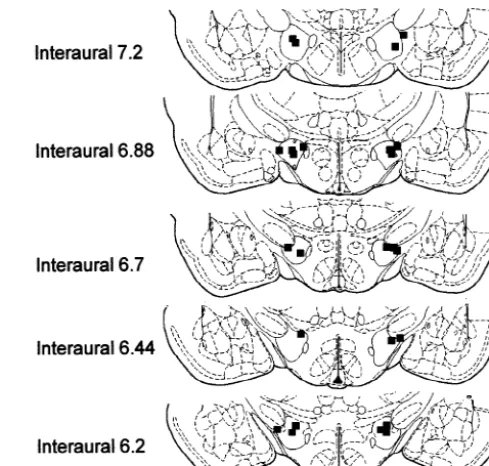
Fig. 3. Schematic representation from Paxinos and Watson showing the injection sites corresponding to the dorsal LH in animals exposed to the DEV diet. The symboljis used to designate injection sites included in the data analysis.
4. Discussion
Accumulating evidence suggests that the LH (and / or
Fig. 2. Effects of threonine injections into the dorsal LH on intake of
zona incerta) may be part of a relay initiated in the APC
DEV diet. Significant increases are indicated by P values for the 0.25 and
that is involved in early detection of amino acid imbalance
1 nmol doses for the 0–6 h period. Data are presented as mean6S.E.M
[1,4,17,18,33,53]. Neuronal activity increases in the LH
and were analyzed by a one-factor ANOVA.
following APC injection of threonine, at a dose that stimulates DEV intake [34], but little change was seen in animals fed a standard diet or those receiving saline powdered DEV intake by 28 and 26% (n512–14) during injections. Tract tracing studies from the area of the APC the first 3 h at 0.25-nmol (P50.059) and 2-nmol doses that we have found sensitive to threonine injections show (P50.071). After 6 h, threonine had significantly increased projections to the LH, medial and reticular thalamic nuclei DEV intake by approximately 26 and 24% at the 0.25 [1]. Differential activation of neurons was seen in the (P50.037) and 1-nmol (P50.047) doses, respectively. Fig. dorsal LH in response to cues associated with lysine in rats 3 shows the distribution of injection sites within this area deficient in lysine, with nondifferential activation being of the LH. Food intake at 24 h following saline injections evident when balanced amino acid solutions were offered was 12.1461.27 g, which was not significantly different [17]. Microinjection of balanced amino acid solutions from intake at this time following threonine injections at directly into the perifornical hypothalamus decrease food any dose. intake [18]. Taken together, these data suggest that reple-tion of DLAA is recognized by a pathway that includes the APC and the LH.
coursing in and / or around the DMH are important in feeding response to IMB is not clear. The pyramidal cells, mediating the anorectic response to amino acid deficiency which contain the excitatory neurotransmitter glutamate, [4]. Similar to the APC, changes in the levels of the DLAA are the primary output neurons of the APC [19], which [15] and c-FOS [53] in the DMH occur after eating IMB, project to the LH [39]. Blockade of the NMDA and the and microinjections of the DLAA into the DMH were AMPA receptors within the APC following bilateral in-shown to increase DEV intake [7]. jections of the NMDA and AMPA receptor antagonists NPY and somatostatin have both been found to alter AP5 and NBQX respectively result in increased IMB intake of IMB following injections into the APC. NPY intake [52]. Recent studies by Stanley et al. provide mRNA in the APC increases in animals fed a threonine evidence that the LH is the most sensitive site to glutamate deficient diet and NPY decreases IMB intake following [45] and that different feeding mechanisms via the kainate, APC injection (1–1.5 nmol in 0.5 ml) up to 6 h postinjec- AMPA, and NMDA receptors [41,44] are involved in the tion [9]. These results contrast to the well known stimulat- control of food intake within the LH. AP5 stimulates ory effects of NPY following injections into the periforni- feeding at doses that do not alter the decrease in feeding cal area and the LH on food intake [43], but are consistent following LH injections of kianate or AMPA. These with data showing a stimulatory effect on food intake injections provide evidence implicating endogenous gluta-following injections of antisense oligonucleotides to the matergic activity within the LH at the NMDA receptor in NPY-Y1 into the amygdala, an area which also sends and the regulation of food intake [44]. Furthermore, c-FOS receives neuronal projections to the APC [18]. Therefore, activity was increased in the piriform cortex in response to one potential mechanism that may act, in part, to initiate NMDA administration into the LH by reverse-dialysis, the stimulatory response on intake of DEV and neuronal suggesting a common pathway including the APC and the activity within the LH following THR injections into the LH perhaps mediates the glutamatergic responses to APC [34], may involve an increase in NPY action in the feeding behavior [24]. Additional studies will need to be LH with a corresponding decrease in the amygdala. More done in order to determine how the glutamatergic system experiments will need to be completed in order to clarify in the LH may be involved in the recognition of amino
this issue. acid imbalance.
The effects of somatostatin on intake of IMB or of food In summary, our results suggest that the LH / zona intake in general vary according to injection site, dose of incerta may be part of a circuit involved in the short term administration, and feeding paradigm. APC injections of anorectic response to the severe amino acid deficiency. somatostatin-28 produced a biphasic response to IMB, Further studies examining the use of reverse-phase dialysis with a stimulation of IMB at 1 pmol and an inhibition of of threonine into the LH on both the intake of DEV and the IMB at 2 pmol [9]. Other studies found SOM-14 to inhibit release of neurotransmitters in this area will help to clarify food intake following ICV [[3] (10 000 pmol) [11,12], (3 the mechanism(s) underlying this response to amino acid pmol)] or LH administration (900 pmol [22]), the LH deficiency.
being the same site where increases in somatostatin were found in satiated relative to hungry rats [22]. Alternatively,
previous studies report increases in food intake following Acknowledgements
ICV administration of either a somatostatin analogue
(SMS-201-995 [10]) or SOM-14 [3] (10 000 pmol) The authors would like to acknowledge the work of [11,12], (1 pmol) with a corresponding decrease following Jennifer E. Hanks and Dr. James W. Sharp. This work was ICV injection of somatostatin antiserum [10]. Recent data supported by National Institutes of Health Grants shows that a somatostatin-like peptide found within the DK07355, DK35747, and NS33347.
hypothalamus contains the CART fragment 82-103 (cocaine and amphetamine related transcript [25]), a
peptide found to inhibit feeding when administered ICV References
and also found to reduce NPY-induced feeding [26]. NPY
varicosities were also observed around cell bodies con- [1] S.M. Aja, Neurotransmitters and neural circuitry supporting amino-privic feeding. University of California, Davis, Dissertation, 1999.
taining CART, suggesting possible interactions between
[2] B.K. Anand, J.R. Brobeck, Hypothalamic control of food intake in
the two systems in the control of food intake [26]. The
rats and cats, Yale J. Biol. Med. 24 (1951) 123–128.
variations between the behavioral studies utilizing [3] G. Aponte, P. Leung, D. Gross, T. Yamada, Effects of somatostatin somatostatin and / or somatostatin antiserum do not provide on food intake in rats, Life Sci. 35 (1984) 741–746.
a clear understanding of the role somatostatin may have [4] L.L. Bellinger, J.F. Evans, C.M. Tillberg, D.W. Gietzen, Effects of dorsomedial hypothalamic nuclei lesions on intake of an imbalanced
within the LH in mediating the anorexic response to DEV,
amino acid diet, Am. J. Physiol. 46 (1999) R250–R262.
therefore further studies are necessary.
[5] J.L. Beverly, D.W. Gietzen, Q.R. Rogers, Effect of dietary limiting
The glutamatergic system appears to have an integral amino acid in prepyriform cortex on food intake, Am. J. Physiol. role in the recognition of amino acid imbalance within the 259 (1990) R709–R715.
dose of amino acids injected into prepyriform cortex influence food [26] P.D. Lambert, P.R. Couceyro, K.M.D. McGirr, S.E. -Vechia, Y. intake, Physiol. Behav. 53 (1993) 899–903. Smith, M.J. Kuhar, CART peptides in the central control of feeding and interactions with neuropeptide Y, Synapse 29 (1998) 293–298. [7] J.E. Blevins, K.D. Dixon, J.A. Barrett, B.G. Truong, D.W. Gietzen,
Effects of threonine injections in the medial hypothalamus of amino [27] S.F. Leibowitz, Hypothalamic paraventricular nucleus lesions acid imbalanced diets in rats. Brain Res. (2000) submitted. produce overeating and obesity in the rat, Physiol. Behav. 27 (1981)
1031–1040. [8] J.E. Blevins, P.J. Havel, D.W. Gietzen, Injections of leptin into the
anterior piriform cortex inhibit food intake in rats, Nutr. Neurosci. 2 [28] P.M.B. Leung, Q.R. Rogers, Food intake: regulation by plasma (5) (1999) 357–367. amino acid pattern, Life Sci. 8 (1969) 1–9.
[9] S.L. Cummings, B.G. Truong, D.W. Gietzen, Neuropeptide Y and [29] P.M.B. Leung, Q.R. Rogers, Effect of amino acid imbalance and somatostatin in the anterior piriform cortex alter intake of amino deficiency on food intake of rats with hypothalamic lesions, Nutr. acid-deficient diets, Peptides 19 (1998) 527–535. Rep. Int. 1 (1970) 1–10.
[10] J. Danguir, Food intake in rats is increased by intracerebroventricu- [30] P.M.B. Leung, Q.R. Rogers, The effect of amino acids and protein lar infusion of the somatostatin analogue SMS 201-995 and is on dietary choice, in: Y. Kawamura, M.R. Kare (Eds.), Umami: A decreased by somatostatin antiserum, Peptides 9 (1988) 211–213. Basic Taste, Marcel Dekker, New York, 1987, pp. 565–610. [11] D. Feifel, F. Vaccarino, Growth hormone-regulatory peptides [31] P.M.B. Leung, Q.R. Rogers, A.E. Harper, Effect of amino acid
(GHRH and somatostatin) and feeding: a model for the integration imbalance in rats fed ad libitum interval-fed or force-fed, J. Nutr. 95 of central and peripheral function, Neurosci. Biobehav. Rev. 18 (1968) 474–482.
(1994) 421–433. [32] J. Lutz, J.K. Tews, A.E. Harper, Simulated amino acid imbalance [12] D. Feifel, F. Vaccarino, J. Rivier, W. Vale, Evidence for a common and histidine transport in rat brain slices, Am. J. Physiol. 229 (1975)
neural mechanism mediating growth hormone-releasing factor-in- 229–234.
duced and somatostatin-induced feeding, Neuroendocrinology 57 [33] L.L. Meliza, P.M.B. Leung, Q.R. Rogers, Effect of anterior pre-(1993) 299–305. pyriform and medial amygdaloid lesions on acquisition of taste-[13] D.W. Gietzen, Neural mechanisms in the responses to amino acid avoidance and response to dietary amino acid imbalance, Physiol.
deficiency, J. Nutr. 123 (1993) 610–625. Behav. 26 (1981) 1031–1035.
[14] D.W. Gietzen, K.D. Dixon, B.G. Truong, A.C. Jones, J.A. Barrett, [34] M. Monda, A. Sullo, V. De Luca, M.P. Pellicano, A. Viggiano, D.S. Washburn, Indispensable amino acid deficiency and increased L-threonine injection into the PPC modifies food intake, lateral seizure susceptibility in rats, Am. J. Physiol. 271 (1996) R18–R24. hypothalamic activity, and sympathetic discharge, Am. J. Physiol. [15] D.W. Gietzen, L.F. Erecius, Q.R. Rogers, Neurochemical changes 273 (1997) R554–R559.
after imbalanced diets suggest a brain circuit mediating anorectic [35] E.S. Nassett, P.T. Ridley, E.A. Schenk, Hypothalamic lesions related responses to amino acid deficiency in rats, J. Nutr. 128 (1998) to ingestion of an imbalanced amino acid diet, Am. J. Physiol. 213
771–781. (1967) 645–650.
[16] D.W. Gietzen, P.M.B. Leung, T.W. Castonguay, W.J. Hartman, Q.R. [36] G. Paxinos, C. Watson, The rat brain in stereotaxic coordinates, Rogers, Time course of food intake and plasma and brain amino Academic Press, Orlando, FL, 1986.
acid concentrations in rats fed amino acid imbalanced or deficient [37] Y. Peng, Y. Gubin, A.E. Harper, M.G. Vavich, A.R. Kemmerer, Food diets, in: M.R. Kare, J. Brand (Eds.), Interaction of the Chemical intake regulation: amino acid toxicity and changes in rat brain and Senses With Nutrition, Academic Press, New York, 1986, pp. plasma amino acids, J. Nutr. 103 (1973) 608–617.
415–456. [38] Y. Peng, A.E. Harper, Amino acid balance and food intake: effect of [17] D.W. Gietzen, P.M.B. Leung, Q.R. Rogers, Dietary amino acid amino acid infusions on plasma amino acids, Am. J. Physiol. 217
imbalance and neurochemical changes in three hypothalamic areas, (1969) 1441–1445.
Physiol. Behav. 46 (1989) 503–511. [39] J.L. Price, B.M. Slotnick, M.-F. Revial, Olfactory projections to the [18] L.B. Haberly, J. Price, Association and commissural fiber systems of hypothalamus, J. Comp. Neurol. 306 (1991) 447–461.
the olfactory cortex of the rat, J. Comp. Neurol. 178 (1978) [40] E. Scharrer, C.A. Baile, J. Mayer, Effect of amino acids and protein 711–740. on food intake of hyperphagic and recovered aphagic rats, Am. J. [19] L.B. Haberly, Olfactory cortex, in: G.M. Shepherd (Ed.), The Physiol. 218 (1970) 400–404.
Synaptic Organization of the Brain, Oxford University Press, New [41] B.G. Stanley, L.H. Ha, L.C. Spears, M.G. Dee II, Lateral hypo-York, 1998, pp. 377–416. thalamic injections of glutamate, kainic acid, D,L -alpha-amino-3-[20] A.E. Harper, Protein and amino acids in the regulation of food hydroxy-5-methyl-isoxazole propionic acid or N-methyl-D-aspartic intake, in: D. Novin, W. Wyrwicka, G. Bray (Eds.), Hunger: Basic acid rapidly elicit intense eating in rats, Brain Res. 613 (1993) Mechanisms and Clinical Implications, Raven Press, New York, 88–95.
1976, pp. 103–113. [42] B.G. Stanley, D. Lanthier, S.F. Leibowitz, Multiple brain sites [21] A.W. Hetherington, S.W. Ranson, The relation of various hypo- sensitive to feeding stimulation by opioid agonists: A cannula-thalamic lesions to adiposity in the rat, J. Comp. Neurol. 76 (1942) mapping study, Pharmacol. Biochem. Behav. 31 (1989) 825–832. 475–499. [43] B.G. Stanley, W. Magdalin, A. Seirafi, W.J. Thomas, S.F. Leibowitz, [22] L.T. Ho, Y.F. Chern, M.T. Lin, The hypothalamic somatostatinergic The perifornical area: The major focus of (a) patchily distributed pathways mediate feeding behavior in the rat, Experientia 45 (1989) hypothalamic neuropeptide Y-sensitive feeding system(s), Brain Res.
161–162. 604 (1993) 304–317.
[23] B.J. Hrupka, Y.M. Lin, D.W. Gietzen, Q.R. Rogers, Small changes in [44] B.G. Stanley, V.L. Willett III, H.W. Donias, M.G. Dee II, M.A. Duva, essential amino acid concentrations alter diet selection in amino Lateral hypothalamic NMDA receptors and glutamate as physiologi-acid-deficient rats, J. Nutr. 127 (1997) 777–784. cal mediators of eating and weight control, Am. J. Physiol. 270 [24] A.M. Khan, M.A. Duva, R. Kaplan, A. Sukhaseum, M.O. Walker, (1996) R443–R449.
B.G. Stanley, Patterns of Fos-like immunoreactivity (FLI) associ- [45] B.G. Stanley, V.L. Willett III, H.W. Donias, L.H. Ha, L.C. Spears, ated with eating elicited by NMDA reverse dialyzed into the lateral The lateral hypothalamus: a primary site mediating excitatory amino hypothalamus (LH), Soc. Neurosci. Abs. 25 (1999) 1883. acid-elicited eating, Brain Res. 630 (1993) 41–49.
[47] J.K. Tews, J. Greenwood, O.E. Pratt, A.E. Harper, Threonine entry Kondoh et al., Recognition of deficient nutrient intake in the brain of into rat brain after diet-induced changes in plasma amino acids, J. rat with L-lysine deficiency monitored by functional magnetic Neurochem. 48 (1987) 1879–1886. resonance imaging, electrophysiologically and behaviorally, Amino [48] J.K. Tews, Y.-W.L. Kim, A.E. Harper, Induction of threonine Acids 10 (1996) 73–81.
imbalance by dispensable amino acids: relation to competition for [52] B.G. Truong, The role of neurotransmitters systems in the anterior amino acid transport into the brain, J. Nutr. 109 (1979) 304–315. piriform cortex in the intake of amino acid imbalanced diets in rats. [49] J.K. Tews, Q.R. Rogers, J.G. Morris, A.E. Harper, Effect of dietary University of California, Davis, Dissertation, 1999.
protein and GABA on food intake, growth, and tissue amino acids in [53] Y. Wang, S.L. Cummings, D.W. Gietzen, Temporal-spatial pattern of cats, Physiol. Behav. 32 (1984) 301–308. c-fos expression in the rat brain in response to indispensable amino [50] G. Tobin, K.N. Boorman, Carotid and jugular amino acid infusions acid deficiency I. The initial recognition phase, Molec. Brain Res.