www.elsevier.com / locate / bres
Research report
The effects of potassium on the rat middle cerebral artery
*
E.M. Golding , M.L. Steenberg, T.D. Johnson, R.M. Bryan Jr
Department of Anesthesiology, Baylor College of Medicine, Houston, TX 77030, USA
Accepted 2 August 2000
Abstract
1 1
After traumatic brain injury, extracellular K in brain can dramatically increase. We studied the effects of increased K on the isolated pressurized rat middle cerebral artery (MCA). MCAs (200–250 mm OD) were isolated, cannulated with glass micropipettes, and
1 1 1
pressurized. K was increased in the extraluminal bath using three paradigms: (1) isotonic K (Kiso) where increases in K were offset
1 1 1 1
by decreases in Na , (2) hypertonic K (Khyper) where K was increased without a concomitant adjustment of Na , and (3) Ksuc, a
1
solution using Kisobut with the addition of sucrose to obtain a hypertonic solution. Increases in K in the extraluminal bath produced
1
significant dilations (approximately 20%) at 21 mM K in all three groups (Kiso, Khyper, and Ksuc). With the Khyperand Ksucgroups, the
1 25
magnitude of the dilation diminished with further increases in K .L-NAME (10 M), an inhibitor of nitric oxide synthase, had no effect
1
on the response of the Khyper and Ksuc groups at 21 mM but significantly enhanced constrictions of the MCAs above 40 mM K
1
compared to the control. The Kisogroup was not affected byL-NAME at any K concentration and showed profound constrictions above
1 1
40 mM K . We conclude that changes in the K concentration and osmolality of the extracellular fluid may have profound effects on the cerebral vasculature. 2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Trauma
1
Keywords: Cerebral arteries; Inward rectifier K channels; Nitric oxide; Osmolality
1
1. Introduction Although the effects of increased K on vascular
smooth muscle have been extensively studied
1
Extracellular K , which is normally 3–5 mM, increases [4,14,15,21,25,30,37,41,43,44], newly acquired informa-to 10–12 mM when neurons are activated [39]. The tion warrants a re-evaluation of this relationship between
1 1
increased K at the vessel wall activates inward rectifier K K and vascular cells (smooth muscle and endothelium). It channels (K s), hyperpolarizes the vascular smooth mus-ir has become apparent over the last 20 years that the cle, and dilates cerebral arteries / arterioles endothelium can release factors which constrict or relax [4,18,21,22,25,28–30,36]. the vascular smooth muscle [12]. The direct and indirect
1
During pathological conditions, such as stroke and effects of increased K on the endothelium were either not
1
traumatic brain injury (TBI), extracellular K can increase known at the time and / or were not taken into considera-to 50–80 mM in brain [1,13,16,19,33,39,40]. The extracel- tion when interpreting the results of the above-mentioned
1
lular K concentration may normalize several minutes experiments. This issue becomes very significant in light after injury or remain elevated in excess of 30 min when of recent reports showing that vascular smooth muscle can the injury involves cortical contusion [40]. At these higher influence endothelium through myo-endothelial junctions
1 21
concentrations, K no longer hyperpolarizes and dilates (gap junctions) [9]. Increases in Ca in the vascular
1
cerebral vessels. Instead, it depolarizes the vascular smooth smooth muscle (which occur with extracellular K con-muscle and constricts [14,44]. centrations above 20–30 mM) can be transmitted to the endothelium via the myoendothelial junctions [9].
In-21
creased Ca in the endothelium may stimulate the release
*Corresponding author. Tel.: 11-713-798-7720; fax: 1
1-713-798-of NO and possibly other relaxing factors [11,12]. Thus,
7644.
E-mail address: [email protected] (E.M. Golding). the release of endothelial factors during conditions of
1
increased K (for instance stroke and TBI) could influence measure of perfusion pressure. A flow meter ([11,
Gil-1
the overall response to K . mont Instruments, Barrington, IL, USA), connected to the The purpose of this investigation was to study the tubing leading to the output reservoir, measured luminal
1
effects of changes in the concentration of extracellular K flow [5]. The luminal perfusate was gassed in the input on the contractile state of the rat middle cerebral artery. reservoir. In addition, the luminal perfusate traveled Specifically we tested two hypotheses: first, the constric- through gas permeable silastic tubing in the bath prior to
1
tion produced by increased extracellular K (30–80 mM perfusing the lumen of each artery to ensure that it was as occurs following TBI) in the rat middle cerebral artery properly gassed and equilibrated to 378C. Samples of PSS (MCA) is attenuated by the stimulated production of NO in from the bath and luminal perfusate were analyzed for endothelium and second, that increases in osmolality that pO , pCO , and pH using a Corning model 280 analyzer2 2
are observed after TBI [20,45], are responsible for the NO (Medfield, MA, USA).
component. The vessels were magnified using an inverted
micro-scope equipped with a video camera and monitor. Outside diameters of the arteries were measured directly from the video screen.
2. Materials and methods
The arteries were allowed at least 1 h to stabilize before conducting any experiments. During this time period the 2.1. Animals and harvesting arteries
diameters decreased to approximately 75% of the initial diameter after pressurization. This development of sponta-The experimental protocol was approved by the Animal
neous tone was indicative of a viable artery. Protocol Review Committee at Baylor College of
Medi-cine. Male Long Evans rats weighing between 275 and 350 g were anesthetized with isoflurane. After loss of the
2.3. General experimental outline and design righting reflex, each rat was decapitated; the brain was
removed from the cranium and placed in cold physiologi- 1
For concentration–response curves, the K concentra-cal saline solution (PSS). The left and right middle
tion of the extraluminal bath was increased by the addition cerebral arteries (MCAs) were carefully removed
begin-of KCl to the extraluminal bath. Three methods were used ning at the circle of Willis and continuing distally for 1 1
for increasing K : (1) isotonic K (Kiso) where increases
approximately 5–6 mm. Left and right posterior cerebral 1 1 1
in K were offset by decreases in Na , (2) hypertonic K arteries and segments of second and third order branches 1
(Khyper) where K was increased without a concomitant of arteries in the mesenteric bed were also harvested. 1
adjustment of Na , and (3) a solution (Ksuc) using Kisobut with the addition of sucrose to obtain a hypertonic 2.2. Mounting the arteries solution. The amount of sucrose added was calculated to produce the same osmotic effect as the addition of adding Arteries were placed in an arteriograph (Living Systems KCl to the extraluminal perfusate without any adjustment Inc., Burlington, VT, USA) where a micropipette was of other ions. The diameter was measured 5 min after inserted into the proximal end of each artery and secured changing the KCl concentrations in the bath.
with a 10-O nylon suture. The lumen was gently perfused One or two concentration–response curves were con-with PSS to remove blood and other contents and the distal ducted per MCA. Between each concentration–response end was cannulated with a second glass micropipette and curve, the bath was washed with fresh PSS and the MCA secured. A segment of each artery lying between branch was allowed 30 min to recover before the next con-points was positioned between the tips of the two mi- centration–response curve was performed. In studies cropipettes. Each artery was bathed in PSS which was where two concentration–response curves were conducted, continually circulated from a reservoir where it was the MCAs were divided into control and experimental equilibrated with a gas consisting of 20% O and 5% CO2 2 groups. In control vessels, two control concentration– with a balance of N . After gassing, the pCO and pO2 2 2 response curves were obtained; in the experimental vessel were approximately 7.37 and 140 mmHg, respectively. one control curve was obtained followed by the ex-The PSS in the bath was maintained at 378C using a perimental concentration–response curve (L-NAME, etc.).
1
was confirmed by the absence of a dilation to the addition 3. Results
25
of 10 mol / L UK14,304, an a2 agonist [5].
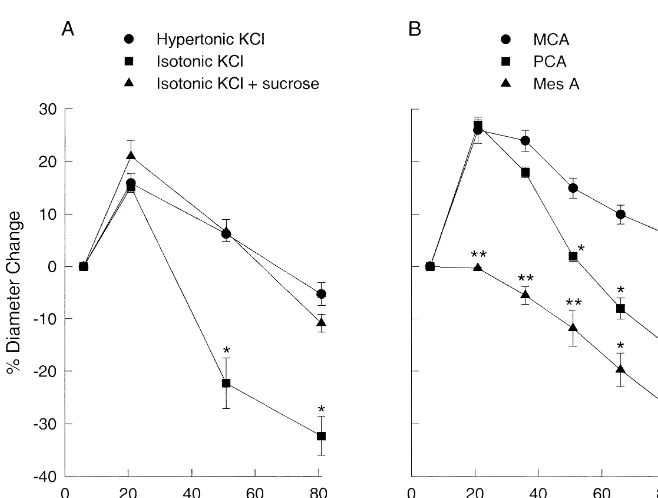
Fig. 1 shows the response of the MCAs to increases in
1
extracellular K . The data are expressed as a percent 2.4. Reagents and drugs change from the resting diameter; positive numbers signify dilations and negative numbers signify constrictions. The
G 1
Sucrose, ouabain, serotonin (5-HT), andL-NAME [N - resting outside diameters (K 5.88 mM) for Khyper, Kiso,
nitro-L-arginine methyl ester] were obtained from Sigma and Ksuc were 25066 mm (n510), 250611 mm (n56),
1
(St Louis, MO, USA).L-NAME was dissolved in PSS and and 23868mm (n57), respectively. At K concentrations
added to both the luminal and extraluminal baths. The PSS of 21 mM, the MCA diameters increased 15–20%
regard-1
consisted of the following [34]: NaCl 119 mM, NaHCO3 less of the method of K addition. At greater concen-24 mM, KCl 4.7 mM, KH PO 1.18 mM, MgSO 1.172 4 4 trations the diameters for the Khyper and Kiso groups mM, CaCl 1.6 mM, glucose 5.5 mM, and EDTA 0.0262 constricted from the maximum dilation at 21 mM. MCAs
mM. in the Kiso group were significantly more constricted at 51
and 81 mM compared to Khyper and Ksuc (Fig. 1A). The response of the MCAs to Khyper at 51 and 81 mM 2.5. Statistics prompted us to compare the Khyper response of the MCA to that of another cerebral artery, the posterior cerebral artery Data are expressed as the mean6S.E.M. For comparison (PCA), and a peripheral artery from the mesenteric bed
1
of responses to KCl, the repeated measures analysis of (Fig. 1B). Khyper of 21 mM K in the extraluminal bath variance was initially attempted. However, in many of the increased the diameter of the MCAs and PCAs by 2662% comparisons the equal variance or normality tests failed (n511) and 2761% (n54), respectively. With subsequent
1
indicating that parametric statistical designs were not increases in the K concentration, the diameter of the appropriate. In these situations, we used the Kruskal– MCAs and PCAs decreased toward baseline; however, the
1
Wallis non-parametric test with a Bonferroni correction for decrease in diameter with increasing K concentrations multiple comparisons (Minitab, State College, PA, USA). was sharper with the PCAs than with the MCAs. At 66 and
1
The acceptable level of significance was defined as P, 81 mM K , the PCAs were constricted significantly from
0.05. the resting diameter. The diameter change for the PCAs
was significantly different from the MCAs at 51, 66, and L-NAME could have been due to the increased tone (more
1
81 mM K . In this study the MCAs did not return to constricted MCA) and not to the inhibition of NO.
1
baseline; even at 81 mM K the MCAs were significantly Therefore, to test for this possibility we constricted the
27 26
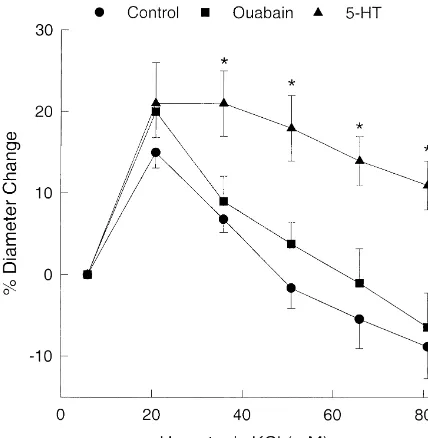
dilated (662%, P,0.05 using Bonferroni correction). MCAs with either serotonin (between 10 and 10 M)
26
However, in some studies mean diameter was near or or 10 M ouabain. Serotonin and ouabain constricted the slightly below the resting baseline at the highest Khyper MCAs by 17% (n59) and 12% (n55), respectively. This concentration (see Fig. 1A). is compared to a 11% constriction produced byL-NAME.
1
Unlike the MCAs and PCAs, the pressurized perfused The response to 21 mM K after constriction with either
1
mesenteric arteries did not dilate to increased K , due serotonin or ouabain was similar to the response in control most likely to the absence of K s on the vascular smoothir MCAs (Fig. 3) or after treatment withL-NAME (Fig. 2A).
1 1
muscle. Additions of K produced significant constrictions At K concentrations greater than 21 mM, the response in beginning at 36 mM Khyper and the arteries continued to ouabain-treated MCAs was similar to the control response. contract with increasing concentrations of Khyper (Fig. 1B). However, the 5-HT-treated group showed more persistent
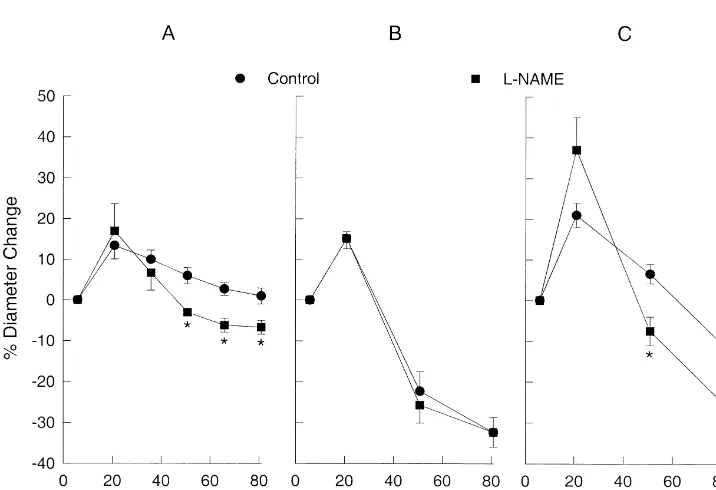
25 1
Fig. 2 shows the effects of L-NAME (10 M), an dilations at increased K concentrations, not less, than the
1
inhibitor of nitric oxide synthase, on the response to Khyper, control group. Clearly, the more constricted MCAs at K Kiso, and Ksuc.L-NAME did not affect the percent change concentrations above 21 mM in the L-NAME treated
in diameter to Khyper 21 and 36 mM; however, it did vessels was not due to the more constricted state of the
1
produce a significant reduction in the diameter compared vessel prior to the addition of K .
1
to the control response at K concentrations of 51, 66, and Fig. 4 describes studies designed to determine if the
25
81 mM (Fig. 2A). Addition of 10 M L-NAME, sig- endothelium was the source of the NO involved with the
nificantly reduced the diameter by 11% (n59, P50.01, see Khyper response described in Fig. 2A. The endothelium was legend Fig. 2A). L-NAME had no effect on the Kiso removed by passing air through the lumen [5] and was
1
concentration–response curve at any K concentration confirmed in each vessel with UK14,304, an a2 agonist (Fig. 2B). However,L-NAME significantly constricted the which dilates MCAs through a mechanism requiring intact
MCAs compared to the control response at Ksuc con- endothelium [5,6]. Before removal of the endothelium
25
centrations of 51 and 81 mM (Fig. 2C) in a manner similar 10 M UK14,304 elicited a 12% dilation; after removal to that ofL-NAME on the Khyper concentration response. with air the dilation was reduced to 1% (P,0.001). After
The altered response to Khyper after the addition of removal of the endothelium the concentration–response
25
Fig. 2. (A) The effects of 10 ML-NAME, a nitric oxide synthase inhibitor, on the hypertonic KCl (Khyper) concentration–response curve in rat middle cerebral arteries. The resting diameters before the addition of Khyperin the control andL-NAME groups were 21863mm (n59) and 19367mm (n59),
25
respectively. *P,0.05 compared to Control response. (B) The effect of 10 ML-NAME on the isotonic KCl (Kiso) concentration–response curve. Resting diameters before the addition of Kisoin the Control andL-NAME groups were 250611mm (n56) and 213611mm (n56), respectively. (C) The effect of
25
of the endothelium appeared similar (L-NAME group in
2
Fig. 2A and Endo in Fig. 4A), we conducted further experiments to determine if the endothelium accounted for the entire NO involved in the response. Concentration–
1
response curves for K were conducted in MCAs after
2
removal of the endothelium in the absence (Endo , Fig.
2
4B) and presence of L-NAME (Endo 1L-NAME, Fig.
4B). Although the addition of L-NAME appeared to
constrict the vessels slightly more at 51, 66, and 81 mM
1
K , the differences were not statistically significant from
2
the Endo Control group. NO participating in the response to Khyper was derived predominantly, if not exclusively, from the endothelium.
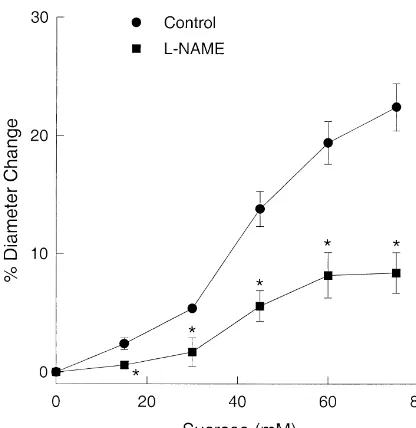
Fig. 5 shows that MCAs dilated when the osmolality was increased by adding sucrose to the extraluminal bath. The addition of L-NAME significantly attenuated the
dilation indicating that NO is involved with the dilation due to increased osmolality.
Fig. 3. The effects of constricting MCAs with either serotonin (5-HT, 27 26 26
between 10 and 10 M) or ouabain (10 M) on the hypertonic KCl 4. Discussion
(Khyper) concentration–response curve. 5-HT (n59) and ouabain (n55) constricted the MCAs by 17% (P50.01) and 11% (P50.04), respectively.
A number of pathological conditions including TBI,
*P,0.05 compared to the corresponding response in the control group.
hypoxia-ischemia, hypoglycemia, seizure, and spreading
1 1
curve for K (Fig. 3A) was similar to the curve generated depression may increase the concentration of K in in the presence of L-NAME; i.e. endothelium removal extracellular fluid of the central nervous system to levels
produced a more constricted state at 51, 66, and 81 mM between 50 and 80 mM [1,13,16,19,33,39,40,46]. This
1
K compared to the control while not significantly affect- increase represents a value which is over five-fold greater
1
ing the response to 21 and 36 mM K . than the concentration seen during normal physiological Although the Khyper concentration–response curves with activation. Since the glial endfeet project from the
paren-1
L-NAME and concentration–response curves after removal chyma to the pial surface and glia can ‘siphon’ K from
2 1
Fig. 4. (A) The effects of removing the endothelium (Endo ) on the K concentration–response curve (n56). The endothelium was removed by flowing G
air through the lumen of the middle cerebral arteries (MCAs). *P,0.05 compared to Control. (B) The effects of N -nitro-L-arginine methyl ester
25 1
(Fig. 1) [10,18,22,29,36]. The mesenteric artery (Mes A), as with many peripheral vessels, do not have K s and thusir
1
do not dilate to lower concentrations of K (Fig. 1B).
1
A major difference in the response to K in the concentration range from 40 to 80 mM in the MCA was
1
whether the osmolality changed when K was increased (Khyper vs. Kiso). Fig. 1A demonstrates this difference by showing that the MCAs were relatively more dilated with
1
Khyper than with Kiso at the higher K concentrations. In order to confirm that osmolality was the factor that produced this difference, we used the Ksuc paradigm (Kiso
with sucrose added to increase the osmolality). The
1
response to increased K was identical when the osmolali-ty was increased, Khyper or Ksuc, regardless of whether
1
Na was decreased (Fig. 1A). Therefore, the difference between the response to Khyper and Kiso was due to the increase in osmolality in the bathing solution.
Part of the mechanism for maintaining the more dilated
1
state during K and K at K concentrations above 40
25 hyper suc
Fig. 5. The effect of 10 ML-NAME on the concentration–response
mM involved NO (Fig. 2). Inhibition of NO synthesis with
curve to sucrose. Resting diameters before the addition of sucrose in the
L-NAME produced a more constricted state (expressed as a Control andL-NAME groups were 21565mm (n518) and 18066mm
(n512), respectively. *P,0.05 compared to the control response. ratio to the resting diameter) at higher concentrations of
1
K when compared to the corresponding control responses
1
one location to another, increases of K in the extracellu- for Khyper and Ksuc (Fig. 2). L-NAME did not have any
1
lar fluid of the brain parenchyma after any of these effect when K was increased without a concomitant pathological conditions can be transmitted to the pial increase in the osmolality of the bath solution (Kiso). Thus, arteries and arterioles on the surface of the brain the increase in osmolality of the bathing solution during [23,32,35,42]. Khyper and Ksuc was associated with the involvement of In this study, we tested the hypothesis that constrictions NO. Studies where the osmolality of the bathing solution
1 1
produced by increased extracellular K in the rat middle was increased without a change in K support a role for cerebral artery are attenuated by the stimulated production NO. Addition of sucrose to the bathing solution dilated of NO in endothelium. The hypothesis follows from the MCAs through a mechanism which involved NO (Fig. 5).
21
idea that increases in cytoplasmic Ca in vascular smooth Although there were two potential sources for the NO,
21
muscle can increase Ca in endothelium through myo- endothelium or perivascular nerves, it appeared that NO
21
endothelial junctions (gap junctions) [9]. Increased Ca in was derived primarily, if not exclusively, from the
endo-1
the endothelium would stimulate the release of NO [11,12] thelium (Fig. 4). We conclude that an increase in K alone and attenuate contraction of the MCA. Myoendothelial did not stimulate the synthesis and release of NO from the junctions are commonly found in both peripheral and endothelium. Rather, it was the increase in osmolality cerebral arteries [2,3,8,26,27,38]. during Khyper and Ksuc that was responsible for the NO
1
Although NO appeared to attenuate the constrictor component of the response at K concentrations greater
1
response with Khyper and Ksuc (.40 mM K ), it was not than 40 mM.
1
involved with the response to Kiso (Fig. 2). If our original Following TBI, increases in both extracellular K hypothesis was valid, then it would be expected that NO [17,19,31,33,39,40,46] and osmolality [20,45] have been would be involved with all three methods for increasing reported. We show in this study that cerebral arterial tone is
1 21 1 1
K (Khyper, Kiso, and Ksuc). If Ca (or a messenger that dependent on the K concentration and the K response is
21
would increase endothelial Ca ) was moving through the critically dependent on the osmolality of the bathing
1
gap junctions from smooth muscle to endothelium and solution. An increase in extracellular K concomitant with stimulating nitric oxide synthase, then it would be ex- an increase in the osmolality of the ECF following TBI pected to also occur with Kiso. Since this apparently did could be protective by maintaining a more dilated state of not occur, we must conclude that our first hypothesis is not the vessel provided the endothelium has not been dam-valid. The participation of NO with the Khyper and Kiso aged. Further studies are required to ascertain whether such
1
response likely involves hypertonicity of the bathing responses to extracellular K are effected in the MCA
1
solution rather than being directly related to the increase in isolated following TBI. While K appears to be one of
1
extracellular K (see below). The initial dilation to in- several possible agents for coupling increases in cerebral
1
1 glutamate following concussive brain injury, J. Neurosurg. 73
association between K and maintenance of tissue viability
(1990) 889–900.
following TBI.
[20] Y. Katayama, T. Mori, T. Maeda, T. Kawamata, Pathogenesis of the mass effect of cerebral contusions: rapid increase in osmolality within the contusion necrosis, Acta Neurochir. (Wien) 71 (Suppl.) (1998) 289–292.
1 11
Acknowledgements [21] U. Knabe, E. Betz, The effect of varying extracellular K , Mg , 11
Ca on the diameter of pial arterioles. in: E. Betz (Ed.), Vascular Smooth Muscle, Springer–Verlag, New York, 1972, pp. 83–85.
This study was supported by PHS grant PO1 NS 27616. 1
[22] H.J. Knot, P.A. Zimmermann, M.T. Nelson, Extracellular K -in-duced hyperpolarizations and dilatations of rat coronary and cerebral
1
arteries involve inward rectifier K channels, J. Physiol. 492 (1996) 419–430.
References [23] S.W. Kuffer, J.G. Nicholls, A.R. Martin, From Neuron to Brain,
Sinauer Assoc, Sunderland, MA, 1984.
[24] W. Kuschinsky, M. Wahl, Local chemical and neurogenic regulation [1] J. Astrup, S. Rehncrona, B.K. Siesjo, The increase in extracellular
of cerebral vascular resistance, Physiol. Rev. 58 (1978) 656–689. potassium concentration in the ischemic brain in relation to the
[25] W. Kuschinsky, M. Wahl, O. Bosse, K. Thurau, Perivascular preischemic functional activity and cerebral metabolic rate, Brain
potassium and pH as determinants of local pial arterial diameter in Res. 199 (1980) 161–174.
cats, Circ. Res. 31 (1972) 240–247. [2] F. Aydin, W.I. Rosenblum, J.T. Povlishock, Myoendothelial
junc-[26] T.L. Little, E.C. Beyer, B.R. Duling, Connexin 43 and connexin 40 tions in human brain arterioles, Stroke 22 (1991) 1592–1597.
gap junctional proteins are present in arteriolar smooth muscle and [3] J.L. Beny, Information networks in the arterial wall, News Physiol.
endothelium in vivo, Am. J. Physiol. 268 (1995) H729–H739. Sci. 14 (1999) 68–73. [27] T.L. Little, J. Xia, B.R. Duling, Dye tracers define differential [4] E. Betz, Ionic interactions in pial vascular smooth muscles, in: E. endothelial and smooth muscle coupling patterns within the
arterio-Betz (Ed.), Ionic Actions on Vascular Smooth Muscle, Springer– lar wall, Circ. Res. 76 (1995) 498–504.
Verlag, New York, 1976, pp. 75–77. [28] S.P. Marrelli, T.D. Johnson, A. Khorovets, W.F. Childres, R.M. [5] R.M. Bryan Jr, M.Y. Eichler, M.W.G. Swafford, T.D. Johnson, M.S. Bryan Jr., Altered function of inward rectifier potassium channels in Suresh, W.F. Childres, Stimulation of a2 adrenoceptors dilates the cerebrovascular smooth muscle after ischemia / reperfusion, Stroke rat middle cerebral artery, Anesthesiology 85 (1996) 82–90. 29 (1998) 1469–1474.
[6] R.M. Bryan Jr, M.L. Steenberg, M.Y. Eichler, T.D. Johnson, M.W.G. [29] J.G. McCarron, W. Halpern, Potassium dilates rat cerebral arteries by Swafford, M.S. Suresh, Permissive role of NO ina2-adrenoceptor- two independent mechanisms, Am. J. Physiol. 259 (1990) H902– mediated dilations in rat cerebral arteries, Am. J. Physiol. 269 H908.
(1995) H1171–H1174. [30] J. McCulloch, L. Edvinsson, P. Watt, Comparison of the effects of [7] D.W. Busija, D.D. Heistad, Factors involved in the physiological potassium and pH on the calibre of cerebral veins and arteries,
regulation of the cerebral circulation, Rev. Physiol. Biochem. Pflugers Arch. 393 (1982) 95–98.¨
Pharmacol. 101 (1984) 161–211. [31] T.K. McIntosh, Neurochemical sequelae of traumatic brain injury: [8] E. Dahl, The fine structure of intracerebral vessels, Z. Zellforsch. therapeutic implications, Cerebrovasc. Brain Metab. Rev. 6 (1994)
Mikrosk. Anat. 145 (1973) 577–586. 109–162.
[9] K.A. Dora, M.P. Doyle, B.R. Duling, Elevation of intracellular [32] E.A. Newman, High potassium conductance in astrocyte endfeet, calcium in smooth muscle causes endothelial cell generation of NO Science 233 (1986) 453–454.
in arterioles, Proc. Natl. Acad. Sci. USA 94 (1997) 6529–6534. [33] P. Nilsson, L. Hillered, Y. Olsson, M.J. Sheardown, A.J. Hansen, 1 21
[10] F.R. Edwards, G.D.S. Hirst, G.D. Silverberg, Inward rectification in Regional changes in interstitial K and Ca levels following rat cerebral arterioles: involvement of potassium ions in autoregula- cortical compression contusion trauma in rats, J. Cerebr. Blood Flow tion, J. Physiol. 404 (1988) 455–466. Metab. 13 (1993) 183–192.
[11] F.M. Faraci, Endothelium-derived vasoactive factors and regulation [34] G. Osol, I. Laher, M.J. Cipolla, Protein kinase C modulates basal of the cerebral circulation, Neurosurgery 33 (1993) 648–658. tone in resistance arteries from the cerebral circulation, Circ. Res. 68 [12] F.M. Faraci, Regulation of the cerebral circulation by endothelium, (1991) 359–367.
Pharmacol. Ther. 56 (1992) 1–22. [35] O.B. Paulson, E.A. Newman, Does the release of potassium from [13] A.J. Hansen, The extracellular potassium concentration in brain astrocyte endfeet regulate cerebral blood flow?, Science 237 (1987)
cortex following ischemia in hypo- and hyperglycemic rats, Acta 896–898.
Physiol. Scand. 102 (1978) 324–329. [36] J.M. Quayle, J.G. McCarron, J.E. Brayden, M.T. Nelson, Inward 1
[14] E.D. Hogestatt, K.E. Andersson, Mechanisms behind the biphasic rectifier K currents in smooth muscle cells from rat resistance-contractile response to potassium depolarization in isolated rat sized cerebral arteries, Am. J. Physiol. 265 (1993) C1363–C1370. cerebral arteries, J. Pharmacol. Exp. Ther. 228 (1984) 187–195. [37] T. Ryman, L. Brandt, K.E. Andersson, P. Mellergard, Regional and [15] E.D. Hogestatt, K.E. Andersson, L. Edvinsson, Mechanical prop- species differences in vascular reactivity to extracellular potassium,
erties of rat cerebral arteries as studied by a sensitive device for Acta Physiol. Scand. 136 (1989) 151–159.
recording of mechanical activity in isolated small blood vessels, [38] L.G. Spagnoli, S. Villaschi, L. Neri, G. Palmieri, Gap junctions in Acta Physiol. Scand. 117 (1983) 49–61. myo-endothelial bridges of rabbit carotid arteries, Experientia 38
¨
[16] K.A. Hossman, Total ischemia in brain, in: R.J. Zulch (Ed.), Brain (1982) 124–125.
1
and Heart Infarct, Springer–Verlag, Berlin, 1977, pp. 107–122. [39] E. Sykova, Extracellular K accumulation in the central nervous [17] D.A. Hovda, D.P. Becker, Y. Katayama, Secondary injury and system, Prog. Biophys. Molec. Biol. 42 (1983) 135–189.
acidosis, J. Neurotrauma 9 (1990) S47–S60. [40] H. Takahashi, S. Manaka, K. Sano, Changes in extracellular [18] T.D. Johnson, S.P. Marrelli, M.L. Steenberg, W.F. Childres, R.M. potassium concentration in cortex and brain stem during the acute Bryan Jr, Inward rectifier potassium channels in the rat middle phase of experimental closed head injury, J. Neurosurg. 55 (1981) cerebral artery, Am. J. Physiol. 274 (1998) R541–R547. 708–717.
[42] S.S. Varon, G.G. Somjen, Neuron–glia interactions, Neurosci. Res. [45] H. Yonas, J.V. Snyder, D. Gur, W.F. Good, R.E. Latchaw, S.K. Program Bull. 17 (1979) 1–239. Wolfson Jr, A. Grenvik, B.C. Good, Local cerebral blood flow [43] P.E. Vinall, F.A. Simeone, Evidence that intraluminal pressure alterations (Xe-CT method) in an accident victim, J. Comp. Ass.
affects high potassium- and serotonin-induced contractions different- Tomography 8 (1984) 990–991.
ly in the bovine middle cerebral artery: an in vitro study, Stroke 18 [46] W. Young, I. Koreh, Potassium and calcium changes in injured
(1987) 92–100. spinal cords, Brain Res. 365 (1986) 42–53.