Nitrogen nutrition and water stress effects on leaf
photosynthetic gas exchange and water use efficiency in
winter wheat
Z.P. Shangguan
a,*, M.A. Shao
a, J. Dyckmans
baState Key Laboratory of Soil Erosion and Dryland Farming on the Loess Plateau,Institute of Soil and Water Conser 6ation, Chinese Academy of Sciences,Northwest Sci-Tech Uni6ersity of Agriculture and Forestry,Yangling,Shannxi712100,P.R.China
bInstitute of Soil Science and Forest Nutrition,Uni
6ersity of Go¨ttingen,Bu¨sgenweg2,37077Go¨ttingen,Germany Received 14 December 1999; received in revised form 21 June 2000; accepted 21 June 2000
Abstract
The responses of gas exchange and water use efficiency to nitrogen nutrition for winter wheat were investigated under well-watered and drought conditions. The photosynthetic gas exchange parameters of winter wheat are remarkably improved by water and nitrogen nutrition and the regulative capability of nitrogen nutrition is influenced by water status. The effects of nitrogen nutrition on photosynthetic characteristics and on the limited factors to photosynthesis are not identical under different water status. Intrinsic water use efficiency (WUEi) of the plants at the
high-N nutrition was decreased by a larger value than that of the plants in the low-N treatment due to a larger decrease in photosynthetic rate than in transpiration rate. Carbon isotope composition of plant material (dp) is
increased by the increase of drought intensity. Thedpat a given level ofCi/Cais reduced by nitrogen deficiency. Leaf
carbon isotope discrimination (D) is increased by the increase of nitrogen nutrition and decreased by the increase of drought intensity. Transpirational water use efficiency (WUEt) is negatively correlated withDin both nitrogen supply
treatments and increased with the nitrogen supply. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Carbon isotope discrimination; Drought; Nitrogen supply; Photosynthesis;Triticum aesti6um; Wheat
www.elsevier.com/locate/envexpbot
1. Introduction
Drought stress and nitrogen deficiency are ma-jor constraints to winter wheat production and yield stability in most rainfed regions in China.
Similar problems are encountered worldwide (Fischer and Turner, 1978; Lawlor, 1995). An efficient use of limited water resources and better growth under both limited water and nitrogen supply are desirable traits for crops in drought environments.
Physiological responses of plants to either drought or nitrogen deficiency have been well documented. However the interactions between these two factors on plant morphological and
* Corresponding author. Tel.:+86-29-7019107; fax: + 86-29-7012210.
E-mail addresses: [email protected] (Z.P. Shangguan), [email protected] (J. Dyckmans).
physiological responses have received relatively little attention (Mcdonald and Davies, 1996). In beans (Shimshi, 1970), coffee (Tesha and Kumar, 1978), and winter wheat (Shangguan, 1997), the stomatal conductance (gs) increased with nitrogen
nutrition under well-watered conditions and be-came more sensitive to leaf water potential. It decreased as soil water became less available. Other works on tea (Nagarajah, 1981) and cotton (Radin and Ackerson, 1989) have indicated an opposite response, i.e. the stomatal sensitivity to leaf water potential was decreased by high nitro-gen nutrition. A strong correlation was found between leaf conductance and leaf nitrogen con-tent; however, this relationship was weaker than that between stomatal conductance and water po-tential (Radin and Parker, 1979; Bolton and Brown, 1980; Morgan, 1986; Shangguan and Chen, 1990; Ciompi et al., 1996). Sugiharto et al. (1990) found a significiant positive correlation between the photosynthetic capacity of leaves and their leaf nitrogen concentration suggesting that most of the nitrogen used for synthesis of compo-nents of the photosynthetic apparatus. In particu-lar, Rubisco, the leaf protein playing the major role in carbon assimilation, was strongly affected by nitrogen deficiency (Seemann et al., 1987). Although CO2 and NO3
− assimilation are linked,
it is not completely clear to what extent they are coupled (Lawlor, 1995). Therefore, further eluci-dation of the relation of leaf nitrogen content to the gas exchange and water use is needed.
Water use efficiency indicates the performance of a crop growing under any environmental con-straint. At the leaf level, intrinsic water use effi-ciency (WUEi) is defined as the ratio of
photosynthetic rate (Pn) to transpiration rate. To
achieve the same Pn at a lower gs, a higher
Rubisco activity and capacity for electron trans-port is required and thus a higher concentration of nitrogen in the leaf does not necessarily mean a proportional increase in the rate of photosynthesis (Shangguan et al., 1999). In crop plants with C3
photosynthetic pathway, carbon isotope discrimi-nation (D) has been used to provide time-inte-grated estimates of plant intrinsic water use efficiency (Farquhar and Richards, 1984; Far-quhar et al., 1989). Foliar D values of C3 plants
have also been used as an integrated measure of the response of photosynthetic gas exchange to environmental variables such as humidity (Winter et al., 1982), salinity (Guy et al., 1986), light intensity (Zimmerman and Ehleringer, 1990), soil water availability (Meinzer et al., 1992), and ele-vated CO2 (Picon et al., 1996). A high but
nega-tive correlation was found between carbon isotope discrimination (D) and WUEt (Ehdaie and Hall,
1991; Ismail and Hall, 1992) and D with WUEi
(Wright et al., 1988, 1994). Meinzer et al. (1990) reported simultaneous reductions in stomatal con-ductance and D with increased WUE for water stressed cowpea. Rao and Wright (1994) showed significant effects of location and water regime on
D for cowpea. Ehleringer et al. (1991) reported a high correlation between D and Ci/Ca. To our
knowledge, there is little information on the ef-fects of the two major environmental constraints on photosynthetic gas exchange and D in winter wheat.
Since different stress histories could signifi-cantly have effects on a number of physiological mechanisms in wheat (Morgan, 1984), the present study was designed to eliminate the uncertain effect of nitrogen nutrition stress with growing plants in solution culture and imposing water stress with polyethylene glycol (PEG 6000). In this study, it was hypothesized that: (1) there would be interaction between drought and nitrogen nutri-tion on photosynthetic gas exchange and water use efficiency, and the effects of nitrogen nutrition on plants depend on solution water status; (2) WUEt and WUEi would be increased by the
nitrogen nutrition supply. For verifying the hy-potheses winter wheat was grown under different combinations of nitrogen nutrition and water sup-ply levels and the study was focused on the effects of nitrogen deficiency and water stress on leaf water status, photosynthesis, nitrogen content, and water use.
2. Materials and methods
2.1. Plant material and growth conditions
Xiaoyuan 6) were initially germinated in dark-ness at a constant temperature of 25°C on moist filter paper in dishes for 5 days. Upon emer-gence, the uniform seedlings were transferred to plastic pots, 20 cm in height and 10 cm in di-ameter, containing a mixture of vermiculite and perlite (1:1, v/v) (four seedlings per pot). All plants were irrigated weekly with a nutrient so-lution and placed in a growth chamber. The nu-trient solutions were modified half-strength Hoagland solutions containing either 15 mM NO3− (high-N) or 1.5 mM NO3− (low-N). All
other ions in the solutions were constant, except for SO4
2−
and Cl−
, which were used in equal portions to maintain charge balance. Between nutrient additions, deionized water was applied as needed. Pots were covered with plastic beads to reduce evaporation from the soil surface. The conditions in the growth chamber were: a photo-synthetic photon flux density of 700 mmol m−2
s−1 over plants; day/night temperature: 25/189
2°C; midday relative humidity: 6095%; and a 12-h light period.
The first measurement was performed 45 days after planting. In the following measurements, PEG (6000) was added to the solutions in both nitrogen treatments and the seedlings had been kept in the solutions for 9 days. Drought stress was achieved by adding different amount of PEG, the osmotic potential of the solutions were −0.24 MPa (well watered) and −1.25 MPa (droughted), respectively.
2.2. Leaf water potential and gas exchange mea
-surements
Leaf water potential (8w) was measured with a
pressure chamber (LI−COR 3005, Inc., Lincoln, NE) on the first fully expanded leaves. A port-able infrared CO2 analyzer (LCA−3, ADC,
Hoddesdon, UK) was used to measure Pn, gs, E
and intercellular CO2 concentration (Ci) during
the 9 days. The leaf was then removed for carbon isotope analysis. The youngest fully expanded leaf of three plants of each treatment were enclosed into the gas exchange chamber between 10:00 and 11:30 h for gas exchange measurements. Before
the gas exchange measurements, the print of the leaf was taken for leaf area measurement by an area meter. Plant WUEi was determined as the
ratio of Pnto E.
2.3. Carbon isotope composition analysis
In order to calculate WUEt, all leaves of the
plants were used for d13
C and nitrogen determi-nations within each water and nitrogen treatment. The leaves were oven-dried at 70°C for at least 48 h and then finely ground. Relative abundancies of
13C and12C were analyzed by mass spectrometry
(Delta S, Finnigan Mat). Carbon isotope compo-sition (d) was expressed as the13C/12C ratio
rela-tive to that of the Pee Dee Belemnite standard (PDB). The resulting d13C values were used to
calculate D (Farquhar and Richards, 1984):
D (‰)= da−dp
1000+dp
×1000 (1)
wheredaanddprefer to the isotopic compositions
of air (−8.0‰) and the plant material, respectively.
D is related to the time-integrated value of the ratio of theCito ambient CO2concentration (Ca)
and thus to plant WUEi (Farquhar et al., 1989):
D=a+(b−a)Ci
where a and b are the discrimination coefficients against 13
CO2 during diffusion into the leaf
and carboxylation. The values of a and b are estimated to be 4.4 and 27.0‰, respectively (Farquhar et al., 1989). 6 (mmol mol−1) is the mean value of leaf-to-air water vapor pressure difference during the growing period.
Plant transpirational water use efficiency (WUEt) is related to D by (Farquhar and
to plant mass, and F is the proportion of net daytime fixation of carbon lost by mitochondrial respiration, fine root mortality or exudates by the whole plant.
2.4. Nitrogen measurements
For determining leaf nitrogen concentration, 200 mg of powdered material was analyzed with a modified Kjeldahl analysis using concentrated sul-phuric and salicylic acid and Na2SO4, K2SO4and
Se as a catalyst in a ratio of 62:1:1 (w/w). The N-concentration of the digests was determined on a continuous flow analyzer (Skalar, Breda, Netherlands).
2.5. Statistical analysis
S.E., variance, regression and correlation coeffi-cients, and significant differences among regres-sion coefficients were calculated by standard methods with the DAPS statistical package (Feng and Tang, 1997).
3. Results and discussion
3.1. Water relations and nitrogen concentration of lea6es
Leaf water potential decreased significantly with water stress development in all treatments. On the 9th day of drought stress, 8w was 0.25
MPa lower in the drought and high-N supply
than that in the drought and low-N supply treat-ment (Table 1). At the same soil water conditions, drought that plants experienced was more severe under high-N supply than under low-N supply. These results are in agreement with Morgan (1984, 1986) who reported that 8wof N-deficient
leaves were less affected by decrease in leaf water content compared to leaves grown under a high nitrogen regime. Some of the xeromorphic charac-teristics are modified, such as cell wall thickness, volumetric modulus of elasticity and cell volume, which have been changed by plants growing in nitrogen deficiency media. These structural changes of the plant tissue could have profound effects on plant internal water relations. There-fore, the different 8w values for each treatment
support the hypothesis that the effect of water stress is related to the level of nitrogen supply. Under well-watered conditions, leaf nitrogen con-tent at high-N supply was significantly (PB0.01) higher than that at low-N supply while the de-creased water availability led to a lower leaf nitro-gen content (Table 1). Leaf nitrogen concentration on the 9th day of the drought pe-riod was reduced by 40.3% for the low-N supply and by 25.4% for the high-N supply treatments, respectively. These results are in agreement with Morgan (1984) who reported that drought in spring wheat reduced leaf nitrogen content. How-ever, opposite result have been reported in two wheat cultivars (Van den Boogaard et al., 1995), i.e. that drought stress led to higher leaf nitrogen content. The mechanism that leads to these con-tradictory results are not clear and further study is needed.
Table 1
Water potential (8w) and nitrogen concentration of winter wheat leavesa
ANOVA Well-watered Droughted
Treatment
N High-N Low-N
High-N Drought Interaction
Low-N
−0.27c −0.32c −1.42b
8w(MPa) −1.67a * ** *
6.25a
4.74b *
Leaf nitrogen concentration (10−2g g−1dry 2.83c 4.66b ** **
wt)
aEach value is the mean of three measurements taken on the 9th day after imposition of drought stress. Results of Fisher’s test
are shown where means followed by the same letter are not significantly different at the 5% level. *PB0.05;
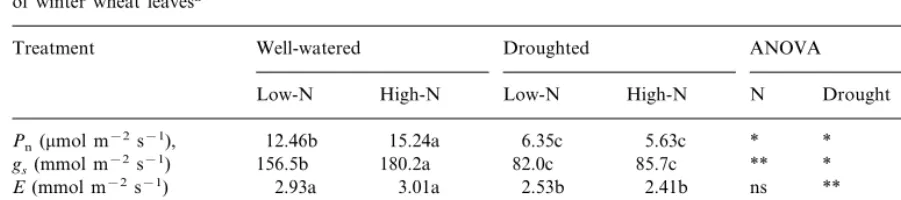
Table 2
Rate of photosynthesis (Pn), leaf conductance for water vapour (gs), transpiration rate (E) and intrinsic water-use efficiency (WUEi)
of winter wheat leavesa
Droughted ANOVA
Treatment Well-watered
High-N Low-N
Low-N High-N N Drought Interaction
Pn(mmol m−2s−1), 12.46b 15.24a 6.35c 5.63c * * **
180.2a 82.0c 85.7c
156.5b **
gs(mmol m−2s−1) * **
3.01a 2.53b 2.41b
E(mmol m−2s−1) 2.93a ns ** ns
209.7c 221.1b 239.8a
217.6b **
Ci(mmol mol−1) * **
5.06a 2.51c 2.34c
WUEI(mmol mol−1) 4.25b * * **
aMeasurements were carried out at ambient CO
2and O2concentrations (345mmol mol−1and 21%, respectively) and at 700mmol
m−2 s−1photosynthetic photon flux density on the 9th day after imposition of drought stress. Each value is the mean of three
replications. Results of Fisher’s test are shown where means followed by the same letter are not significantly different at the 5% level. *PB0.01;
**PB0.05; two-way ANOVA where the effects of nitrogen, water and nitrogen/water interaction are significantly different.
3.2. Leaf photosynthetic gas exchange
Under well-watered conditions,Pn andgs were
increased in the high-N treatment as compared to the low-N treatment. WUEi of the plants was
significantly higher in the high-N treatment than that in the low-N treatment (Table 2). Under drought conditions,Pnof the plants in the high-N
treatment was significantly inhibited while no sig-nificant nitrogen effect was noticed forgs. At low
N supply WUEi was higher than that at high N
supply. The observation thatgsof plants at
high-N supply appeared to be greater than that of plants at low N supply under well-watered condi-tions is in agreement with previous studies (Wong et al., 1979; Bolton and Brown, 1980). Wong et al. (1979) attributed this relation to the determina-tion of stomatal aperture by the mesophyll capac-ity to fix CO2because high plant nitrogen content
results in great photosynthetic capacity and fairly stable Ci between nitrogen treatments. Lower Ci
of wheat plants in high-N solution indicated greater photosynthetic capacity of the mesophyll. However, in this study, stomatal control was not sufficient to maintain Ci constant between
nitro-gen treatments. Treatment differences in non-stomatal sensitivity to reduction in 8w caused
WUEi to either increase or decrease from 8w
induced changes in gs in the high-N and low-N
treatment, respectively. WhilePnand WUEiwere
initially greater for the high-N treatment because
at the start of the stress cycle the apparent greater sensitivity of mesophyll photosynthetic capacity made decrease in 8w. Then gs resulted in similar
values ofPnand WUEiat lowgs. For the drought
stress treatments gs and Pn both decreased. The
reaction to drought is different between high- and low-N treatments. WUEi of the plants at high-N
solution was decreased more than that of the plants in the low-N treatment due to stronger decrease in Pnthan in E.
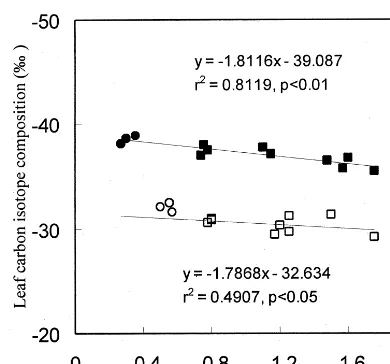
3.3. dp and the gas exchange-deri6ed 6alues of Ci/Ca
Because of the lag in time-integration scales between the A/E and isotope data, no quantita-tive interpretation can be derived from the dP
versusCi/Carelationships obtained in the present
study, as can be done when isotope discrimination is assessed simultaneously with gas exchange (Lloyd et al., 1992). Nitrogen deficiency strongly increased dp at a given Ci/Ca derived from gas
exchange measurements. Averagely speaking, dp
was difference between the high-N treatment and the low-N treatment was 6.9‰. dp were weakly
negatively correlated with theCi/Cavalues derived
phe-Fig. 1. Relationship between leaf carbon isotope composition and gas exchange-derived values of Ci/Ca of winter wheat.
Points are the mean of three measurements taken daily after imposition of water stress. Symbols: ( ) high-N and well-wa-tered; () high-N and droughted; () low-N and well-wa-tered; () low-N and droughted.
patchy leaf function and to gm, conductance for
CO2 diffusion in liquid phase from the
intercellu-lar air spaces to the sites of carboxylation in the chloroplasts was negligible in this case. gm was
decreased during drought (Shangguan et al., 1999). Such an effect would also tend to decrease leaf carbon isotope discrimination — and thus to increase dP — while decreasing theA/Eratio (or
increasing the gas exchange-derivedCi/Ca) (Lloyd
et al., 1992; Parkhurst, 1994).
3.4. Transpirational water use efficiency and carbon isotope discrimination
D increased with nitrogen supply. Nine days after imposition of drought,Ddifferences between the high-N and the low-N treatment were 1.5 and 2.3‰ in well-watered and drought conditions, respectively. In the drought treatments, D was 3.96 and 3.29‰ lower as compared to the well-watered treatments for low-N and high-N supply, respectively (Table 3). WUEt of the plants in the
high-N treatment was increased by 62.2% in the case of the well-watered plants but only by 42.0% in the drought treatment. Drought increased WUEt by 67.7% for low-N supply and by 46.8%
for high-N supply. WUEt was increased by N
supply both for the leaf gas exchange and at the whole plant and time integrated level. dp was
increased with the increase of drought intensity (Fig. 2). This reflects the effect of drought on isotope discrimination of the carbon photoassimi-lated during the drying cycle. This interpretation is also supported by a public garden study in which water-stressed western larch seedlings nomenon was also observed in Pinus pinaster
subject to drought and elevated CO2 (Picon et al.,
1996). In contrast, the results of Meinzer et al. (1992), Picon et al. (1996) onCoffea arabica and
Quercus petraea, respectively, are not in accor-dance with the simple two-step discrimination model.
However, Vivin et al. (1995) using Q. robur, a species with a leaf structure identical to that of
Q.petraea, they found a good agreement between season-long WUE determined gravimetrically and dP, using the simple two-step discrimination
model. This result suggests that the effects due to
Table 3
Transpiration efficiency (WUEt) and isotope discrimination (D) of winter wheat plantsa
ANOVA Droughted
Well-watered Treatment
Interaction High-N
Low-N High-N
Low-N N Drought
14.91b * * *
D(‰) 16.53ab 18.20a 12.57c
13.36a ** ** **
WUEt(mg g−1) 5.61c 9.10b 9.41b
aEach value is the mean of three measurements taken on the 9th day after imposition of drought stress. Results of Fisher’s test
are shown where means followed by the same lower case letter are not significantly different at the 5% level. *PB0.05;
Fig. 2. Relationship between leaf carbon isotope composition and leaf water potential of winter wheat. Points are the mean of three measurements taken daily after imposition of water stress. Symbols: ( ) high-N and well-watered; () high-N and droughted; () low-N and well-watered; () low-N and droughted.
difference in the values of WUEtbetween the two
nitrogen treatments (Fig. 3). In the high-N treat-ment, WUEtwas not increased by drought despite
of the decreasedDvalues. According to Farquhar et al. (1989), D is mainly caused in C3 species by
(i) fractionation due to CO2diffusion; (ii) changes
in stomatal resistance or assimilation rate, which affect the ratio of internal to ambient concentra-tion of CO2 (Ci/Ca); and (iii) fractionation by the
enzyme ribulose-1,5-bisphosphate carboxylase-oxygenase (Rubisco).
This discrepancy between WUEt andDmay be
explained based on Eq. (3). Ca and 6 were the
same in all treatments and for a given species,kis contant among the treatments. Because it is also likely that the term (1−F) was increased by nitrogen supply and the parameter F was de-creased by drought (Bowman et al., 1989; Von Caemmerer et al., 1997), it can be con-culuded that (b−D) is decreased and that D, which is also positively linear with Ci/Ca, is
in-creased by nitrogen supply, no matter of what water availability. The linear relationship between
Dand WUE was in agreement with the theory of the isotope effect (Farquhar and Richards, 1984) and other experiments (Wright et al., 1988, 1994). The relationship betweenDand nitrogen was not significant although as reported earlier by Eh-leringer (1990). D values in plant tissues (leaves, stubble and roots) were higher (less negative) in NH4
+-grown plants of T. aesti6um and Zea mays
than those in those supplied with NH4NO3 or
NO3−(Yin and Raven, 1998). It can be concluded
that nitrogen form and water conditions can in-teract to influence WUEt, but the underlying
physiological mechanisms need further elucida-tion.
Acknowledgements
This research was funded by the major state basic research development projects of China (Project No: G19 990 117) and the key project of resources, ecological and environmental research of the Chinese Academy of Sciences (Project No: KZ 951-B1-211).
Fig. 3. Relationship between transpiration efficiency (WUEt)
and leaf carbon isotope discrimination () of winter wheat. Points are the mean of three measurements taken daily after imposition of water stress. Symbols: ( ) high-N and well-wa-tered; () high-N and droughted; () low-N and well-wa-tered; () low-N and droughted.
showed 2‰ less discrimination of13C than that of
well-watered seedlings (Zhang et al., 1994). WUEt
References
Bolton, J.K., Brown, R.H., 1980. Photosynthesis in grass species differing in carbon dioxide fixation pathways. V. Response ofPanicum maximum,Panicum milioidesand tall fescue to nitrogen nutrition. Plant Physiol. 66, 97 – 100. Bowman, W.D., Hubick, K.T., Von Caemmerer, S., Farquhar,
G.D., 1989. Short-term changes in leaf carbon isotope discrimination in salt- and water-stressed C4 grasses. Plant Physiol. 90, 162 – 166.
Ciompi, S., Gentili, E., Guidi, L., Soldatini, G.F., 1996. The effect of nitrogen deficiency on leaf gas exchange and chlorophyll fluorescence parameters in sunflower. Plant Sci. 118, 177 – 184.
Ehdaie, B., Hall, A.E., 1991. Water-use efficiency and carbon isotope discrimination in wheat. Crop Sci. 31, 1282 – 1288. Ehleringer, J.R., Klassen, S., Clauton, C., Sherrill, D., Fuller-holbrook, M., Fu, Q., Cooper, T.A., 1991. Carbon isotope discrimination and transpiration efficiency in com-mon beans. Crop Sci. 31, 1611 – 1615.
Ehleringer, J.R., 1990. Correlation between carbon isotope discrimination and leaf conductance to water vapor in common beans. Plant Physiol. 93, 1422 – 1425.
Farquhar, G.D., Richards, R.A., 1984. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust. J. Plant Physiol. 11, 539 – 552. Farquhar, G.D., Ehleringer, J.R., Hubick, K.T., 1989. Carbon
isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40, 503 – 537.
Feng, G.G., Tang, Y.Q., 1997. Data Analysis Platform. Chi-nese Agriculture Press, Beijing.
Fischer, R.A., Turner, N.C., 1978. Plant productivity in the arid and semiarid zones. Annu. Rev. Plant Physiol. 29, 277 – 317.
Guy, R.D., Reid, D.M., Krouse, H.R., 1986. Factors affecting
13C/12C ratios of inland halophytes. I. controlled studies on
growth and isotopic composition ofPuccinellia nuttalliana. Can. J. Bot. 64, 2693 – 2699.
Ismail, A.I., Hall, A.E., 1992. Correlation between water-use efficiency and carbon discrimination in diverse cowpea genotypes and isogenic lines. Crop Sci. 32, 7 – 12. Lawlor, D.W., 1995. Photosynthesis, producitivity and
envi-ronment. J. Exp. Bot. 46, 1449 – 1461.
Lloyd, J., Syvertsen, J.P., Kriedemann, P.E., Farquhar, G.D., 1992. Low conductances for CO2diffusion from stomata
to the sites of carboxylation in leaves of woody species. Plant Cell Environ. 15, 873 – 899.
Mcdonald, A.J.S., Davies, W.J., 1996. Keeping in touch: response of the whole plant to deficits in water and nitro-gen supply. Adv. Bot. Res. 22, 229 – 300.
Meinzer, F.C., Goldstein, G., Grantz, D.A., 1990. Carbon isotope discrimination in Coffea genotypes grown under limited water supply. Plant Physiol. 92, 130 – 135. Meinzer, F.C., Saliendra, N.Z., Crisosto, C.H., 1992. Carbon
isotope discrimination and gas exchange inCoffea arabica during adjustment to different soil moisture regimes. Aust. J. Plant Physiol. 19, 171 – 184.
Morgan, J.A., 1984. Interaction of water supply and N in wheat. Plant Physiol. 76, 112 – 117.
Morgan, J.A., 1986. The effects of N nutrition on the water relations and gas exchange characteristics of wheat. Plant Physiol. 80, 52 – 58.
Nagarajah, S., 1981. The effect of nitrogen on plant water relations in tea (Camellia sinensis). Plant Physiol. 51, 304 – 308.
Parkhurst, D.F., 1994. Diffusion of CO2and other gases inside
leaves. New Physiol. 9, 339 – 348.
Picon, C., Guehl, J.M., Ferhi, A., 1996. Leaf gas exchange and carbon isotope composition responses to drought in a drought-avoiding (Pinus pinaster) and a drought-tolerant (Quercus petraea) species under present and elevated atmo-spheric CO2 concentrations. Plant Cell Environ. 19, 182 –
190.
Radin, J.W., Ackerson, R.C., 1989. Water relations of cotton plants under nitrogen deficiency II. Stomatal conductance, photosynthesis, and abscisic acid accumulation during drought. Plant Physiol. 67, 115 – 119.
Radin, J.W., Parker, L.L., 1979. Water relations of cotton plants under nitrogen deficiency. II. Environmental inter-actions on stomata. Plant Physiol. 64, 499 – 501.
Rao, N.R., Wright, G.C., 1994. Stability of the relationship between leaf area and carbon isotope discrimination across environments in peanut. Crop Sci. 34, 98 – 103.
Seemann, J.R., Sharkey, T.D., Wang, J.T., Osmond, C.B., 1987. Environmental effects on photosynthesis, nitrogen use efficiency, and metabolite pools in leaves of sun and shade plants. Plant Physiol. 84, 796 – 802.
Shangguan, Z.P., Chen, P.Y., 1990. Effects of water stress on photosynthesis of wheat leaves and their relation to drought resistance. Acta Bot. Boreal Occident Sin. 10, 1 – 7. Shangguan, Z.P., Shao, M.A., Dyckmans, J., 1999. Interaction of osmotic adjustment and photosynthesis in winter wheat under soil drought. J. Plant Physiol. 154, 753 – 758. Shangguan, Z.P., 1997. Regulation of nitrogen nutrition on
photosynthetic characteristics of winter wheat in dryland. Plant Nutr. Fert. Sci. 3, 105 – 110.
Shimshi, D., 1970. The effect of nitrogen supply on some indices of plant – water relations of beans (Phaseolus 6ulgarisL.). New Phytol. 69, 413 – 424.
Sugiharto, B., Miyata, K., Nakamoto, H., Sasakawa, H., Sugiyama, T., 1990. Regulation of expression of carbon-as-similating enzymes by nitrogen in maize leaf. Plant Physiol. 92, 963 – 969.
Tesha, A.J., Kumar, D., 1978. Effect of fertilizer nitrogen on drought resistance inCoffea arabica L. J. Agric. Sci. 90, 625 – 631.
Van den Boogaard, R., Kostadinova, R.S., Veneklaas, E., Lambers, H., 1995. Association of water use efficiency and nitrogen efficiency with photosynthetic characteristics of two wheat cultivars. J. Exp. Bot. 46, 1429 – 1438. Vivin, P., Gross, P., Aussenac, G., Guehl, J.M., 1995.
Whole-plant CO2 exchange, carbon partitioning and growth in
Quercus robur seedlings exposed to elevated CO2. Plant
Von Caemmerer, S., Ludwing, M., Millgate, A., Farquhar, G.D., Price, D., Badger, M., Furbank, R.T., 1997. Carbon isotope discrimination during C4 photosynthesis: insights
from transgenic plants. Aust J. Plant Physiol. 24, 487 – 494. Winter, K., Holtum, J.A.M., Edwards, G.E., O’Leary, M.H., 1982. Effect of low relative humidity ond13C value in two
C3grasses and inPanicum milioidess, a C3– C4intermediate
species. J. Exp. Bot. 33, 88 – 91.
Wong, S.C., Cowan, J.R., Farquhar, G.D., 1979. Stomatal conductance correlates with photosynthetic capacity. Na-ture 282, 424 – 426.
Wright, G.C., Hubick, K.T., Farquhar, G.D., 1988. Discrimi-nation in carbon isotope of leaves correlates with water-use efficiency of field-grown peanut cultivars. Aust. J. Plant Physiol. 15, 815 – 825.
Wright, G.C., Nageswara Rao, R.C., Farquhar, G.D., 1994. Water use efficiency and carbon isotope discrimination in peanut under water stress conditions. Crop Sci. 34, 92 – 97. Yin, Z.H., Raven, J.A., 1998. Influences of different nitrogen sources on nitrogen- and water-use efficiency, and carbon isotope discrimination in C3Triticum aesti6umL. and C4
Zea maysL. plants. Planta 205, 574 – 580.
Zhang, J.W., Fins, L., Marshall, J.D., 1994. Stable carbon isotope discrimination, photosynthesic gas exchange, and growth differences among western larch families. Tree Physiol. 14, 531 – 539.
Zimmerman, J.K., Ehleringer, J.R., 1990. Carbon isotope ratios are correlated with irradiance levels in the Panama-nian orchid Catasetum 6iridifla6um. Oecologia 83, 247 – 249.