L
Journal of Experimental Marine Biology and Ecology 246 (2000) 103–123
www.elsevier.nl / locate / jembe
Elemental composition of Mysis mixta (Crustacea,
Mysidacea) and energy costs of reproduction and
embryogenesis under laboratory conditions
*
Elena Gorokhova , Sture Hansson
Department of Systems Ecology, Stockholm University, 106 91 Stockholm, Sweden Received 2 May 1999; received in revised form 3 September 1999; accepted 30 November 1999
Abstract
Mysis mixta were reared under laboratory conditions (temperature: 9–108C; salinity: 7‰, ad libitum food). Dry weight, ash, total carbon and nitrogen content of mysids (muscle tissue, eggs, and embryos of different developmental stages) have been analyzed. We found significant variations in ash content and elemental composition during growth and maturation for both sexes. The proportion of carbon in abdominal muscle decreased gradually from juveniles with body weight of 3–4 mg (42.9%) to males and gravid females (|40.0%). The nitrogen content was
relatively constant (11.4% in average) with significant differences only between juveniles (11.3%) and mature females (11.6%). In embryos, carbon and nitrogen content were highest in early stages (58.6 and 14.3%, respectively). By the end of the marsupial development, carbon had decreased to 51.4% and nitrogen to 12.6%. The C:N ratio reflected the change in somatic carbon content, and the ratio decreased 6.2% from juveniles to gravid females, indicating lipids to be an energy source during maturation and reproduction. The weight-specific female investment in reproduction increases with body size. In gravid females, intersegmental growth during brooding period was observed, while males appear to store energy only for copulation and die after mating. Ontogenetic variation in body composition has implications for elemental budgets of M. mixta, its value as prey for fish and in modeling energy and nutrient cycling. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Mysis mixta; Energy costs; Reproduction; Embryogenesis
1. Introduction
The changes that accompany development and reproduction in crustaceans are related
*Corresponding author. Tel.: 146-8-164-256; fax: 146-8-158-417.
E-mail address: [email protected] (E. Gorokhova)
to considerable energetic losses and variations in elemental ratios (Nicol et al., 1995). Information on the chemical and biochemical composition of animals at different developmental stages is needed to describe these changes, to understand ecological and life history traits, and to evaluate how these patterns are further affected by environmen-tal factors. Some aspects of elemenenvironmen-tal composition and fluctuations associated with maturation and egg production have been reported for both macro- and microinverteb-rates (e.g. for copepods, Arashkevich and Drits, 1997; krill, Ikeda, 1984, 1985; Falk-Petersen, 1981; cephalopods, Bouchaud, 1991; decapod larvae, Anger and Schun, 1992; Minagawa et al., 1993).
In the Baltic Sea, mysids play an important role as major forage species for herring and smelt (Aneer, 1980; Arrhenius and Hansson, 1993) and are important zooplankton predators (Hansson et al. 1990; Rudstam et al., 1992). Among the mysids, Mysis mixta (Lilljeborg, 1852) is the numerically dominant pelagic species (Rudstam et al., 1986), but very little direct information on its elemental composition is available, and in general this kind of data exists mainly for freshwater species (Salonen et al., 1976; Hakala, 1979).
Mysis mixta is a boreal relict species of North-Atlantic origin, which inhabits Eastern Atlantic regions from the White Sea, Spitsbergen, Scandinavia and central and southern part of the Baltic Sea and westward to Iceland. In the Western Atlantic, it has been found in Greenland coastal waters and the eastern United States down to Cape Cod (Wigley and Burns, 1971, Blaxter et al., 1980). Generally, it is considered an annual species, although some individuals survive a second year (Grabe and Hatch, 1982; Salemaa et al., 1986; Shvetsova and Shvetsov, 1990). The species is semelparous and a cold-season breeder. In December, the population consists of mating mature males and females, and the males die shortly after copulation. The overwintering population is almost entirely composed of gravid females (Shvetsova, 1980, Rudstam et al., 1986). The young offspring are released in early spring through the beginning of summer, with a peak presumably in March, although protracted spawning periods (to July–August) have been observed (Shvetsova, 1980; Salemaa et al., 1986). Spawned females gradually disappear from the population and by the end of June, very few individuals of the previous year’s generation are normally found (Simm and Kotta, 1992). Newly released juveniles grow slowly and start to develop secondary sexual characters in June–September and complete maturation by November–December (Simm and Kotta, 1992).
terms of dry weight, carbon, nitrogen and ash content of eggs, embryos at different developmental stages, juvenile, subadults, and adult individuals of M. mixta.
2. Materials and methods
2.1. Collecting and rearing of mysids
Specimens of M. mixta were collected in a coastal area of the northern Baltic proper (588499N, 178359E, bottom depth of 25–35 m), in August 1995 and August 1996, with
2
a large plankton net (1 m opening, mesh size 0.5 mm). Immediately after collection, specimens covering the entire representative size range were length measured and prepared for ash content, carbon and nitrogen analysis. Simultaneously, materials were collected for the development of regression models between various body variables, such as total body length, carapace length, uropod length, wet and dry weight. The rest of the animals were transported to the laboratory where the animals collected in 1995 and 1996 were maintained for 8 and 5 months, respectively, as part of a study on mysid growth, moulting and stable isotope fractionation. These animals were kept in darkness in natural brackish water (6–7‰) at 9–108C, i.e. conditions similar to those in their natural environment. The mysids were fed with a surplus of a freshly hatched brine shrimp (Artemia spp., San Francisco Bay Brand), which were also regularly sampled for analyses of C and N content. Details on rearing, feeding regime, consumption rates, and food composition are published elsewhere (Gorokhova, 1998, 1999). Initial values of the animals used in the experiments were assumed to be those of mysids from the same size group (60.5 mm) sampled at the start of the experiments.
2.2. Experiment I
During the period from August 1995 until April 1996, groups of 30–50 mysids (initial length 7–12 mm) were kept in 50-l aerated aquaria. The conditions provided permitted for growth, gonad development and reproduction. After 5–9 months, in December– April, adult individuals (16.960.9 mm) from the laboratory culture were sampled for analyses, separating males, non-gravid, gravid, and postspawned females. Mysid length, dry weight, fecundity, size and stage of development for embryos present in the brood pouch were recorded. Embryos were dissected out and transferred into pre-weighed tin capsules.
2.3. Experiment II
In August to December 1997, specimens of M. mixta (initial length 8–11 mm) were kept individually in 1-l plastic beakers. The food was changed daily and the water was renewed weekly. Every week, three to five individuals were sacrificed, sized, staged in one of the categories described below, and prepared for the analysis. The experiment lasted for 18 weeks.
2.4. Developmental stages of mysids and embryos
Seven life cycle stages were considered according to Mauchline (1980) and Mees et al. (1994). (1) Juveniles: no secondary sexual characteristics. (2) Immature males: 4th pleopods are elongated but do not reach the posterior edge of the last abdominal segment; the lobus masculinus is not fully developed and lacks setation. (3) Immature females: oostegites are visible only from the ventral side. (4) Adult males: 4th pleopods reach the posterior edge of the last abdominal segment; the lobus masculinus is setose. (5) Adult females: marsupia are visible from the lateral side but empty. (6) Gravid females: marsupia are filled with eggs / embryos. (7) Postspawned females: embryos have been released from marsupia.
Embryo stages were classified using descriptions of Mauchline (1980), Cuzin-Roudy and Tchernigovtzeff (1985), and Wittmann (1981a). (1) Embryonic egg: embryos in the initial state of marsupial development (stages of early embryo and ovoid eggs, still within the egg membrane). (2) Eyeless larvae: newly hatched and developing nauplioid larvae with eye pigmentation and large yolk mass. Wittmann referred to this stage as ‘naupliar’, and Cuzin-Roudy and Tchernigovtzeff as ‘naulioid phase’. (3) Eyed larvae: moulted nauplioid larvae with the eyes on stalks and almost absorbed yolk. In the terminology of Wittmann and Cuzin-Roudy and Tchernigovtzeff this was ‘postnaupliar’ and ‘postnauplioid’ stages, respectively.
2.5. Length measurements
Measurements of the total mysid body length (BL, mm) were taken from the tip of the rostrum to the posterior edge of the last abdominal segment, using calipers (60.02 mm) under a stereomicroscope. Length measurements of the carapace, uropods, and embryos were made using a binocular microscope fitted with an ocular micrometer. Carapace length (CL, mm) was measured as the distance from the tip of the rostrum to the mediodorsal margin of the carapace. Uropod length (UL, mm) was from the posterior margin of the last abdominal segment to the posterior edge of the outer uropod, excluding the setae. Larval size was measured in seawater as the largest diameter for the egg stage and the length from the terminal to the frontal tip of the body for the eyeless and eyed stages.
2.6. Drying and weighting
weight (6–8 days for mysids and 3 days for eggs / embryos and Artemia). The dry weights (DW, mg) were determined using a Sartorius M3P microbalance to the nearest microgram. When preparing mysid abdominal tissue, abdomens were carefully separated while dried and transferred to the pre-weighed tin capsules. Whole abdomen or its aliquot, depending on size, 15–20 eggs / embryos, or 30–50 Artemia nauplii comprised each sample. All the samples were kept frozen (2208C) until analysis.
2.7. CN elemental analyses
Elemental carbon and nitrogen content were measured by combusting the tin capsules in a CHN-analyzer (CHN-900, 600-800-300, Leco Corporation). For calibration, EDTA of analytical quality (Sigma-Aldrich) was used. The standard deviation among replicate standard samples was within 0.2% for both carbon and nitrogen.
2.8. Ash content
After dry weight determinations, five to ten replicate samples from the same set that had been analyzed for elemental composition, were incinerated at 5008C for 4 h in a muffle furnace (Hirota and Szyper, 1975). The remaining ash, containing only inorganic substances, were cooled in dessicator and weighed in the same way as DW.
2.9. Energy densities
Caloric values of mysid body, muscle tissue and eggs / embryos were calculated from formula given in Salonen et al. (1976), using values of mysid DW, carbon, and ash content. The proportion of inorganic carbon was assumed to be the same as in Mysis relicta (0.044% of DW, Salonen et al., 1976) and to be independent on mysid size. For calculations of the total energy of ovigerous females, we used the observed fecundity, eggs / embryos energy content and energy content of the female muscle tissue.
2.10. Data analysis
3. Results
3.1. Growth, development and body size
Juveniles brought into the laboratory in August developed secondary sexual charac-teristics during September–November, underwent gonadogenesis (experiment I and II), and mated (experiment I). In the later, by the middle of December, about 30% of females were ovigerous. Simultaneously, mortality of males increased and, no males were present in the culture by the mid-February. At this point, all females were fertilized and bearing embryos. The first offspring hatched in the middle of March and by the end of April, all the females were postspawned.
There were no significant difference between the sampling occasions and between the experiments with regard to the mysid size in different life stages (juveniles, immature and mature males and immature and mature females) (ANOVA, p.0.05 in all cases). Pooled data on size variability within each category from the field and laboratory observations are presented in Table 1.
Embryos (all stages) ranged in size from 0.35 to 3.00 mm, with an overlap between neighboring stages (Table 1). As free-living mysids, larvae were released at a mean size of 2.960.08 mm, corresponding well to the smallest M. mixta observed in the field samples (3.0 mm) in both 1995 and 1997. The embryo undergoes a substantial dry weight reduction (32%) during the marsupial development (Table 1).
In some female specimens, oostegites can be seen already at a size of 8.5–8.7 mm, but in most mysids, sex is not distinguishable until they have reached 10–12 mm. Fully mature individuals also varied in size from about 14 to 16 mm and 13 to 17 mm in males and females, respectively. The size dimorphism in M. mixta does not appear until the brooding: there were no significant differences in length ( p.0.6) or DW ( p.0.3) between males and non-gravid females. No significant body length difference was found between mature and gravid females ( p.0.05), while the DW of ovigerous and larvigerous females was significantly higher due to the presence of eggs or embryos
Table 1
M. mixta: mean size6S.D. in body length (BL) and dry weight (DW), and size ranges of different life-cycle categories (n denotes the number of observations)
Life stage Mean6S.D., range n
BL (mm) DW (mg)
Eggs 0.5460.13, 0.35–0.71 0.03560.002, 0.031–0.037 11
Eyeless embryos 1.5860.21, 1.26–2.19 0.03160.003, 0.029–0.034 16 Eyed embryos 2.6660.28, 2.07–3.00 0.02460.003, 0.020–0.028 12
Juveniles 6.161.83, 2.9–9.7 0.5260.38, 0.05–2.81 58
( p,0.001). The length of postspawned females did not differ significantly from that of females bearing embryos ( p.0.05) while their weight was lower ( p,0.0003), reflecting loss of marsupial content during hatching.
The allometric relationships between DW and body length (BL) are shown in Table 2. No significant differences ( p.0.5) were found between log–log linear regressions of DW against BL for juveniles and subadults, neither between those for males and non-ovigerous females nor for wild and laboratory reared subadults. Consequently, all these categories were pooled for a common regression line as a power function (Table
2
2). The regression of DW to BL for gravid females was poor (r 50.51) and differed from that for the other life-cycle categories as indicated by significantly different slopes ( p,0.05).
Regression lines for the carapace length to body length were similar (both slopes and intercepts are not significantly different, p.0.3 for slopes and p.0.05 for intercepts) between females having egg-like embryos and younger stages (juveniles, immature and mature males and females; Table 2). The line for females carrying eyed embryos, however, had significantly different intercept ( p,0.02) indicating elongated abdomen, i.e. increase in linear dimension during brooding period. Overall, females with eggs were significantly smaller than those with eyed larvae (length 15.6761.08 vs. 16.1260.97 mm, p,0.01) yielding the intermoult growth of 2.9% in body length.
3.2. Reproductive life history traits
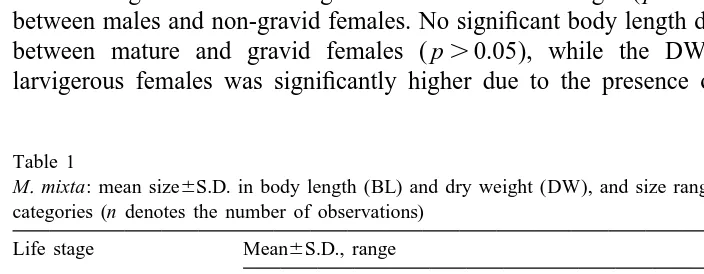
The brood size in experiment I ranged from 14 to 35 (27.265.3) eggs or embryos per female. The number of eggs and embryos per individual female (F), tended to increase with female size, though the relationship with either BL or CL was poor (Table 2). However if only the eyed embryos were considered, the regression improved slightly (Table 2). Furthermore, the average number of eggs per brood compared to that of
Table 2
M. mixta: morphometric relationships describing dry weight (DW, mg), carapace length (CL, mm), uropod length (UL, mm) and fecundity (F, number of young per brood) as functions of mysid body length (BL, mm)
a for different life-cycle categories
2
Life-cycle stages (age and sex) Type of regression a b r n
b
Juveniles, immature and DW5a*BL 0.0032 2.850 0.98 190
mature males and females
b
Gravid females DW5a*BL 0.7140 1.582 0.51 39
Juveniles, immature and CL5a*BL1b 0.42 0.12 0.98 64
mature males and females
Gravid females with eggs CL5a*BL1b 0.40 0.43 0.94 11
Gravid females with eyed embryos CL5a*BL1b 0.42 20.05 0.82 12
All stages UL5a*BL2b 0.28 20.39 0.98 67
Gravid females F5a*BL1b 3.76 231.98 0.34 39
Gravid females with eyeless F5a*BL1b 4.46 243.73 0.44 28
and eyed embryos
a 2
n denotes number of observations and r is the corresponding regression coefficient. Value for all linear
Fig. 1. M. mixta. Number of embryos in all developmental stages, fertilized eggs, and eyed embryos per brood. The box extends from the 25th percentile to the 75th percentile, with a horizontal line at the median. Whiskers show the range of the data.
embryos was significantly higher (29.265.1 vs. 24.364.5; p,0.04; Fig. 1) indicating either embryo loss from marsupia or prenatal mortality during marsupial development. Larger females carried larger eggs, with a linear relationship between egg DW and
2
female DW: Eggs DW50.0012?Female DW10.022 (r 50.61, p,0.005).
3.3. Carbon and nitrogen content
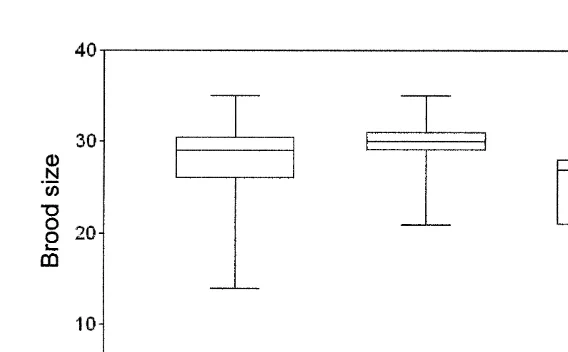
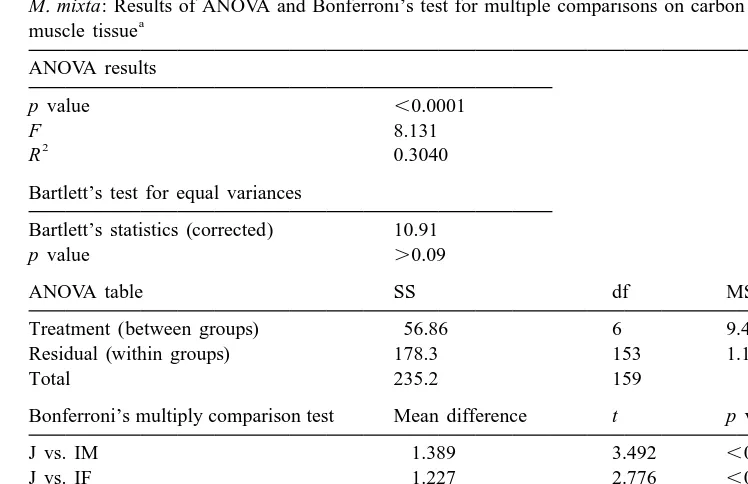
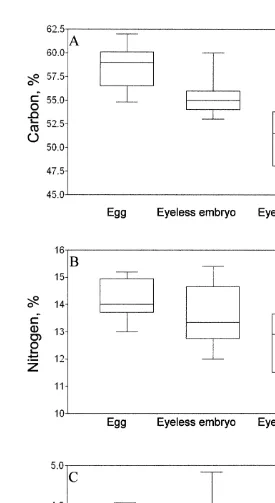
The elemental composition did not differ between years and within the different life stage categories (juveniles, immature males and females; p.0.05 in all cases). Pooled data on the carbon and nitrogen content within each category from field and laboratory (experiments I and II) observations are presented in Fig. 2. There was a considerable variation in carbon content in somatic tissues both within and between different life-cycle stages of M. mixta (Fig. 2a). The proportion of carbon in abdominal muscle decreased gradually from juveniles (42.961.12%) to mature males and gravid females (40.061.61%). The carbon content in muscle tissue did not differ between sexes until after the peak of the mating period (Table 3, Fig. 2a). In specimens sampled from the end of December to the mid-January (males: 38.5560.76, n511 and gravid females: 39.2760.88, n515), a significantly lower percentage of carbon was found in the males ( p,0.04). The nitrogen content was relatively constant (11.4% in average) with significant differences only between juveniles and mature females (11.3 vs. 11.6% respectively; Table 4). The C:N ratio reflected the change in somatic carbon content, and the ratio decreased 6.2% from juveniles and subadult to mature males and gravid females (Fig. 2c). Thus, at the conditions provided, the specific content of carbon (C, %)
2
Table 3
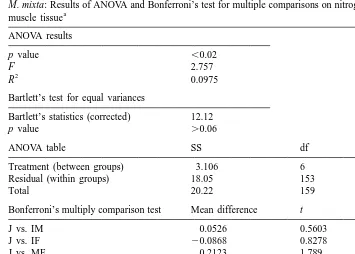
M. mixta: Results of ANOVA and Bonferroni’s test for multiple comparisons on carbon content in abdominal a
Treatment (between groups) 56.86 6 9.476
Residual (within groups) 178.3 153 1.166
Total 235.2 159
Bonferroni’s multiply comparison test Mean difference t p value
J vs. IM 1.389 3.492 ,0.01
J, juveniles; IM, immature males; IF, immature females; MM, mature males; MF, mature females; GF, gravid females; PF, postspawned females.
2
DW111.34; r 50.03, p,0.03), resulting in a significant change in C:N ratio 2
(C:N5 20.033?Body DW13.81; r 50.23, p,0.0001).
In embryos, carbon and nitrogen content were highest in early stages (58.6 and 14.3%, respectively), and decreased to 51.4% for carbon ( p,0.0001, Fig. 3a) and to 12.6% for nitrogen ( p,0.04, Fig. 3b) to the end of the marsupial development. In eggs, there was no correlation between DW versus carbon and nitrogen content (Pearson r5 20.13,
2 2
p.0.7, R 50.02 and Pearson r50.09, p.0.8, R 50.01 for C and N, respectively). Variations in the C:N ratios in embryos were weak (Fig. 3c).
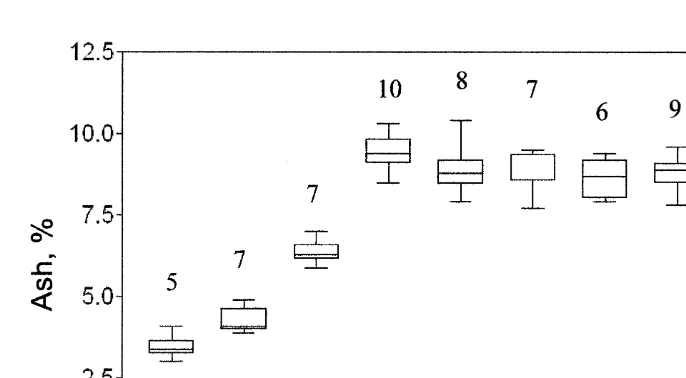
3.4. Ash content
Table 4
M. mixta: Results of ANOVA and Bonferroni’s test for multiple comparisons on nitrogen content in abdominal a
Treatment (between groups) 3.106 6 0.5177
Residual (within groups) 18.05 153 0.1345
Total 20.22 159
Bonferroni’s multiply comparison test Mean difference t p value
J vs. IM 0.0526 0.5603 .0.05
J, juveniles; IM, immature males; IF, immature females; MM, mature males; MF, mature females; GF, gravid females; PF, postspawned females.
3.5. Energy content
21 The weight-specific energy content of the mysid muscle tissue fell from 20.0 KJ g
21 21
DW (20.9 KJ g AFDW) in the young mysids in August (field samples) to 17.6 KJ g 21
DW (18.9 KJ g AFDW) in mature animals in the winter (laboratory-reared mysids). 21
The lowest caloric value (17.9 KJ g AFDW) was obtained for males in the end of mating period. The total energy content of ovigerous females (somatic tissues and
21 21
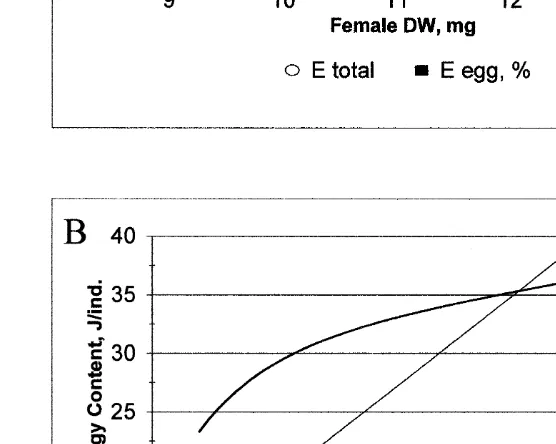
fertilized eggs) was 18.7 KJ g DW (or 207.7 J ind ) in the beginning of the marsupial development (December–January). By this time energy contained in ripe eggs composed about 10–17% of the total female energy content (Fig. 5a). The relationship between female body size and the energy they devote to reproduction (Fig. 5b), based on regressions describing fecundity and egg size as functions of female DW and egg elemental composition, could be established. Larger females invest more to their offspring both in terms of the absolute values and relative to their DW.
21
The energy density of embryos varied from 29.7 KJ g AFDW in earliest embryos to 21
Fig. 4. M. mixta. Ash content for all ontogenetic stages (field samples, experiments 1 and 2). E, egg; ELE, eyeless embryo; EE, eyed embryo; J, juveniles; IM, immature males; IF, immature females; MM, mature males; MF, mature females; GF, gravid females; PF, postspawned females. The box and whiskers parameters are the same as in Fig. 1. Numbers above plots show the number of analyses.
of embryos and their prenatal mortality, the energy content of brood decreased by 50% during the complete embryogenesis.
4. Discussion
Only a few mysid species have been laboratory reared for studies of development and growth: Leptomysis lingvura (Wittmann, 1981a,b), Neomysis intermedia (Toda et al., 1987), Siriella armata (Cuzin-Roudy and Saleuddin, 1989), Rhopalophthalmus ter-ranatalis (Wooldridge, 1986), and Holmesmysis costata (Turpen et al., 1994). The results from such studies under controlled conditions are usually of lower variability than results from studies on natural populations, where inter-population differences might occur due to genetic or environmental variations. However, the investigators have not focused on the changes in chemical composition associated with development and reproduction. According to previous observations, behaviour, size distribution, and chemical composition of Mysis species including M. mixta, are influenced by various factors, for example, by season and life cycle stage (Hakala, 1979; Gardner et al., 1985; Adare and Lasenby, 1994). The results presented here show that age and sexual maturity has marked effects on the chemical composition of the somatic tissues of M. mixta even if environmental conditions and food supply remain invariable.
21 Fig. 5. M. mixta. Panel A shows calculated total energy content (E) of gravid female (E tot,s, J ind ) and
21
energy accumulated in the eggs in relation to female DW (E egg, %,j, J mg ). Panel B: empirical models 21
for energy invested in reproduction as an absolute value (thin line, J ind ) and relative to female size (thick 21
(Wittmann, 1981b). Indeed, if accumulation of lipids for overwintering require an environmental stimulus (e.g. decrease of temperature and / or light period), then this process might be inhibited in the laboratory. Another possible source of bias in our observations is an inadequacy of the Artemia as a sole food item for M. mixta. Artemia nauplii have commonly been used as a diet for marine crustaceans including mysids (for review, see Leger et al., 1986). However, many studies indicate that the dietary quality of Artemia varies with its geographical origin (Beck and Bengtson, 1982; Leger and Sorgeloos, 1984). The nutritional value of Artemia nauplii is most often correlated with the lipid quality, particularly variations in the composition of highly unsaturated fatty acids (Leger et al., 1986; Kreeger et al., 1991). When testing culture and dietary conditions for mysid crustaceans, survival, growth, and reproduction output are usually used as indicators (Johns et al., 1981; Leger et al., 1986; Kreeger et al., 1991). In our study, the low mortality, successful breeding, and the size attained by adults indicate that the laboratory treatment provided conditions that fulfill the requirements of M. mixta for growth and reproduction. Moreover, growth rates and fecundity in laboratory cultured mysids match previous estimates from field populations (Shvetsova, 1980; Rudstam, 1989; Simm and Kotta, 1992; Gorokhova and Hansson, 2000). Consequently, the results of this study may be of general relevance until comparable field data become available.
4.1. Growth, development and body size
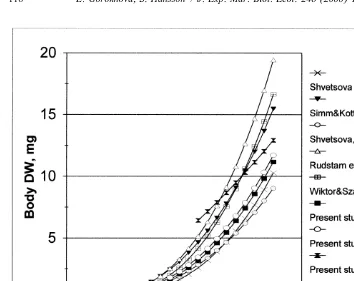
Despite differences in laboratory and natural conditions, timing of sexual maturation, mating and brood duration were virtually synchronous with phenological events observed in the wild (Salemaa et al., 1986; Simm and Kotta, 1992). At the same time, the selection of specimens within a narrow size range for experiments, together with identical growth conditions, resulted in lower size variability of the reared individuals (Table 1) compared to that of M. mixta in the wild (12–22 mm in October, mostly matured individuals, Wiktor and Szaniawska, 1988). In general, the ranges of length and weight found in laboratory reared M. mixta agree with field observations from the Baltic (Shvetsova, 1980; Rudstam et al., 1986; Wiktor and Szaniawska, 1988) and that of Grabe and Hatch (1982) in New Hampshire coastal waters. However, the length–weight regressions between these studies varied considerably (Fig. 6), most probably due to the difficulties related to positioning of a mysid when measuring length. Until the brooding period begins, mysids show uniform sizes and biometrical relationships regardless of sex, while females with a brood pouch require different equations for interconversions. Inter-moult (5intersegmental) growth of |3% in length was observed in gravid females, during the brooding period. Mauchline (1973a) and Fenton (1994) examined inter-moult growth in several mysid species and found it to range between 3–10% (7% in average) and between 2–14% (6% in average), respectively. The question as to what extent field population follows this growth pattern under general food depletion during most part of the cold period needs to be carefully analyzed together with studies on food consumption and assimilation.
4.2. Reproductive life history traits
Fig. 6. M. mixta. Total length to body dry weight relationships obtained in different studies. To compare the varying length–weight and length–length relationships to those obtained by Shvetsova (1987), Shvetsova et al. (1992), Simm and Kotta (1992), Rudstam et al. (1986), and Wiktor and Szaniawska (1988), differences in the lengths measured must be taken into account. The total body length in these studies corresponds to the sum of body length (BL) and either uropod (UL) or telson length (TL) measured in our study (Table 2).
(Mauchline, 1980; Wittmann, 1984). Due to the low variability in the female size in our study, the relationships between brood size and female length and between egg DW and female DW were weak. Considerable variations in the brood size of M. mixta have been reported: 9–97 eggs by Mauchline (1980), 21–255 by Grabe and Hatch (1982), 8–87 by Shvetsova et al. (1992), 8–42 by Shvetsova (1980), and 13–53 by Simm and Kotta (1992). The average fecundity obtained in the laboratory (27.2) was surprisingly close to the values reported from the Gulf of Riga for females of the corresponding length (28.4 embryos, Simm and Kotta, 1992; 19.6 embryos, Shvetsova, 1980).
(17% in numbers) but no comparative field data are available to conclude if this value is different in natural population. However, the differences between fecundity estimates using egg numbers and that based on embryo numbers are important for assessment of the recruitment stock and parental investment in the offspring.
4.3. Energy costs of embryogenesis and reproduction
The utilization of the yolk material by the developing embryo results in a decrease in egg dry weight. Unfortunately, no data on variations in M. mixta egg and embryo DW and elemental or biochemical composition are available, but compared with data from other marine crustaceans of similar size, our data correspond well with values given by Khmeleva and Romanova (1978). The latter authors compare water content, dry mass, ash, and caloric content in fertilized eggs and embryos of marine Idoteidae (seven species), Gammaridae (one species) and Decapoda (six species) from different localities. In their study, the decrease in DW during embryo development varied from 24.0% in amphipods to 41.2% in isopods. At the same time, ash content increased two to four times from 3.0–5.4 to 8.2–19.5%. Consequently, the caloric value was reduced 14.2 to 16.7%. The overall loss of embryo energy content was estimated to be 35.9% in decapods, 44.6% in amphipods, and 49.1% in isopods. Similar changes were observed in developing embryos of M. mixta: loss of 31.4% of the initial DW and 13% in caloric value, together with an increase from 3.4 to 6.4% in ash content. This resulted in a 40% energy loss for intermediary metabolic needs and metamorphoses during the marsupial development.
Female M. mixta invests a significant amount of energy in the offspring. The proportional amount of energy devoted to reproduction increase with female size in different manners (Fig. 5b). Using the same regression of carbon against energy as we used, Hakala (1979) estimated that the carbon loss of M. relicta females for egg production was 8%. Apparently, our estimates of egg production costs in M. mixta (|14%) were higher. The lower caloric values in males after mating than in gravid females indicate that male energy losses for spermatogenesis and copulation is even higher.
4.4. Elemental composition and energy content
The ranges and means of the elemental composition of M. mixta muscle tissues (C: 37–44% and N: 10–13%) are somewhat different from those reported for freshwater mysids. These differences were rather expected, due to analyzing abdominal muscle instead of the whole body (somatic plus generative tissues), the latter being more common. Salonen et al. (1976) and Hakala (1979) studied Mysis relicta from Lake
¨ ¨ ¨
Wiktor and Szaniawska (1988) reported the weight-specific energy content of M. 21
mixta (size 4–30 mm) from the southern Baltic to be 24.8 KJ g DW on average, which is higher than our estimates. However, for the size group 6–13 mm, which is similar to 21 animals used in our experiments, their and our values are similar (20.6 and 20.0 KJ g DW). In contrast to Wiktor and Szaniawska (1988), the caloric value of M. mixta
21
reported by Rumohr et al. (1987), 15.1 KJ g DW, was much lower than our estimates. The weight specific energy content of other mysid species varies. Observations on another North-Atlantic species, Mysis stenolepsis (Tyler, 1973) and on freshwater Neomysis mercedis from Lake Washington (Chigbu and Sibley, 1996) were consistent
21
with our findings for M. mixta. Values ranging from 13.5 to 20.8 KJ g DW have been found for Neomysis integer in different parts of the Baltic (Healey, 1972; Szaniawska et al., 1986; Rumohr et al., 1987).
To a considerable extent, the variation in the carbon-to-dry weight ratio mirrors variation in lipid content, as lipids are richer in C (70–80%) than carbohydrates (40–44%) and proteins (50–54%, Winberg, 1971). A seasonal cycle in the carbon content of M. relicta was described by Hakala (1979), who found values between 45 and 58% in a population with a 2-year life cycle. In immature animals, carbon accumulated in the autumn, while adults lost carbon during August to December. Hakala (1979) also described ontogenetic variation in the energy content of M. relicta with the highest
21
values for young embryos (27.85 KJ g DW) and lowest in the young mysids (20.77 21
KJ g DW). Seasonal changes in the percent total lipid content of M. relicta with 1-and 2-year life cycles (southern Ontario, Adare 1-and Lasenby, 1994) followed the same pattern. In that study, juvenile mysids had approximately 10% less lipid than adults, a difference similar to that recorded between adult female and juveniles of the deposit-feeding amphipods Pontoporeia hoyi (Quigley et al., 1989) and Monoporeia affinis and P. femorata (Hill et al., 1992). In adult M. relicta, the percent lipid reached a peak between July and the end of August, and then gradually declined until November– December (Gardner et al., 1985; Adare and Lasenby, 1994). The authors concluded that this decline reflected seasonal changes in the quality and quantity of food or resulted from physiological changes coupled to reproduction and development. The reduction of
˚
lipids in Meganyctiphanes norvegica (Bamstedt, 1976; Falk-Petersen, 1981), copepods (Arashkevich and Drits, 1997), as well as the decrease in carbon described for cladocerans (Manca et al., 1994) have also been linked to sexual maturation.
Our carbon data, with higher values in August than in winter and a decline with size (and with developing of ovarian / testis tissues), indicate a similar change in lipid content in Baltic M. mixta with a 1-year life cycle as in the second year of life in M. relicta. In our experiment, however, the mysids had a surplus of food and the decline can not be
˚
females that survive a second year reproduce twice is unknown; it would be to a female’s advantage to devote a high proportion of her energy reserves to somatic growth during first year and to oogenesis during second year of life. Further studies using both elemental composition and in situ growth analysis would lead to a more comprehensive understanding of the mysid energetics.
Acknowledgements
This study was financially supported by the Helge Ax:son Johnson and the Wallenberg Foundations, the Swedish Natural Science Research Council and the European Union through MAST / BASYS. We also thank the Stockholm Centre for Marine Research for
¨ ´
providing facilities and technical support at the Asko Laboratory. Ulrika Hamren is thanked for field and laboratory assistance. [SS]
References
Adare, K., Lasenby, D., 1994. Seasonal changes in the total lipid content of the Opossum Shrimp, Mysis relicta (Malacostraca, Mysidacea). Can. J. Fish. Aquat. Sci. 51, 1935–1941.
Aneer, G., 1980. Estimates of feeding pressure on pelagic and benthic organisms by Baltic herring (Clupea
harengus v. membras L.). Ophelia 1, 265–275.
Anger, K., Schun, M., 1992. Bioenergetics of abbreviated larval development in the bromeliad crab,
Metopaulias depressus (Decapoda, Grapsidae). Comp. Biochem. Physiol. A, Physiology 103, 507–518.
Arashkevich, E.G., Drits, A.V., 1997. Biochemical composition, respiration rate and gonad development in late-stage copepodites of Calanoides carinatus (Kroyer) from the Benguela upwelling region. Okeanologiya 37, 571–577.
Arrhenius, F., Hansson, S., 1993. Food consumption of larval young and adult herring and sprat in the Baltic Sea. Mar. Ecol. Prog. Ser. 96, 25–137.
˚
Bamstedt, U., 1976. Studies on the deep-water pelagic community of Korsfjorden, Western Norway. Changes in the size and biochemical composition of Meganyctiphanes norvegica (Euphausiacea) in relation to its life cycle. Sarsia 61, 15–30.
Beck, A.D., Bengtson, D.A., 1982. International study on Artemia. XXII. Nutrition in aquatic toxicology: diet quality of geographical strains of the brine shrimp Artemia. Spec. Tech. Publ. Am. Soc. Test. Mater. 766, 161–169.
Blaxter, J.H., Russel, F.S., Yonge, M., 1980. The biology of mysids. Adv. Mar. Biol. 17, 1–511.
Bouchaud, O., 1991. Energy consumption of the cuttlefish Sepia officinalis L. (Mollusca, Cephalopoda) during embryonic development, preliminary results. Bull. Mar. Sci. 49 (1–2), 333–340.
Chigbu, P., Sibley, T.H., 1996. Biometrical relationships, energy content and biochemical composition of
Neomysis mercedis from Lake Washington. Hydrobiologia 337 (1–3), 145–150.
Cuzin-Roudy, J., Tchernigovtzeff, C., 1985. Chronology of the female molt cycle in Siriella armata M. Edw. (Crustacea: Mysidacea) based on marsupial development. J. Crustacean Biol. 5, 1–14.
Fenton, G.E., 1994. Breeding biology of Tenagomysis tasmaniae Fenton, Anisomysis mixta australis (Zimmer) and Paramesopodopsis rufa Fenton from south-eastern Tasmania (Crustacea: Mysidacea). Hydrobiologia 287 (3), 259–276.
Gardner, W.S., Nalepa, W.A., Frez, E., Cichocki, A., Landrum, P.F., 1985. Seasonal patterns in lipid content of Lake Michigan macroinvertebrates. Can. J. Fish. Aquat. Sci. 42, 1827–1832.
Gorokhova, E., 1998. Exploring and modelling the growth dynamics of Mysis mixta. Ecol. Model. 110, 45–54. Gorokhova, E., 1999. Mysid growth, stable isotope fractionation, and bioenergetics: implications for food web
studies. PhD thesis, Stockholm University, Stockholm, pp. 1–29.
Gorokhova, E., Hansson, S., 2000. An experimental study on variations in stable carbon and nitrogen isotopes fractionation during growth of Mysis mixta and Neomysis integer. Can. J. Fish. Aquat. Sci. (in press). Grabe, S.A., Hatch, E.R., 1982. Aspects of the biology of Mysis mixta (Lilljeborg 1852) (Crustacea,
Mysidacea) in New Hampshire coastal waters. Can. J. Zool. 60, 1275–1281.
Hakala, I., 1979. Seasonal variations in the carbon and energy contents and assimilation of a Mysis relicta ¨ ¨ ¨
population in Paajarvi, southern Finland. Ann. Zool. Fennici 16, 129–137.
Hansson, S., Larsson, U., Johansson, S., 1990. Selective predation by herring and mysids, and zooplankton community structure in a Baltic Sea coastal area. J. Plankton Res. 12, 1099–1116.
Healey, M.C., 1972. Bioenergetics of sand goby (Gobius minutus) population. J. Fish. Res. Board Canada 29, 187–194.
Hill, C., Quigley, M.A., Cavaletto, J.F., Gordon, W., 1992. Seasonal changes in lipid content and composition in the benthic amphipods Monoporeia affinis and Pontoporea femorata. Limnol. Oceanogr. 37, 1280–1289. Hirota, J., Szyper, J.P., 1975. Separation of total particulate carbon into inorganic and organic components.
Limnol. Oceanogr. 20, 896–900.
Ikeda, T., 1984. Sequences in metabolic rates and elemental composition (C, N, P) during the development of
Euphausia superba Dana and estimated food requirements during its life span. J. Crustacean Biol. 4,
273–284.
Ikeda, T., 1985. Metabolic rate and elemental composition (C and N) of embryos and non-feeding early larval stages of Antarctic krill (Euphausia superba) Dana). J. Exp. Mar. Biol. Ecol. 90, 119–127.
Johns, D.M., Berry, W.J., Walton, W., 1981. International study on Artemia. XVI. Survival, growth and reproductive potential of the mysid Mysidopsis bahia Molenock fed various geographical strains of the brine shrimp Artemia. J. Exp. Mar. Biol. Ecol. 53, 209–219.
Khmeleva, H., Romanova, Z., 1978. Izmenenie massy i kalorijnosti nekotorykh rakoobraznykh za period embriogeneza (in Russian, with English summary). Biologiya Morya 46, 54–60.
Kreeger, K.E., Kreeger, D.A., Langdon, C.J., Lowry, R.R., 1991. The nutritional value of Artemia and
Tigriopus californicus (Baker) for two Pacific mysid species, Metamysidopsis elongata (Holmes) and Mysidopsis intii (Holmquist). J. Exp. Mar. Biol. Ecol. 148, 147–158.
Leger, P., Sorgeloos, P., 1984. International Study on Artemia. XXIX. Nutritional evaluation of Artemia nauplii from different geographical origin for the marine crustacean Mysidopsis bahia. Mar. Ecol. Prog. Ser. 15, 307–309.
Leger, P., Bengtson, D.A., Simpson, K.I., Sorgeloos, P., 1986. The use and nutritional value of Artemia as a food source. Oceanogr. Mar. Biol. Annu. Rev. 24, 521–623.
Manca, M., Spagnuolo, T., Comoli, P., 1994. Variations in carbon and nitrogen content with body length of
Daphnia hyalina-galeata s.l. from laboratory and field observations. J. Plankton Res. 16 (10), 1303–1314.
Mauchline, J., 1973a. Intermoult growth of species of Mysidacea (Crustacea). J. Mar. Biol. Assoc. UK 53, 569–572.
Mauchline, J., 1973b. The broods of the British Mysidacea. J. Mar. Biol. Assoc. UK 53, 801–817. Mauchline, J., 1980. The biology of Mysids. Adv. Mar. Biol. 18, 3–373.
Mees, J., Abdulkerim, Z., Hamerlynck, O., 1994. Life history, growth and production of Neomysis integer in the Westerschelde estuary (SW Netherlands). Mar. Ecol. Prog. Ser. 109, 43–57.
Morgan, M.D., 1980. Life history characteristics of two introduced populations of Mysis relicta. Ecology 61 (3), 551–561.
Nicol, S., Delamare, W.K., Stolp, M., 1995. The energetic cost of egg-production in Antarctic krill (Euphausia
superba Dana). Antarctic Sci. 7 (1), 25–30.
Quigley, M.A., Cavaletto, J.F., Gardner, W.S., 1989. Lipid composition related to size and maturity of the amphipod Pontoporeia hoyi. J. Great Lakes Res. 15, 601–610.
Rudstam, L.G., 1989. A bioenergetic model for Mysis growth and consumption applied to a Baltic population of Mysis mixta. J. Plankton Res. 11, 971–983.
Rudstam, L.G., Hansson, S., Larsson, U., 1986. Abundance, species composition and production of mysid shrimps in a coastal area of the northern Baltic proper. Ophelia 4, 225–238.
Rudstam, L.G., Hansson, S., Johansson, S., Larsson, U., 1992. Dynamics of planktivory in a coastal area of the northern Baltic Sea. Mar. Ecol. Prog. Ser. 80, 159–173.
Rumohr, H., Brey, T., Ankar, S., 1987. A compilation of biometric conversion factor for bentic invertebrates for the Baltic Sea. BMB Publ. 9, 1–24.
¨
Salemaa, H., Tyystjarvi-Muronen, K., Aro, E., 1986. Life histories, distribution and abundance of Mysis mixta and Mysis relicta in the northern Baltic Sea. Ophelia 4, 239–247.
Salonen, K., Sarvala, J., Hakala, I., Viljanen, M.L., 1976. The relation of energy and organic carbon in aquatic invertebrates. Limnol. Oceanogr. 21, 724–730.
Shvetsova, G.M., 1980. Orazmnozhenii dvukh vidov reliktovykh arkticheskikh mizid Rizhskogo zaliva (in Russian). Rybokozyajstvennye Issledovaniya Bassejna Baltijskogo Morya 15, 108–117.
Shvetsova, G.M., 1987. Opredelenie massy tela mizid I morskogo tarakana po ikh dline I razmeram otdel’nykh chastej tela. Fishereiliche Untersuchungen der DDR und der UdSSR in der Ostsee (in Russian, with German abstract). Fischerei-Forschung Rostock 25 (4), 66–67.
Shvetsova, G.M., Shvetsov, F.G., 1990. Produktsiya reliktovoj mizidy Mysis mixta Lilljeborg v vostochnoj i jugo- vostochnoj chastyakh Baltijskogo morya (in Russian, with English abstract). Gidrobiologicheskij Zh. 26 (1), 24–29.
Shvetsova, G., Shvetsov, F., Hoziosky, S. (Eds.), 1992. Distribution, abundance and annual production of
Mysis mixta Lilljeborg in Eastern and Southeastern Baltic, Biol. Oceanogr. Committee, International
Council for the Exploration of the Sea, Paris, Abstract.
¨ Simm, M., Kotta, I., 1992. The life cycle and production of Mysis mixta in the Gulf of Riga. In: Kohn, V.J.,
Jones, M.B., Moffat, A. (Eds.), International Expert Conference, Hiddensee, Germany, Taxonomy, Biology and Ecology of (Baltic) Mysids (Mysidacea: Crustacea), Rostock University Press, Rostock, pp. 55–60. Szaniawska, A., Wiktor, K., Jaruszewska-Nasinska, G., 1986. Seasonal changes of caloric value of Neomysis
integer (Leach) from Gdansk Bay. Zesz.Nauk. BiNoZ Oceanografia 11, 75–97.
Toda, H., Arima, T., Takahashi, M., Ichimura, S., 1987. Physiological evaluation of temperature effect on the growth processes of the mysid, Neomysis intermedia Czerniawsky. J. Plankton Res. 9, 51–63.
Turpen, S., Hunt, J.W., Anderson, B.S., Pearse, J.S., 1994. Population structure, growth, and fecundity of the kelp forest mysid Holmesmysis costata in Monterey Bay, California. J. Crustacean Biol. 14 (4), 657–664. Tyler, A.V., 1973. Caloric values of some North American invertebrates. Mar. Biol. 19, 258–261.
Wigley, R.L., Burns, B.R., 1971. Distribution and biology of mysids (Crustacea, Mysidacea) from the Atlantic coast of the United States in the NMFS Woods Hole collection. Fish Bull. 69 (4), 717–746.
Wiktor, K., Szaniawska, A., 1988. Energy content in relation to the population dynamics of Mysis mixta (Liljeborg) from the southern Baltic. Kieler Meeresforsch. Sonderh. 6, 154–161.
Winberg, G.G., 1971. Symbols, Units and Conversion Factors in Studies of Freshwater Productivity, IBP Central Office, London.
Wittmann, K.J., 1981a. Comparative biology and morphology of marsupial development in Leptomysis and
other mediterranean Mysidacea (Crustacea). J. Exp. Mar. Biol. Ecol. 52, 243–270.
Wittmann, K.J., 1981b. On the breeding physiology of marsupial development in mediterranean Leptomysis (Mysidacea: Crustacea) with special reference to the effects of temperature and egg size. J. Exp. Mar. Biol. Ecol. 53, 261–279.
Wittmann, K.J., 1984. Ecophysiology of marsupial development and reproduction in Mysidacea (Crustacea). Oceanogr. Mar. Biol. Rev. 22, 393–428.