30 Müller, M. and Knudsen, S. (1993) The nitrogen response of a barley C-hordein promoter is controlled by positive and negative regulation of the GCN4 and endosperm box. Plant J. 4, 343–355
31 Rao, V.V. et al. (1992) Developmental changes of L-lysine–ketoglutaric acid reductase in rat brain and liver. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 103, 221–224
32 Markovitz, P.J. et al. (1984) Familial hyperlysinemias. J. Biol. Chem. 259, 11643–11646
33 Deleu, C. et al. (1999) Three new osmotic stress-regulated cDNAs identified by differential display polymerase chain reaction in rapeseed leaf discs. Plant Cell Environ. 22, 979–988
34 Feller, A. et al. (1994) Repression of the genes for lysine biosynthesis in Saccharomyces cerevisiae is caused by limitation of Lys14-dependent transcriptional activation. Mol. Cell. Biol. 14, 6411–6418
35 Verhage, M. et al. (2000) Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science 287, 864–869
36 Lam, H.M. et al. (1998) Glutamic acid receptor genes in plants. Nature 396, 125–126
37 Nanjo, T. et al. (1999) Biological functions of proline in morphogenesis and osmotolerance revealed in antisense transgenic Arabidopsis thaliana. Plant J. 18, 185–193
U
nderstanding the phylogenetic relationships among theprinci-pal lineages, or clades (Box 1), of angiosperms is essential for elucidating the evolutionary events that underlie the divers-ification and ascension of this ecologically dominant plant group. We also need to reconstruct flowering-plant phylogeny to facilitate com-parative studies of plant development, metabolism, reproduction, pathology and genomics. For these and other reasons, reconstructing angiosperm phylogeny has been a major goal of plant systematists.
The state of knowledge before 1999
Attempts to unravel the overall phylogeny of angiosperms through cladistic analysis date back more than a decade1,2. Goals of such
studies include identifying the composition of major lineages, the relationships among them and the earliest lineages (first-branching clades) of flowering plants. Analyses reported before 1999 were typi-cally based on relatively small non-molecular2,3 or single-gene4–6
data matrices, with some exceptions7,8
. Many results generated dur-ing this period constituted noteworthy advances that were largely upheld by subsequent work. For example, several clades were iden-tified, including the eudicots, rosids and asterids; some previously proposed groups, including the Hamameliidae and Dilleniidae, were also shown to be assemblages of distantly related species2,4–6,8,9.
However, although a potentially accurate picture of angiosperm phylo-geny was taking shape, the plant-systematic and larger biological communities did not place great confidence in it.
In addition to obvious instances of conflict among the earlier stud-ies, systematists were aware of several other problems that tempered
their enthusiasm. One major concern was that statistical support for putative clades and the relationships among them was generally low, if investigated. A second concern was that earlier studies relied exclusively on parsimony as an optimality criterion in data analysis. However, in parsimony analyses of DNA sequences, long branches in a tree separated by short internodes can attract each other artifac-tually because of chance substitutions of identical nucleotides at homologous sequence positions10,11. Such long-branch attraction can
be engendered by using distantly related outgroups. This is because the branch leading to the outgroups attracts another long branch to the base of the ingroup (Box 1). Alternatively it can be engendered by insufficient taxon sampling, because taxonomically large groups are represented only by sparse, long branches in an analysis9,12–14
. A third concern about these earlier studies was that the available analysis protocols and computer programs employed were not well suited to analysing complex phylogenies (those with large numbers of taxa5,15,16
). Consequently, analyses of some complex phylogenies had to be stopped by the investigators before they could be com-pleted4–6
. Finally, it became clear that the amount of data being ana-lyzed was not sufficient to resolve the phylogenetic problems addressed, both because there were too few phylogenetically infor-mative characters9,12,15and because some of the apparently
informa-tive characters were potentially biased and misleading9,17
.
Breakthroughs during the past year
Beginning in late 1999, several more-rigorous, multigene studies have been published that address phylogenetic relationships among
Recent progress in reconstructing
angiosperm phylogeny
Robert K. Kuzoff and Charles S. Gasser
In the past year, the study of angiosperm phylogeny has moved from tentative inferences based on relatively small data matrices into an era of sophisticated, multigene analyses and significantly greater confidence. Recent studies provide both strong statistical support and mutual corroboration for crucial aspects of angiosperm phylogeny. These include identifying the earliest extant lineages of angiosperms, confirming Amborella as the sister of all other angiosperms, confirming some previously proposed lineages and redefining other groups consistent with their phylogeny. This phylogenetic framework enables the exploration of both genotypic and phenotypic diversification among angiosperms.
Paulo Arruda*, Edson L. Kemper, Fabio Papes and Adilson Leite are at the Centro de Biologia Molecular e Engenharia Genética, Universidade Estadual de Campinas, 13083-970, Campinas, SP, Brazil. Paulo Arruda is also in the Departamento de Genética e Evolução, IB, Universidade Estadual de Campinas, 13083-970, Campinas, SP, Brazil.
principal angiosperm lineages18–24. This surge in publications has
been facilitated by extensive collaboration among international researchers, automated sequencing technology, advances in phylo-genetic analysis software and access to increasingly powerful per-sonal computers. Some recent inquiries focus on relationships near the phylogenetic root of the flowering plants18,20–22
and others explore a broader range of angiosperm phylogeny19,23,24. These
stud-ies use data matrices of between two18,23
and 17 (Ref. 22) complete, aligned gene sequences, and include up to 560 species19,24. Inferring
trees from such large data matrices and assessing the statistical sup-port for recovered clades have been facilitated by innovations in phylogenetic analysis software16,25–27
. Major relationships elucidated by these analyses are generally not novel but, collectively, they pro-vide abundant corroboration for each other as well as previously unrealized levels of statistical support.
Although the strategies used in recent investigations differ appre-ciably, taken together, they strongly support a suite of conclusions for overall angiosperm phylogeny18–22,24
. First, these recent studies identify Amborella trichopoda as the sister group of all other angiosperms. Second, the next two successive branches in the angiosperm tree are the Nymphaeales and the ‘ITA’ (Illiciaceae, Schisandraceae, Trimeniaceae and Austrobaileyaceae) clade; Amborella, the Nymphaeales and ITA are known as the ‘ANITA’ clades20
(Figs 1 and 2). Third, the composition of several major lin-eages of angiosperms (Table 1) is consistent among all these studies (Table 2). Fourth, these studies, taken together, suggest that several relationships among these lineages have been confidently resolved, although several relationships remain unclear (Fig. 2).
Five recent studies
One of the recent landmark studies18focused on relationships near
the phylogenetic root of angiosperms, using a fairly novel approach to identify these earliest branches that does not involve sampling outgroups. All other seed plants are distantly related to angiosperms and their use as outgroups might hinder phylogeny reconstruction. To circumvent this problem, species were sampled for two paralo-gous phytochrome genes, phyA and phyC, that are duplicated only among angiosperms. Sequences of these genes from 26 species (cho-sen to repre(cho-sent the angiosperm lineages thought to be the most basal) were aligned and analyzed in concert. In the resulting phylo-genetic network (an unrooted tree), there is a branch that connects two largely symmetrical halves; one comprising all the phyA genes, the other all the phyC genes. The position of this connecting branch was used to root both halves, producing two highly concordant trees. Several studies before 1999 indicated the positive effect on phylo-genetic accuracy of increasing the number of characters sampled
per species9,12,13,15. In a recent study22, the sequences of
17 chloroplast genes (~13.4 kb) were sampled from each of 21 species, representing three gymnosperm and 18 putative basal angiosperm lineages. Primary goals of this study were to identify the first-branching lineages of angiosperms, to assess the effects of greatly increased character sampling and to determine the phylogen-etic usefulness of 14 previously untested chloroplast genes. Impor-tantly, phylogenetic analyses were conducted with and without gym-nosperm outgroups, to explore whether this would affect the relationships at the base of the angiosperms22
.
On the basis of several Monte Carlo computer simulations (method reviewed in Ref. 28), it has been argued that the judicious addition of species to phylogenetic analyses of taxonomically large groups can break up otherwise long branches and improve the accu-racy of results12–14. A third recent study19attempted to improve the
accuracy of an inferred angiosperm phylogeny by increased sam-pling of both the number of species (560) and the nucleotides per species (4733) relative to earlier studies4–8
. The genes sampled were
Box 1. Glossary
Tree
A hypothesis of phylogenetic relationships (branching order) for a group of species (sometimes genes) inferred through phylogenetic analysis25–27,32of DNA or protein sequences, non-molecular data, or
a combination of these, sampled from each species (Fig. I). The root is generally determined by the position of the branch connecting out-group (black) and inout-group (colored) species. The first branch (earli-est-diverging lineage) within the ingroup is the sister group to all other ingroup species. The most recent common ancestor (MRCA) of all ingroup species is represented by the node adjacent to the root of the tree. A tree is composed of hierarchically nested clades (phylo-genetic branches or lineages) of organisms, each comprising a MRCA for that group and all its descendants. All nodes in a com-pletely resolved tree are bifurcating. An unresolved node is a poly-tomy (clade Y).
Bootstrapping
A technique for assessing how strongly phylogenetic data support clades in a tree29. The characters in the original data set are sampled,
with replacement, until a new data set of the same size as the orig inal is generated. The generated data set is analyzed phylogeneti-cally and a summary tree produced. These two steps, constituting one replicate, are repeated a specified number of times. Bootstrap values represent the percentage of summary trees supporting a particular clade. Replicates can propagate biases present in the original data matrix.
Parsimony jackknifing
A technique for assessing how strongly phylogenetic data support clades in a tree25. Replicate data matrices are generated through
inde-pendent and random deletion of characters from the original data matrix until a user-defined fraction [typically 0.5 or 1 2(1 4e)] of the original characters remain. Summary trees are produced for each replicate data matrix through parsimony analysis. Jackknife values are the percentage of summary trees that support a clade. Replicates can propagate biases present in the original data matrix.
Trends in Plant Science
First branch of ingroup
Clade X
Clade Y
Sister to rest
of ingroup
Clade Z
MRCA of A to G
MRCA of B to F
Fig. I.
Species A Outgroup Species
Species G
Species B
Species C
Species D
Species E
Species F Root
rbcL and atpB from the chloroplast genome, and nuclear 18S rDNA. Because of the increased sampling of species, this study was the most-rigorous test of the monophyly of major angiosperm groups yet published. The many species included in this study merited the use of alternatives to standard computational techniques. For example, they used a recently developed search algorithm called the Ratchet16
(implemented by NONA 2.0, Ref 26) that finds shorter trees in par-simony analyses of complex phylogenies more quickly than other available algorithms. Also, because traditional heuristic parsimony bootstrapping29
with this number of species was impractical, this study used a more rapid method, parsimony jackknifing25(Box 1) to
assess the support for inferred clades (Table 2).
The evolution of individual genes or genomes used in phylogeny reconstruction is generally not well understood and might have a negative impact on phylogenetic analyses. In a fourth recent study20,
five genes from three genomes (nuclear, chloroplast and mitochon-drial) were sampled from 105 species, representing all major gym-nosperm and putative basal angiosperm lineages. The sampled genes, encompassing 8733 aligned nucleotides, were the same as those used in the third study, above19
, with the addition of two
mito-chondrial genes, matR and atp1. Sampling three genomes with potentially different his-tories and modes of inheritance (the nuclear genome is biparentally inherited but the chloroplast and mitochondrial genomes are generally uniparentally inherited) might miti-gate the effects of possible cryptic biases pre-sent in the individual genes or genomes. In addition, separate phylogenetic analyses were conducted for each of the five genes included in the study20.
For reasons discussed above, a fifth study21
also sampled five genes representing all three plant genomes (totaling 6564 nucleotides) for 51 species from several major angiosperm and gymnosperm lineages. These genes are nuclear 18S rDNA, chloroplast atpB and three mitochondrial genes (mtSSU, cox1 and rps2). In addition, the sequence data were analyzed with and without gymnosperm outgroups and a series of likelihood ratio tests (method reviewed in Ref. 30) were conducted to deter-mine whether the position of Amborella as sis-ter to all other angiosperms was significantly better than alternative candidates for this posi-tion. Although the other studies discussed above relied solely on parsimony, which can produce misleading results under certain cir-cumstances10,11, the data in this study21 were
reanalyzed using a model-based approach to phylogenetic inference called maximum-likelihood estimation31,32
(MLE). This analy-sis should be less sensitive to branch-length disparity, provided that the model of sequence evolution used is appropriate.
Mutual corroboration
Although there is always room to question phylogenetic results10,11,22, taken together, the
five studies reviewed above provide a well-supported picture of angiosperm phylogeny (Fig. 2; Table 2) that withstands a variety of potential criticisms. Although the effects of taxon12–14
and character9,12,13,15
sampling can dramatically affect the accuracy of inferred phylogenies, altering these factors across a wide range (21 versus 560 species and 2.2 ver-sus 13.4 kb per species; Table 2) did not alter the relationships among basal branches or the composition of major angiosperm lineages.
Long-branch attraction, whether it is due to the use of distantly related outgroups or to the presence of long ingroup branches, also does not appear to have had a negative impact on these phylogenetic results:
• Relationships near the root of the tree and the composition of major lineages were not affected by the removal of distantly related outgroups from analyses18,21,22.
• The first three branches in the trees from the five studies summa-rized above are not especially long compared with other branches in each of these studies18–22
.
• MLE provides corroboration for the branch positions that were inferred through parsimony analysis and several likelihood ratio tests detected no better rootings21.
Hence, although long-branch attraction among the earliest angio-sperm lineages cannot be decisively ruled out, it is unlikely that it affected these results.
Fig. 1. Flowers of representatives from ANITA clades. Amborella, the Nymphaeales and the
ITA (Illiciaceae, Schisandraceae, Trimeniaceae and Austrobaileyaceae) clade are known as the ‘ANITA’ clades20
. (a) Female flower of Amborella trichopoda (photograph courtesy of Sandra K. Floyd). Amborella comprises one species of dioecious, woody shrub endemic to New Caledonia. Flowers have a spirally arranged, undifferentiated perianth, a small hypanthium and either numerous more-or-less laminar stamens (male flower) or 4–8 distinct, urn-shaped carpels, with unfused margins (female flower). (b) Flower of Nymphaea sp. (Nymphaeales; photograph courtesy of Gregory M. Plunkett). Nymphaea comprises ~50 species of aquatic, rhizomatous cosmopolitanly distributed herbs. Flowers are bisexual with several undifferenti-ated tepals, numerous more-or-less laminar stamens, numerous united carpels, with fused mar-gins, and an inferior ovary. (c) Flower of Austrobaileya scandens (Austrobaileyaceae, ITA clade; photograph courtesy of Susana Magallón). Austrobaileya comprises one species of ever-green liana found in NE Australia. Flowers have numerous, undifferentiated, spirally arranged tepals, several spirally arranged, more-or-less laminar stamens, and 10–13 distinct carpels with unfused margins. (d) Flower of Illicium sp. (Illiciaceae, ITA clade; photograph courtesy of Douglas E. Soltis), Illicium comprises ~42 species of shrubs and small trees, distributed in east-ern Asia and southeast-ern North America. Flowers are bisexual, with a spirally arranged, undiffer-entiated perianth, spirally arranged stamens with short, thick filaments, and carpels with partially fused margins. All descriptions are based on Refs 33,51.
(a) (b)
Finally, these principal findings are not likely to be the result of cryptic-gene or genome biases. The positions of Amborella and the other ANITA clades in independent analyses of at least five individual genes rep-resenting all plant genomes6,18,20,23
and five multigene matrices18–22strongly suggests that
all these sources of data contain a significant, concordant phylogenetic signal. By far the simplest explanation for the congruence of these studies and their strong statistical sup-port is that they have each accurately inferred elements of the underlying organismal phy-logeny.
Impact and implications
Phenotypic evolution
The collective findings of the above studies provide a phylogenetic framework that enables additional research on several classi-cal problems in angiosperm evolution. For example, there is tremendous interest in reconstructing the patterns of diversification among angiosperms for a variety of attributes including floral and vegetative morphology, metabolism, modes of reproduction, con-stituent gene families, and genomes18–22,33.
This can be accomplished by mapping those characters onto individual trees, tracing their evolution throughout the phylogeny and reconstructing character states for ancestral taxa (e.g. using MacClade 3.07, Ref. 34).
Comparisons of features of families in the ANITA clades, in their phylogenetic context, provide insight into the origin and early diversification of flowering plants18,20,21,33
. For example, the vegetative body of the com-mon ancestor of all extant angiosperms prob-ably had a woody habit, vessel-less wood, unilacunar leaf nodes with two traces, leaves with chloranthoid teeth along their margins and no ethereal oils. Flowers of this common ancestor probably had an undifferentiated perianth arranged in more than two cycles or series, perianth appendages that were unfused above the base and anthers that shed pollen
towards the center of the flower. Carpels in these flowers were urn-shaped (ascidiate), were not attached to one another (apocarpous) and had margins that did not fuse completely but were closed at maturity by secretions. An herbaceous habit, ethereal oils, wood with vessels, differentiated sepals and petals, and complete carpel closure probably evolved after the origin of flowering plants. These derived features might have contributed to the rapid diversification of later-evolved angiosperm lineages (Table 1). The emerging pic-ture of the earliest angiosperms differs appreciably from previously proposed models for the first angiosperms (reviewed in Refs 35–37). Recent results from studies of all the seed-plant groups suggest that the extant gymnosperms form a monophyletic group that is the sister lineage to the angiosperms20,36,37 (Fig. 2). This contradicts
some earlier models that placed one group of gymnosperms, the Gnetales, as the sister to all angiosperms. Consequently, future clues to the morphological transitions that led to the origin of the
angiosperms will come largely from fossil33,36 and comparative
molecular-genetic data37,38
.
Genetic basis of phenotypic evolution
In general, understanding genotypic or phenotypic diversification requires knowledge of an organismal phylogeny to specify the locations, frequency and directionality of character state changes within a lineage. The phylogenetic results discussed above provide the requisite framework to dissect the evolution of genes, gene families and genomes, and to relate these events to morphological evolution. Molecular and phylogenetic dissection of petal and ovule evolution illustrate this point (additional examples are reviewed in Ref. 38).
It has been argued on the basis of comparative morphology that, among angiosperms, superficially similar petals have evolved sev-eral times from either stamens (andropetals) or sepals (bracteo-petals)38
. How frequently each has occurred can be elucidated only by the combined analysis of comparative morphology and gene-expression and gene-family evolution in the context of angiosperm phylogeny38–40. Recent results indicate that, among the eudicots,
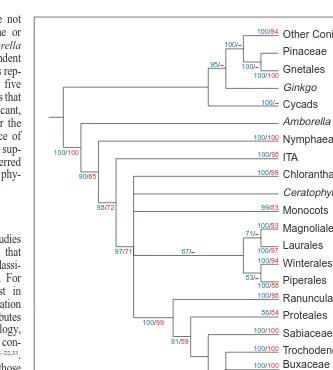
sep-arate lineages of AP3 homologs have been recruited in independent Fig. 2. Simplified ‘supertree’52
based on five recent multigene phylogenies for angiosperms18–22
. The supertree is the strict consensus of 36 shortest trees recovered from a branch-and-bound analysis of clades in individual multigene studies (source trees) using matrix representation with parsimony52
and a weighting scheme based on the support values of branches in the source trees (,50% 51; 51–70% 52; 71–90% 53; 91–100% 54). Support values for clades in the supertree are based on parsimony bootstrap29
(blue) and jackknife25
(red) analyses from two source trees (‘2’ indicates ,50% support). Support val-ues from other source trees are listed in Table 2. Relationships among gymnosperms shown here are consistent with recent results from analyses of seed plants36
. Abbreviations: ITA clade, consists of Illiciaceae, Schisandraceae, Trimeniaceae and Austrobaileyaceae.
Trends in Plant Science
evolutionary events to determine petal identity in the Ranunculales and in the Rosids and Asterids39,40
. Among the grasses, AP3-homolog expression is conserved in petals but other genetic factors have been altered, converting the familiar petals into lodicules38,41
. Although the directionality of change in these cases is fairly uncon-troversial, the exact origins and frequency of these transformations remain unclear. Determining these will require testing additional organisms, selected according to the available phylogeny. In addi-tion, an understanding of the general applicability of other aspects of floral development in model organisms will be achieved through future phylogenetically informed research42.
The Arabidopsis INNER NO OUTER (INO) gene has been shown to be essential for the asymmetric growth of ovule outer integu-ments43
. Gene-expression patterns in wild-type and mutant Ara-bidopsis were compatible with INO acting either to establish abaxial–adaxial polarity in ovules or to promote outer integument outgrowth directly. The phylogenetic results discussed above permit the selection of angiosperm lineages that are radially symmetrical and bitegmic or asymmetrical (polar) and unitegmic in order to test the two models by examining the ancestral function of INO orthologs through comparative gene-expression studies. Tests of these two models through comparative study of expression of INO orthologs isolated from diverse species, selected according to the lat-est phylogenies, are in progress [R. Kuzoff et al., unpublished (http://www.ou.edu/cas/botany-micro/botany2000/section2/ abstracts/28.shtml)].
Genome evolution
A comparison of the molecular phylogeny of group-I introns and overall angiosperm phy-logeny suggests that group-I introns have been independently acquired ~1000 times by various angiosperms44
. Whether these introns have been acquired independently from fun-gal donors or through horizontal transfer from plants is unclear but it has important implications for the potential for genetic transfer from genetically engineered crop species to local flora. Resolving this ques-tion will depend on expanded analyses of group-I introns in the context of angiosperm phylogeny.
Phylogenetic analysis of genome size among the grasses reveals a predominantly uni-directional trend towards genomic ‘obesity’45
. Whether genomes evolve towards greater size in general among angiosperms can now be explored in other well-defined angiosperm lineages. Genome doubling through poly-ploidy is a common phenomenon in the his-tory of angiosperms and is especially prevalent in crop species42. The number of
polyploid origins and their parentage, conse-quences for gene evolution and biochemical impact can now be dissected in the context of overall angiosperm phylogeny.
Classification
Several authors have discussed the inadequa-cies of previously available classification sys-tems for flowering plants9,35,46
. Foremost among their concerns is that these classifi-cations are at odds with robust results of pub-lished phylogenetic analyses. In an effort to address this need, the Angiosperm Phylogeny Group (APG), a consortium of over 40 plant systematists, has pro-posed a working classification of angiosperms into orders and some higher-level groupings46. Their provisional classification system was
based on results of single4–6
and preliminary multigene19
studies. The additional phylogenetic results summarized above are entirely con-cordant with their classification system, suggesting that it should be retained and extended. The proposed APG classification is useful for facilitating communication within and among disciplines of biology and presentation of fruitful research to the broader community.
Future directions
Although much of the overall angiosperm phylogeny is now confi-dently resolved, polytomies (Box 1) among magnoliid clades, Ceratophyllum and the monocots, as well as within the eudicots, require additional study (Fig. 2). The enormous effort that has already been put into these studies might lead to pessimism about the prospects for additional progress. Fortunately, there are several resources that can be brought to bear on the problem, which will clarify relationships further. For example, several available sources of data have not been fully exploited. Morphological data are being reanalyzed in light of the results discussed above to reveal previous misleading homology assessments, enhancing their utility in sub-sequent analyses and facilitating the incorporation of crucial fossil data33,35,36
.
Similar analyses of molecular data9,15,22,23will reveal hidden
ten-dencies in nucleotide substitution and permit the generation of
Table 1. Several lineages (clades) of flowering plants recognized in recent phylogenetic analyses43
Clade Families Examples
(species)
Amborella 1 (1) Amborella trichopoda
Nymphaeales 2 (81) Water lilies (Nymphaea spp.)
‘ITA’a 4 (95) Star anise (Illicium verum)
Chloranthales 1 (75) Chloranthus, Ascarina
Ceratophyllum 1 (6) Ceratophyllum
Monocots 102 (65 000) Grasses, orchids, palms
Magnoliales 6 (2700) Magnolia, tulip tree (Liriodendron tulipifera)
Laurales 7 (3400) Sassafras, avocado (Persea americana)
Winterales 2 (73) Winter’s bark (Drimys)
Piperales 4 (3500) Black pepper (Piper)
Ranunculales 7 (4400) Buttercup (Ranunculus spp.), poppy (Papaver spp.)
Proteales 3 (1600) Sacred lotus (Nelumbo), plane (Platanus spp.)
Caryophyllales 26 (9400) Cactus (Cactaceae), beet (Beta vulgaris), spinach (Spinacia oleracea)
Asterids 107 (87 000) Sunflower (Helianthus spp.), carrot, tomato, holly (Ilex spp.), snapdragon (Antirrhinum majus)
Rosids 149 (77 000) Maple (Acer spp.), apple, pea, rose, Arabidopsis
a
more-realistic models of sequence evolution. These clarified models will inform and increase the accuracy of subsequent model-based phylogenetic analyses30–32
. Also, the molecules used in several of the analyses described above18,21,22and elsewhere47can be sampled from
additional species and combined with existing data for more detailed analyses of relationships within principal angiosperm clades, such as the monocots, eudicots, rosids and asterids. Other complex phy-logenies, in the grasses for example, required as many as eight inde-pendent data sets before complete resolution with strong support was achieved48.
Computational strategies such as compartmentalization5,49,50
, in which large, well-supported clades are replaced in an analysis by an inferred ancestral state for that clade, also appear to be promising. In fact, an expanded analysis of 26S rDNA (Ref. 47) and other sequences20
using such compartmentalization of angiosperm clades
has produced complete resolution among the basal lineages and with stronger support than was achieved previously (M. Zanis et al., pers. commun.). We are optimistic that angiosperm phylogenetics will progress rapidly on its present course, facilitating additional compara-tive studies and fuller exploitation of knowledge garnered from model research organisms.
Acknowledgements
We apologize to those whose work could not be included in this re-view owing to space limitations. We thank Olaf Bininda-Emonds, Jim Doyle, Sean Graham, Toby Kellogg, Jessie McAbee, Greg Plunkett, Vincent Savolainen, Doug and Pam Soltis, and Michael Zanis for helpful discussions, and the Katherine Esau Postdoctoral Fellowship and the National Science Foundation (IBN-9983354) for financial support.
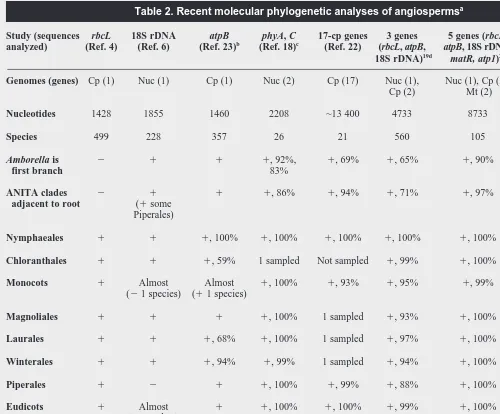
Table 2. Recent molecular phylogenetic analyses of angiospermsa
Study (sequences rbcL 18S rDNA atpB phyA, C 17-cp genes 3 genes 5 genes (rbcL, 5 genes (atpB,
analyzed) (Ref. 4) (Ref. 6) (Ref. 23)b
(Ref. 18)c
(Ref. 22) (rbcL, atpB, atpB, 18S rDNA, 18S rDNA, cox1, 18S rDNA)19d
matR, atp1)20
rps2, mtSSU)21
Genomes (genes) Cp (1) Nuc (1) Cp (1) Nuc (2) Cp (17) Nuc (1), Nuc (1), Cp (2), Nuc (1), Cp (1), Cp (2) Mt (2) Mt (3)
Nucleotides 1428 1855 1460 2208 ~13 400 4733 8733 6564
Species 499 228 357 26 21 560 105 51
Amborella is 2 1 1 1, 92%, 1, 69% 1, 65% 1, 90% 1, 89%
first branch 83%
ANITA clades 2 1 1 1, 86% 1, 94% 1, 71% 1, 97% 1, 92%
adjacent to root (1some
Piperales)
Nymphaeales 1 1 1, 100% 1, 100% 1, 100% 1, 100% 1, 100% 1, 100%
Chloranthales 1 1 1, 59% 1 sampled Not sampled 1, 99% 1, 100% 1 sampled
Monocots 1 Almost Almost 1, 100% 1, 93% 1, 95% 1, 99% 1, 92%
(21 species) (11 species)
Magnoliales 1 1 1 1, 100% 1 sampled 1, 93% 1, 100% 1, .90%
Laurales 1 1 1, 68% 1, 100% 1 sampled 1, 97% 1, 100% 1, .90%
Winterales 1 1 1, 94% 1, 99% 1 sampled 1, 94% 1, 100% 1 sampled
Piperales 1 2 1 1, 100% 1, 99% 1, 88% 1, 100% 1
Eudicots 1 Almost 1 1, 100% 1, 100% 1, 99% 1, 100% 1, 100%
(13 orders)
Rosids 1 Almost Almost Not sampled 1 sampled 1, 60% Not sampled 1
(11 order) (21 order)
Asterids 1 Almost 1, 66% Not sampled 1 sampled 1, 99% Not sampled 1, .90%
(11 order)
aClades that are recovered (
1) or not recovered (2) and bootstrap support values29(unless otherwise indicated) are indicated in each analysis. Names for lineages generally follow Ref. 46, except for Winterales and Chloranthales, which were recognized in more recent analyses19,20
. ‘1 sampled’ indicates clades that were not tested for monophyly, because only one species was sampled.
bAlthough both atpB and rbcL sequences were analyzed23, results summarized here are based on atpB alone.
cBootstrap values were inferred for the clade comprising all species other than Amborella in the separate phyA and phyC trees18; all other bootstrap values in this analysis came from the analysis of concatenated phyA and phyC sequences.
References
01 Dahlgren, R. and Bremer, K. (1985) Major clades of angiosperms. Cladistics 1, 349–368
02 Donoghue, M.J. and Doyle, J.A. (1989) Phylogenetic analysis of angiosperms and relationships of Hamamelidae. In Evolution, Systematics, and Fossil History of the Hamamelidae (Crane, P.R. and Blackmore, S., eds), pp. 17–45, Clarendon Press
03 Nixon, K.C. et al. (1994) A reevaluation of seed plant phylogeny. Ann. MO Bot. Gard. 81, 484–533
04 Chase, M.W. et al. (1993) Phylogenetics of seed plants: an analysis of nucleotide sequences from the plastid gene rbcL. Ann. MO Bot. Gard. 80, 526–580 05 Rice, K.A. et al. (1997) Analysing large data sets: rbcL 500 revisited. Syst. Biol.
46, 554–563
06 Soltis, D.E. et al. (1997) Angiosperm phylogeny inferred from 18S ribosomal DNA sequences. Ann. MO Bot. Gard. 84, 1–490
07 Doyle, J.A. et al. (1994) Integration of morphological and ribosomal RNA data on the origin of angiosperms. Ann. MO Bot. Gard. 81, 419–450
08 Nandi, O.I. et al. (1998) A combined cladistic analysis of angiosperms using rbcL and non-molecular data sets. Ann. MO Bot. Gard. 85, 137–212
09 Chase, M.W. and Cox, A.V. (1998) Gene sequences, collaboration and analysis of large data sets. Aust. Syst. Bot. 11, 215–229
10 Felsenstein, J. (1978) Cases in which parsimony or compatibility methods will be positively misleading. Syst. Zool. 27, 401–410
11 Kim, J. (1996) General inconsistency conditions for maximum parsimony: effects of branch lengths and increasing numbers of taxa. Syst. Biol. 45, 363–374 12 Hillis, D.M. (1996) Inferring complex phylogenies. Nature 383, 130–131 13 Graybeal, A. (1998) Is it better to add taxa or characters to a difficult phylogenetic
problem? Syst. Biol. 47, 9–17
14 Rannala, B. et al. (1998) Taxon sampling and the accuracy of large phylogenies. Syst. Biol. 47, 702–710
15 Soltis, D.E. et al. (1998) Inferring complex phylogenies using parsimony: an empirical approach using three large DNA datasets for angiosperms. Syst. Biol. 47, 32–42
16 Nixon, K. (1999) The parsimony Ratchet: a rapid means for analysing large data sets. Cladistics 15, 407–414
17 Kellogg, E.A. and Juliano, N.D. (1997) The structure and function of Rubisco and their implications for systematic studies. Am. J. Bot. 84, 413–428
18 Mathews, S. and Donoghue, M.J. (1999) The root of angiosperm phylogeny inferred from duplicate phytochrome genes. Science 286, 947–949
19 Soltis, P.S. et al. (1999) Angiosperm phylogeny inferred from multiple genes as a tool for comparative biology. Nature 402, 402–404
20 Qiu, Y-L. et al. (1999) The earliest angiosperms: evidence from mitochondrial, plastid and nuclear genomes. Nature 402, 404–407
21 Parkinson, C.L. et al. (1999) Multigene analyses identify the three earliest lineages of extant flowering plants. Curr. Biol. 9, 1485–1488
22 Graham, S.W. and Olmstead, R.G. Utility of 17 chloroplast genes for inferring the phylogeny of the basal angiosperms. Am. J. Bot. (in press)
23 Savolainen, V. et al. (2000) Phylogenetics of flowering plants based upon a combined analysis of plastid atpB and rbcL gene sequences. Syst. Biol. 49, 306–362
24 Soltis, D.E. et al. Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB sequences. Bot. J. Linn. Soc. (in press)
25 Farris, J.S. et al. (1996) Parsimony jackknifing outperforms neighbor-joining. Cladistics 12, 99–124
26 Goloboff, P. (1998) NONA, Computer Program and Software, Version 2.0, Tucuman, Argentina, P. Goloboff
27 Swofford, D.L. (1998) PAUP*: Phylogenetic Analysis Using Parsimony (and Other Methods), Version 4.0, Sinauer
28 Huelsenbeck, J.P. and Hillis, D.M. (1996) Parametric bootstrapping in molecular phylogenetics: applications and performance. In Molecular Zoology: Advances, Strategies, and Protocols (Ferraris, J.D. and Palumbi, S.R., eds), pp. 19–45, Wiley–Liss
29 Felsenstein, J. (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39, 783–791
30 Huelsenbeck, J.P. and Rannala, B. (1997) Phylogenetic methods come of age: testing hypotheses in an evolutionary context. Science 276, 227–232 31 Yang, Z. (1997) How often do wrong models produce better phylogenies? Mol.
Biol. Evol. 14, 105–108
32 Lewis, P.O. (1998) Alternatives to parsimony for inferring phylogeny using nucleotide sequence data. In Molecular Systematics of Plants (Vol. II) (Soltis, D.E. et al., eds), pp. 132–163, Kluwer
33 Doyle, J.A. and Endress, P.K. Morphological phylogenetic analysis of basal angiosperms: comparison and combination with molecular data. Int. J. Plant Sci. (in press)
34 Maddison, W.P. and Maddison, D.R. (1992) MacClade: Analysis of Phylogeny and Character Evolution, Version 3.07, Sinauer
35 Doyle, J.A. (1998) Phylogeny of vascular plants. Annu. Rev. Ecol. Syst. 29, 567–599
36 Donoghue, M.J. and Doyle, J.A. (2000) Seed plant phylogeny: demise of the anthophyte hypothesis? Curr. Biol. 10, R106–R109
37 Frohlich, M.W. and Parker, D.S. (2000) The mostly male theory of flower evolutionary origins: from genes to fossils. Syst. Bot. 25, 155–170
38 Lawton-Rauh, A.L. et al. (2000) Molecular evolution of flower development. Trends Ecol. Evol. 15, 144–149
39 Kramer, E.M. et al. (1998) Molecular evolution of genes controlling petal and stamen development: duplication and divergence within the APETELA3 and PISTILLATA MADS-box gene lineages. Genetics 149, 765–783
40 Kramer, E.M. and Irish, V.F. (1999) Evolution of genetic mechanisms controlling petal development. Nature 399, 144–148
41 Ambrose, B.A. et al. (2000) Molecular and genetic analyses of the Silky1 gene reveal conservation in floral organ specification between eudicots and monocots. Mol. Cell 5, 569–579
42 Soltis, D.E. and Soltis, P.S. (2000) Contributions of plant molecular systematics to studies of molecular evolution. Plant Mol. Biol.
42, 45–75
43 Villanueva, J.M. et al. (1999) INNER NO OUTER regulates abaxial–adaxial patterning in Arabidopsis ovules. Genes Dev. 13, 3160–3169
44 Cho, Y. et al. (1998) Explosive invasion of plant mitochondria by a group I intron. Proc. Natl. Acad. Sci. U. S. A. 95, 14244–14249
45 Bennetzen, J.L. and Kellogg, E.A. (1997) Do plants have a one-way ticket to genomic obesity? Plant Cell 9, 1509–1514
46 Angiosperm Phylogeny Group (1998) An ordinal classification for families of flowering plants. Ann. MO Bot. Gard. 85, 531–553
47 Kuzoff, R.K. et al. (1998) The phylogenetic potential of entire 26S rDNA sequences in plants. Mol. Biol. Evol. 15, 251–263
48 Grass Phylogeny Working Group A phylogeny of the grass family (Poaceae), as inferred from eight character sets. In Proceedings of the Second
International Conference on the Comparative Biology of the Monocots: Grasses – Systematics and Evolution (Vol. 2) (Jacobs, S.W.L. and Everett, J.E., eds), CSIRO (in press)
49 Mishler, B.D. (1994) Cladistic analysis of molecular and morphological data. Am. J. Phys. Anthropol. 94, 143–156
50 Bininda-Emonds, O.R.P. et al. (1998) Supraspecific taxa as terminals in cladistic analysis: implicit assumptions of monophyly and a comparison of methods. Biol. J. Linn. Soc. 64, 101–133
51 Mabberley, D.J. (1997) The Plant Book: A Portable Dictionary of the Vascular Plants (2nd edn), Cambridge University Press
52 Sanderson, M.J. et al. (1998) Phylogenetic supertrees: assembling the trees of life. Trends Ecol. Evol. 13, 105–109
Robert K. Kuzoff*and Charles S. Gasser are at the Section of Molecular and Cellular Biology, University of California, Davis, CA 95616, USA.