Recent papers have explored early events in the development of simple leaves. Functional compartmentalization of the shoot apical meristem correlates with distinct fields of cells
connected by plasmodesmata. Molecules important in the initiation of phyllotactic pattern are described and the relationship between dorsoventral patterning and lateral leaf expansion is investigated.
Addresses
3609 Plant Sciences, Botany Department, University of Georgia, Athens, GA 30602, USA; e-mail: [email protected]
Current Opinion in Plant Biology2000, 3:31–36
1369-5266/00/$ — see front matter © 2000 Elsevier Science Ltd. All rights reserved.
Abbreviations CZ central zone
knox knotted1class of homeobox
PZ peripheral zone
SAM shoot apical meristem
Introduction
Amid the vast diversity in plant morphology, no organ con-tributes more to phenotypic variation than the leaf. Plants have evolved various spatial arrangements of leaves about the stem (phyllotaxy), and disparate varieties of leaf size, shape and complexity. Despite this inherent variability, at least two developmental characters define most if not all leaves [1,2]: namely leaves arise from a relatively large number of progenitor ‘founder cells’ [3] recruited from the periphery of the shoot apical meristem; leaves are dor-siventrally asymmetrical (bifacial) at their inception.
This review describes recent insights into leaf develop-mental mechanisms, focusing primarily on investigations of simple leaves in the monocot maize plant, and in the dicots Arabidopsis, tobacco and snapdragon (Antirrhinum). Interested readers are directed to more historically com-prehensive reviews [4–6] for discussions of compound leaf development, and simple leaf development from nonmod-el organisms. This review emphasizes newly published research (from 1998 to present) relevant to the develop-ment of morphological diversity between monocot and dicot leaf forms. Two early stages in the development of simple leaves are discussed (see also [4,5]). The first stage is characterized by leaf founder-cell recruitment, wherein the transition from meristematic identity to leaf identity occurs. The second stage engenders the expansion of the founder cells into a young leaf primordium.
Meristem-leaf transition stage
The shoot apical meristems (SAMs) of maize and the dicots Arabidopsis, tobacco and Antirrhinumexhibit similar patterns of zonation and structure [7]. Leaf organogenesis occurs in a
lateral region of relatively high mitotic index called the meristem ‘peripheral zone’ (PZ). The meristem ‘central zone’ (CZ), characterized by a lower rate of cell division, replenishes meristematic cells contributed to developing leaves. The expression profiles of the knotted1 class of homeobox (knox) genes in maize [8–13], and those of orthol-ogous genes in Arabidopsis [14,15], mirror this pattern of meristem zonation. Accordingly, knoxgenes are expressed at very high levels in the CZ, and are downregulated in the PZ of the meristem. These data indicate that the downregula-tion of knox genes might contribute to leaf founder-cell determination during development of simple leaves.
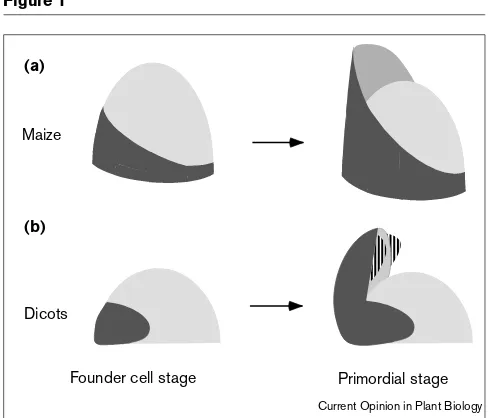
The pattern and extent of knox downregulation in the SAM also reflects morphological differences of leaf bases in monocots and dicots. For example, maize leaves, in which the leaf bases completely ensheath the circumfer-ence of the stem into which they are inserted [1,2], display knoxdownregulation that extends from one meristem flank to the opposite pole ([8,9]; Figure 1). In contrast, the leaf bases of Arabidopsis exhibit far less spreading around the stem than those of maize; this phenotype is correlated with stm1 homeobox downregulation that is markedly more localized ([15]; Figure 1). The molecular pathway leading to founder-cell recruitment, and the specific role for knox genes in this process, remain unknown; however, recent analyses of symplasmic fields in plant meristems illumi-nate a route for the channeled movement of proposed signaling molecules during founder-cell recruitment stages of leaf morphogenesis [16••,17••].
Symplasmic fields are groups of plant cells united by plas-modesmatal connections. Rinne and van der Schoot [16••]
and Gisel et al. [17••] tracked the movement of fluorescent
dyes into vegetative SAMs in birch and Arabidopsis, respectively. These studies showed that the PZ and CZ of the outer meristem layer comprise distinct and separate symplasmic fields. In the early initiation stages of founder-cell recruitment [8,15], however, a transient connection between the PZ and CZ is established [16••]. Therefore,
signaling molecules might theoretically move between symplasmic fields and initiate leaf morphogenesis.
Spatial patterning of founder cell recruitment:
phyllotaxy
The geometrically-precise placement of leaves about the stem — a process that ultimately depends upon the pat-tern of founder-cell recruitment in the SAM — has long intrigued experimental botanists [18,19]. Surgical studies demonstrated that the close proximity of existing leaf pri-mordia inhibits the initiation of new leaves from the SAM [20]. The nature of this purported inhibitory factor, albeit chemical or physical or both, has been the subject of botanical debate.
Recent research into the maize mutationterminal ear1has shed new light on the question of phyllotaxis. Veit et al. [21••] found that the terminal ear1gene encodes a mRNA
binding protein that is expressed in a horseshoe configura-tion subtending leaf primordia. The open end of the horseshoe corresponds to the position of young primordia, including the founder cells of the incipient leaf prior to any visible morphogenesis. These researchers postulate that the TERMINAL EAR protein might function to regulate phyllotaxis in maize shoots via inhibition of leaf initiation within its expression domain. Consistent with this predic-tion, terminal ear mutants produce superfluous numbers of leaves in an disorganized pattern. Characterization of the, as yet unknown, target(s) of the TERMINAL EAR pro-tein promises to divulge crucial information to our understanding of the earliest events in leaf development.
Another maize mutation impacting phyllotaxy is abphyl1 (aberrant phyllotaxy) [22,23•]. This intriguing mutant
dis-plays dechussate (two leaves per node) phyllotaxis, which is common to many dicot plants. Normal maize, like all grasses, produces vegetative leaves in an alternate (a single leaf per node) phyllotaxis. The dechussate phenotype is especially pronounced in abphyl mutant embryos and
seedlings, and is correlated with an abnormally large meris-tem. Reversion of the mutant phenotype to normal phyllotaxy is common in upper leaves, accompanied by concomitant restoration of normal meristem size. Jackson and Hake [23•] note that a correlation between SAM size
and change in phyllotaxy is characteristic of dicot species that exhibit disparate phyllotaxies during their life cycle, although cause and effect can not as yet be determined.
A direct role for biophysics in leaf initiation is championed by researchers studying the interaction of externally administered EXPANSINS and shoot meristems ([24,25,26••]; see also review by Cosgrove, pp 73–78). In
these studies, the placement of beads soaked with EXPANSINS — proteins that increase extensibility of plant cell walls — induced leaf primordial development from tomato meristems. The EXPANSIN-induced organs satisfy several leaf developmental criteria, including dorsoventrality and an influence on the positioning of incipient leaves. In addition, Reinhardt et al.[26••] show
that the expansin gene LeExp18 is expressed in tomato SAMs at the site of incipient leaf formation. Nonetheless, the primordia that emerge from EXPANSIN-treated meristems do not complete the full program of leaf mor-phogenesis, but cease development in early primordial stages [24,25]. The abortion of leaf development is ascribed to the external application of EXPANSINS, which are normally produced endogenously [25]. Kuhlenmeier and colleagues [24,25,26••] argue that their
results support a biophysical model for leaf development [27,28], in which meristematic stresses result in localized buckling of the SAM and subsequent initiation of leaf development. In this view, EXPANSIN-induced cell wall loosening causes bulging of the meristem, which in turn elicits morphogenesis. Additional data are required to clar-ify whether EXPANSIN accumulation is an initiator of, or merely a later response to leaf morphogenetic programs.
The progression from founder cells to a leaf
primordium
The knoxgene family [9,11] was first identified by dominant mutations that cause ectopic expression of these meristem-atic genes in developing leaf primordia [8,10,12,13,29,30]. Although all of the dominant Knox mutations result in simi-lar transformations of distal blade tissue into proximal sheath-like tissue, a fascinating aspect of the Knox mutants is the leaf domain-specific perturbations associated with individual Knox mutant phenotypes (reviewed in [31]). Two newly-cloned members of this mutational class are gnarley1 [12,32•] and liguless3(lg3) [13], each characterized by
domi-nant regulatory mutations that transform leaf cell/tissue identities. Consistent with previous analyses of other Knox mutations, lg3is normally expressed in shoot apices, where-as Gnarley1 and Lg3 mutations result in the ectopic accumulation of KNOX proteins in leaf primordia. Clonal analyses indicate that the Gnarley1mutant gene-product is cell autonomous in the lateral dimension of the leaf, but non-cell autonomous in the transverse dimension [32•].
Figure 1
Early development of simple leaves. Model for stages in early leaf development in (a) maize and (b) typical dicots. In (a) maize, founder cell (darkly shaded) recruitment begins on one meristematic flank and expands laterally to completely surround the SAM (lightly shaded). The hooded primordium that subsequently emerges from the SAM displays dorsoventral asymmetry and envelops the SAM because the lateral domains of the leaf blade and sheath are established during founder cell recruitment. In (b) dicots, however, founder cell recruitment does not proceed extensively around the SAM. The peg-like primordium that emerges, although dorsoventrally asymmetrical, contains a mildly expanded leaf base but does not have a prominent lamina. Interaction of dorsal and ventral domains in the distal regions of the primordium induces expansive growth of the dicot lamina (striped). The petiole forms from the unexpanded region of the primordium, between the proximal leaf base and the distal lamina.
maize maize
Maize
Founder cell stage Primordial stage Dicots
(a)
(b)
An exciting development in knox gene research is the iden-tification of genes regulating knox gene expression [33••–35••,36]. The phantasticagene encodes a member of
a small subfamily of MYB-related transcription factors [33••] required for the proper regulation of a knoxgene in
Antirrhinum[34••,35••]. Waites et al.[33••] show that
phan-tastica is transcribed in developing lateral organs and, therefore, represents the first molecular marker found to be expressed in leaf founder cells.
Leaf dorsoventrality and lateral leaf expansion
Hudson and colleagues [33••,36] describe phantastica
mutant leaves as defective in several aspects of leaf devel-opment, including lateral expansion of the lamina, dorsoventrality and proximal-distal patterning. A severe phenotype of phantastica mutants is a radialized leaf, inter-preted as lacking dorsal leaf identity [36]. These researchers propose that PHANTASTICA promotes dorsal leaf identity, which is required to initiate lateral growth [33••,36]. This model is based upon the premise that dicot
leaves, which emerge from the meristem as peg-shaped buttresses [2–5,7], undergo lateral expansion of the lamina during primordial developmental stages (Figure 1). In this view, dorsal (adaxial) and ventral (abaxial) leaf identity is established at the founder-cell stage. Subsequently, the juxtaposition of dorsal and ventral domains at the margins of the dicot leaf primordium results in the elaboration of leaf blade (lamina) development along a new lateral axis.
In contrast, the leaf blade domains of monocot grasses are established prior to the primordial stage, as illustrated by the flattened stem-encircling primordia of newly initiated grass
leaves ([1–3]; Figure 1). Analyses of the mutant narrow sheath, which conditions the pre-primordial deletion of lateral leaf domains [37,38], also support a model for founder-cell-staged recruitment of leaf blade domains in maize.
Interpretations of the phenotypes of several new leaf pattern mutants are inspired from the proposed model for PHAN-TASTICA function [33••], wherein lateral leaf expansion
requires the interaction of dorsal (adaxial) and ventral (abax-ial) identities. Analyses of the bladeless tobacco leaf mutant lam1 [39,40] demonstrate that the LAM1 ‘dorsalizing factor’ is required in L3-derived layers of the shoot (i.e. the inner-most layers of the SAM) in order to establish dorsal differentiation and lateral growth in leaf primordia.
The dicot model for lateral leaf expansion presupposes that ventralizing, as well as dorsalizing mutations, should likewise condition radialized leaves devoid of lamina. As predicted, the dominant mutation phabulosa1 produces dorsalized (adaxialized) leaves that often display radial phenotypes and fail to develop leaf blades [41•]. A striking
phenotype observed in severe phabulosa mutants is the development of ectopic meristems surrounding the bases of adaxialized leaves. This phenotype, and the adaxialized expression pattern of the putative meristem-forming factor PINHEAD [42••], strongly suggests that the adaxial leaf
domain renders competency to develop SAMs. McConnell and Barton [41•] propose that PHABULOSA may be
required to specify adaxial cell fate, although these predic-tions are tempered by the inherent difficulties in discerning the wild-type function of genes identified by dominant, gain-of-function mutations.
Figure 2
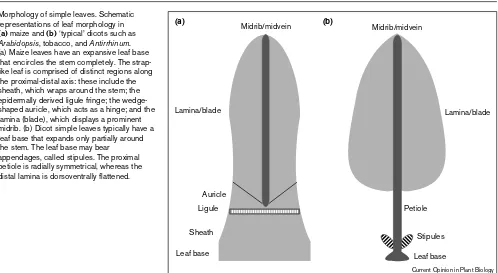
Morphology of simple leaves. Schematic representations of leaf morphology in
(a)maize and (b) ‘typical’ dicots such as
Arabidopsis, tobacco, and Antirrhinum. (a) Maize leaves have an expansive leaf base that encircles the stem completely. The strap-like leaf is comprised of distinct regions along the proximal-distal axis: these include the sheath, which wraps around the stem; the epidermally derived ligule fringe; the wedge-shaped auricle, which acts as a hinge; and the lamina (blade), which displays a prominent midrib. (b) Dicot simple leaves typically have a leaf base that expands only partially around the stem. The leaf base may bear
appendages, called stipules. The proximal petiole is radially symmetrical, whereas the distal lamina is dorsoventrally flattened.
Petiole
Stipules Midrib/midvein
Lamina/blade Midrib/midvein
Lamina/blade
Leaf base Leaf base
Sheath Ligule
Auricle
(a) (b)
The maize allele leafbladeless (ragged seedling1) is the first proposed dorsoventral leaf mutation affecting monocot leaves [43•]. In keeping with a model wherein lateral leaf
domains are established at the founder-cell stage in grass leaves, Timmermans et al. [43•] report that this interesting
mutant disrupts dorsoventrality and founder-cell recruit-ment in maize lateral organs. Severe leafbladeless phenotypes include radial ‘abaxialized’ leaves, although the presence of a ligule (an adaxial leaf elaboration, see Figure 2) on radial leaves might indicate that LEAF-BLADELESS is required only for lateral leaf development and not the establishment of dorsiventrality.
An alternative view of the radialized phantastica mutant phenotype has been offered by analyses of the orthologous gene product in maize [34••,35••,44•]. The recessive rough
sheath2mutation causes ectopic expression of at least three different knoxgenes in mutant leaves [44•], and conditions
leaf phenotypes resembling those observed in dominant neomorphic knox mutants [29,31,44•]. A regulator of knox
gene expression in maize shoots, rough sheath2is shown to encode a MYB-like product orthologous to the PHAN-TASTICA gene product [34••,35••]; however, unlike
phantastica, rough sheath2does not affect leaf dorsoventrali-ty. Timmermans et al. [34••] suggest that the disparate
phenotypes of these orthologous mutations may be a result of differences in developmental timing of expression of these genes, or perhaps different molecular targets of the gene products in the dicot and monocot shoots. Tsiantis et al. [35••] prefer a different interpretation. These
researchers suggest that the rough sheath2 and phantastica genes perform similar roles in leaf development, and the dissimilar mutant phenotypes simply reflect differences in maize and dicot leaf morphology (Figure 2). Specifically, loss of knoxgene repression in rough sheath2 mutant maize leaves causes the acquisition of proximal developmental fates, such as sheath and ligule/auricle identity (Figure 2), in distal leaf domains of the blade [35••,45]. Because the
proximal region in dicot leaves is comprised of the radially symmetrical petiole (Figure 2), however, loss of PHAN-TASTICA function in Antirrhinum results in a ‘proximalized’ and unifacial lamina. In this light, PHAN-TASTICA might not be seen to function as a dorsalizing factor in the leaf at all. Consistent with this alternative view, phantasticatranscripts are not restricted to the dorsal domain of leaf primordia but are expressed throughout the young leaf [33••].
Three members of the YABBYgene family of Arabidopsis display specific, polar expression patterns predicted for genes that specify dorsoventral identity [46••]. Proposed to
encode overlapping functions, filamentous flower1, yabby2 and yabby3are expressed in the abaxial domain of young leaf primordia; moreover, ectopic expression of these genes promotes the abaxialization of lateral organs. Taken together these data comprise strong evidence that these Yabby gene family members are responsible for acquisition of abaxial cell fate in Arabidopsis [46••].
Conclusions
Recent research into early leaf development has provided new evidence that founder-cell recruitment is correlated with the patterned reduction of inhibitory factors and the relaxation of biophysical stresses in the SAM. Although the specific factors responsible for the recruitment of leaf founder cells from meristematic precursors remain unknown, this process might be mediated via signaling in and between symplasmic fields in the shoot apex. Regulatory loci governing the expression patterns of knot-ted1-like homeobox genes, molecular markers of the leaf/non-leaf boundary in simple-leafed plants, promise to generate important clues regarding the mechanisms of early leaf development. Analyses of the often bizarre leaf-patterning mutants are the source of experimental models in which specific cell types comprising lateral, dorsoventral and proximal-distal axes of the developing leaf intersect and interact to assemble a completed organ. The expand-ed use of genomics technology to study the functions of orthologous genes during monocot and dicot leaf develop-ment, combined with saturation mutagenesis [47] promises to yield invaluable insight into the evolution of leaf morphology.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest ••of outstanding interest
1. Kaplan DR:The monocotyledons: their evolution and comparative biology. VII. The problem of leaf morphology and evolution in the monocotyledons.The Quart Rev Biol 1973,48:437-457.
2. Kaplan DR: Leaf morphology and development.In Principles of Plant Morphology [preprint reader]. Berkeley: Odin Readers; 1996. 3. Poethig RS: Cellular parameters of leaf morphogenesis in maize and tobacco.In Contemporary Problems of Plant Anatomy. Edited by White RA, Dickinson WC. New York: Academic Press; 1984:235-238.
4. Sylvester AW, Smith L, Freeling M: Acquisition of identity in the developing leaf.Annu Rev Cell Dev Biol 1996, 12:257-304. 5. Poethig RS: Leaf morphogenesis in flowering plants.Plant Cell
1997, 9:1077-1087.
6. Sinha N: Simple and compound leaves: reduction or multiplication?Trends Plant Sci1997, 2:396-402.
7. Steeves TA, Sussex IM: Organogenesis in the shoot: leaf origin and position.In Patterns in Plant Development, edn 2. Cambridge, UK: Cambridge University Press; 1989:109-121.
8. Smith L, Greene B, Veit B, Hake S:A dominant mutation in the maize homeobox gene, Knotted-1, causes its ectopic expression in leaf cells with altered fates.Development 1992, 116:21-30. 9. Jackson D, Veit B, Hake S: Expression of the maize KNOTTED-1
related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot.Development
1992, 120:405-413.
10. Schneeberger RG, Becraft PW, Hake S, Freeling M: Ectopic expression of the knox homeobox gene rough sheath1 alters cell fate in the maize leaf.Genes Dev1995,9:2292-2304.
11. Kerstetter R, Vollbrecht E, Lowe B, Veit B, Yamaguchi J, Hake S: Sequence analysis and expression patterns divide the maize knotted-1-like homeobox genes into two classes. Plant Cell 1994, 6:1877-1887.
13. Muehlbauer GJ, Fowler JE, Girard L, Tyers R, Harper L, Freeling M: Ectopic expression of the maize homeobox gene Liguleless3 alters cell fates in the leaf.Plant Physiol1999, 119:651-662. 14. Lincoln C, Long J, Yamguchi J, Serikawa K, Hake S: A knotted1-like
homeobox gene in Arabidopsisis expressed in the vegetative meristem and dramatically alters leaf morphology when
overexpressed in transgenic plants. Plant Cell 1994, 6:1859-1876. 15. Long JA, Moan EI, Medford JI, Barton MK: A member of the
KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis.Nature1996, 379:66-69.
16. Rinne PLH, van der Schoot C: Symplasmic fields in the tunica of •• the shoot apical meristem coordinate morphogenetic events.
Development1998, 125:1477-1485.
This paper describes the use of fluorescent-dye loading techniques to reveal the presence of symplasmic fields in the outer meristematic layer of birch meristems. Two distinct fields are illustrated, corresponding to the peripheral zone and the central zone of the seedling meristem and providing a mecha-nism for compartmentalized communication. A transient connection between the two compartments is made during early leaf initiation and the symplasmic fields are broken down with the induction of meristematic quiescence.
17. Gisel A, Barella S, Hempel F, Zambryski PC: Temporal and spatial •• regulation of symplasmic trafficking during development in
Arabidopsis thalianaapices.Development1999, 126:1879-1889. Fluorescent tracer molecules loaded into young leaf primordia of
Arabidopsisare shown to move symplamically into the tunica layer of the vegetative shoot apical meristem. Symplasmic movement was found to be progressively restricted with the onset of flowering. Later, the symplasmic field is re-established in inflorescence meristems, revealing spatial and tem-poral regulation of plasmodesmatal connections.
18. Church AH: On the Relation of Phyllotaxy to Mechanical Laws.
London: Williams and Norgate; 1904.
19. Mitchison GJ: Phyllotaxy and the Fibonacci series.Science1997, 196:270-275.
20. Snow M, Snow R: Experiments on phyllotaxis. I. The effect of isolating a primordium.Phil Trans Royal Soc London Ser B 1931, 221:1-43.
21. Veit B, Briggs SP, Schmidt RJ, Yanofsky MF, Hake S: Regulation of •• leaf initiation by the terminal ear1gene of maize.Nature1998,
393:166-168.
The phenotype and molecular cloning of the terminal ear1 mutation is described. Mutant plants produce superfluous numbers of narrow leaves in deviant phyllotactic patterns. The TE1 gene encodes contains an RNA-bind-ing motif, and is expressed in shoot apices in a pattern consistent with its proposed role as a negative regulator of leaf initiation in maize.
22. Greyson RI, Walden DB: The ABPHYL syndrome in Zea mays. I. arrangement, number, and size of leaves. Amer J Bot1972, 59:466-472.
23. Jackson D, Hake S: Control of phyllotaxy in maize by the abphyl1 • gene.Development 1999, 126:315-323.
A study of abphyl1, a fascinating mutation that causes monocot maize leaves to be produced in dicotyledon-like phyllotactic pattern.
24. Fleming AJ, McQueen-Mason S, Mandel T, Kuhelmeier C: Induction of leaf primordia by the cell-wall protein expansin.Science1997, 276:1415-1418.
25. Fleming AJ, Caderas D, Wehrli E, McQueenMason S, Kuhlemeier C: Analysis of expansin-induced morphogenesis on the apical meristem of tomato. Planta 1999, 208:166-174.
26. Reinhardt D, Wittwer F, Mandel T, Kuhlemeier C: Localized •• upregulation of a new expansingene predicts the site of leaf
formation in the tomato meristem.Plant Cell1998, 10:1427-1437. Describes the expression of the tomato expansin gene LeExp2at the site of incipient leaf primordia on the shoot apical meristem. A biophysical model for phyllotaxis determination proposes a key role for EXPANSINS — proteins that increase cell wall extensibility — in the initiation of leaf morphogenesis.
27. Selker JML, Steucek GL, Green PB: Biophysical mechanisms for morphogenetic progression at the shoot apex.Dev Biol1992, 153:29-43.
28. Green P: Expansin and morphology: a role for biophysics. Trends Plant Sci1997, 2:365-366.
29. Becraft PW, Freeling M: Genetic analysis of Rough sheath1 developmental mutants of maize. Genetics1994, 136:295-311.
30. Fowler J, Freeling M: Genetic analysis of mutations that alter cell fates in maize leaves: dominant Liguless mutations. Dev Genet
1996, 18:198-222.
31. Freeling M: A conceptual framework for maize leaf development.
Dev Biol1992, 153:44-58.
32. Foster T, Veit B, Hake S: Mosaic analysis of the dominant mutant, • Gnarley1-R, reveals distinct lateral and transverse signaling
pathways during maize leaf development.Development1999, 126:305-313.
The authors present a mosaic analysis of Gnarley1, a dominant, gain-of-func-tion mutagain-of-func-tion in a Knotted1-like homeobox gene. X-ray induced sectors of nonmutant tissue on mutant tissue support the idea that lateral veins serve as developmental boundaries and that auricle propagation can be separat-ed from auricle initiation.
33. Waites R, Selvadurai HRN, Oliver IR, Hudson A: The phantastica •• gene encodes a MYB transcription factor involved in growth and
dorsoventrality of lateral organs in Antirrhinum.Cell1998, 93:779-789.
The phantasticagene — which is purported to be required for the establish-ment of dorsoventrality in Antirrhinum leaves, bracts and petals — is found to encode a MYB-domain-containing transcription factor expressed in founder cells and young primordia. Analyses of temperature-sensitive alleles indi-cates an additional role for PHAN in elaboration of the proximodistal axes of snapdragon lateral organs.
34. Timmermans MCP, Hudson A, Becraft PW, Nelson T:ROUGH •• SHEATH2: a myb protein that represses knoxhomeobox genes in
maize lateral organ primordia. Science1999, 284:151-153. The rough sheath2gene is cloned in a PCR-based assay to identify the maize orthologue of phantastica, a leaf-patterning mutation from Antirrhinum.
Despite similar expression pattern, mutations in the orthologous genes result in dissimilar mutant phenotypes.
35. Tsiantis M, Schneeberger R, Golz JF, Freeling M, Langdale JA: The •• maize rough sheath2gene and leaf developmental programs in
monocot and dicot leaves. Science1999, 284:154-156.
The maize orthologue of the phantasticagene from Antirrhinumis cloned by transposon tagging. A model for ROUGH SHEATH2 and PHANTASTICA func-tion argues that both genes are involved in negative repression of homeobox gene expression in lateral organs. Loss-of-function mutations in both rough sheath2and phantastica, therefore, result in ‘proximalized’ leaf phenotypes. 36. Waites R, Hudson A: phantastica: a gene required for
dorsoventrality of leaves inAntirrhinum majus.Development1995, 121:2143-2154.
37. Scanlon MJ, Schneeberger RG, Freeling M: The maize mutant narrow sheathfails to establish leaf margin identity in a meristematic domain.Development 1996,122:1683-1691. 38. Scanlon MJ, Freeling M: The narrow sheath leaf domain deletion: a
genetic tool used to reveal developmental homologies among modified maize organs. Plant J 1998,13:547-561.
39. McHale NA: Lam1 and Fat genes control development of the leaf blade in Nicotiana sylvestris. Plant Cell1993, 5:1029-1038. 40. McHale NA, Marcotrigiano M: Lam1is required for dorsoventrality
and lateral growth of the leaf blade in Nicotiana.Development
1998, 125:4235-4243.
41. McConnel JR, Barton MK: Leaf polarity and meristem formation in • Arabidopsis.Development1998, 125:2935-2942.
A dominant mutation leads to the adaxialization of Arabidopsisleaves. The data support a model whereby shoot apical meristem formation is depen-dent upon signals emanating from adaxial leaf surfaces.
42. Lynn K, Fernandez A, Aida M, Sedbrook J, Tasaka M, Masson P, •• Barton MK: The pinhead/zwillegene acts pleiotropically in
Arabidopsisdevelopment and has overlapping functions with the argonaute1gene. Development1999, 126:469-481.
The PINHEAD protein is required for shoot apical meristem formation in
Arabidopsis.Evidence is presented that PINHEAD function defines a devel-opmental domain also comprising the adaxial surface of young leaf primor-dia, and that this domain is essential for shoot meristem development.
43. Timmermans MCP, Schultes NP, Jankovsky JP, Nelson T:
• leafbladeless1is required for dorsoventrality of lateral organs in maize.Development1998, 125:2813-2823.
44. Schneeberger R, Tsiantis M, Freeling M, Langdale JA: Therough • sheath2 gene negatively regulates homeobox gene expression
during maize leaf development.Development1998, 125:2857-2865. The recessive mutation rough sheath2induces leaf and shoot developmen-tal abnormalities similar to those reported for dominant Knotted1-like home-odomain mutants. The rough sheath2 phenotype is correlated with ectopic expression of homeodomain genes in leaves, indicating that rough sheath2
is a negative regulator of Knotted1-like gene expressive in shoots. 45. Muehlbauer GJ, Fowler JE, Freeling M: Sectors expressing the
homeobox gene liguless3implicate a time dependent mechanism for cell fate acquisition along the proximal-distal axis.
Development1997, 124:5097-5106.
46. Siegfried KR, Eshed Y, Baum S, Otsuga D, Drews GN, Bowman J: •• Members of the YABBYgene family specify abaxial cell fate in
Arabidopsis.Development1999, 126:4117-4128.
Three members of the YABBYgene family are expressed on the abaxial domain of lateral organ primordia. The expression domains of these genes are correspondingly altered in the abaxializing mutant phabulosa, whereas ectopic expression of two YABBYmembers confers development of ectopic abaxial tissue types. These data strongly suggest that the YABBYgene fam-ily may have evolved to encode overlapping functions in the specification of abaxial identity.
47. Berna G, Robles P, Micol JL: A mutational analysis of leaf