Rho GTPases are small GTP-binding proteins of the Ras superfamily. In animal cells, the Rho GTPase family comprises three main subgroups, termed Cdc42, Rac and, perhaps confusingly, Rho1.
Although the signalling outputs of the proteins within these subgroups differ substantially, all share certain fundamental characteristics. One is that they act as molecular switches: on when bound to GTP, off when this nucleotide is cleaved to GDP be-cause of the intrinsic GTPase activity of the enzyme or when no nucleotide is bound. A second is that these switches are controlled by extracellular stim-uli. A third shared property is that, in their activated state, Rho proteins bind to a panoply of signalling proteins that mediate the effects of these GTPases on cell physiology2,3. These effects include a
pro-found alteration in actin dynamics and intracellular transport, activation of protein kinase cascades that regulate gene transcription, cell-cycle transit and, in some cell types, the assembly of the machinery required for reactive oxidant production1. These
signalling properties of the Rho proteins are highly conserved amongst eukaryotes as diverse as yeast, slime molds, worms, flies and man, and, in many cases, Rho proteins recruit and use very similar effector proteins. For these reasons, one might pre-dict that plant Rhos are regulated and function in a manner similar to that seen in other eukaryotes, but, as will be discussed, Rho signalling pathways in plants take some unexpected turns.
Rho proteins: who’s who in plants?
In animal cells, the small GTPases of the Ras superfamily can be divided into five families. These are the Ras, Rab, Arf, Ran and Rho groups. Plants, however, seem to lack one of these protein families altogether, namely Ras. If Ras is indeed absent, this puts plants into a category separate from most other eukaryotes, in which Ras proteins play a central
signalling role in growth and development, and means that basic signalling pathways in organisms in the plant kingdom might be organized according to fundamentally different principles. In the case of the Rho family, in plants there appears to be an over-abundance of Rac-like proteins (in Arabidopsis and
Plant GTPases: the
Rhos in bloom
Aline H. Valster, Peter K. Hepler
and Jonathan Chernoff
In animal cells and in fungi, small GTP-binding proteins of the
Rho family have well-established roles in morphogenesis,
cell-cycle progression, gene transcription and the generation of
superoxide anions. The presence of these proteins in plant cells,
however, has been established only recently, and the role of Rho
GTPases in plants is now coming into view. Already, it is
apparent that there are both striking similarities and fascinating
differences in how Rho GTPases are regulated and used in plant
versus animal and fungal cells. These new findings define certain
core properties that might be common to members of this protein
family in all eukaryotes.
References1 Neutra, M.R. et al. (1996) Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu. Rev. Immunol.14, 275–300
2 Jepson, M.A. and Clark, M.A. (1998) Studying M cells and their role in infection. Trends Microbiol.6, 359–365
3 Owen, R.L. (1999) Uptake and transport of intestinal macromolecules and microorganisms by M cells in Peyer’s patches – a personal and historical perspective. Semin. Immunol.11, 157–163
4 Sansonetti, P.J. and Phalipon, A. (1999) M cells as ports of entry for enteroinvasive pathogens: mechanisms of interaction, consequences for the disease process. Semin. Immunol.11, 193–203
5 Gebert, A. and Pabst, R. (1999) M cells at locations outside the gut.
Semin. Immunol.11, 165–170
6 Freeman, T.C. et al. (1995) H+/di-tripeptide transporter (PepT1)
expression in the rabbit intestine. Pflügers Arch. – Eur. J. Physiol.430, 394–400
7 Mason, C.M. et al. (1994) Heterogeneous Na+, K(+)-ATPase expression in
the epithelia of rabbit gut-associated lymphoid tissues. Pflügers Arch. – Eur. J. Physiol.427, 343–347
8 Smith, M.W. and Syme, G. (1982) Functional differentiation of enterocytes in the follicle-associated epithelium of rat Peyer’s patch.
J. Cell Sci.55, 147–156
9 Freeman, T.C. (1995) Parallel patterns of cell-specific gene expression during enterocyte differentiation and maturation in the small intestine of the rabbit. Differentiation59, 179–192
10 Pappo, J. and Owen, R.L. (1988) Absence of secretory component expression by epithelial cells overlying rabbit gut-associated lymphoid tissue. Gastroenterology95, 1173–1177
11 Brown, D. et al. (1990) Brush-border membrane alkaline phosphatase activity in mouse Peyer’s patch follicle-associated enterocytes. J. Physiol.
427, 81–88
12 Smith, M.W. (1985) Selective expression of brush border hydrolases by mouse Peyer’s patch and jejunal villus enterocytes. J. Cell. Physiol.124, 219–225
13 Kelsall, B.L. and Strober, W. (1996) Distinct populations of dendritic cells are present in the subepithelial dome and T cell regions of the murine Peyer’s patch. J. Exp. Med.183, 237–247
14 Neutra, M.R. (1999) M cells in antigen sampling of mucosal tissues.
Curr. Top. Microbiol. Immunol.236, 17–32
15 Jepson, M.A. et al. (1992) Co-expression of vimentin and cytokeratins in M cells of rabbit intestinal lymphoid follicle-associated epithelium.
Histochem. J.24, 33–39
16 Gebert, A. et al. (1992) Co-localization of vimentin and cytokeratins in M-cells of rabbit gut-associated lymphoid tissue (GALT). Cell Tissue Res.269, 331–340
17 Gebert, A. et al. (1994) Cytokeratin 18 is an M-cell marker in porcine Peyer’s patches. Cell Tissue Res.276, 213–221
18 Giannasca, P.J. et al. (1994) Regional differences in glycoconjugates of intestinal M cells in mice: potential targets for mucosal vaccines. Am. J. Physiol.267, 1108–1121
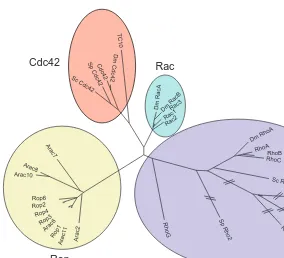
pea, termed Rop, for ‘Rho of plants’, or Arac, for
‘ArabidopsisRac’), but neither Cdc42s nor bona fide
Rhos have yet been discovered (Fig. 1). Although the Rops clearly belong to a distinct subfamily, they all bear the signature GTP-binding amino acid sequence TKLD, a distinguishing mark of the Rac group, and are therefore thought to be most closely related to these GTPases (Fig. 2). Given that Rho itself has not been found in plants, it is therefore of considerable interest that microinjection of a specific Rho inhibitor, C3 exotoxin, affects cytoplasmic stream-ing in pea pollen tubes4and in Tradescantiastamen
hairs (see below). As the C3 toxin does not affect Racs (except under extraordinary in vitro conditions), Rop1 (A.H. Valster, unpublished) or members of the Cdc42 family, these results suggest that either one of the other (perhaps atypical) Rops is the in vivoC3 target, or this toxin is not as specific as currently be-lieved, or plants also encode authentic Rho proteins. Two features stand out regarding Rop proteins in plants: there are an extraordinary number of them and they are extraordinarily alike. At the last count, the Arabidopsisdatabase contained at least 13 distinct Rac-like genes, of which several potentially encode proteins that are nearly identical to one another5–11.
Multiple similar Rac-like genes have also been de-scribed in other plants, representing both monocot and dicot species7,12–14. By contrast, only four Racs
are found in Caenorhabditis elegans, three, so far, in humans, and none at all in budding or fission yeasts.
In Arabidopsis, the proteins encoded by the Rop2 and
Rop4 genes are 98% identical, whereas Rop1 and Rop3 are 96% identical. The only major difference between these proteins and several other closely related Rops/Aracs is found at their C termini, as is manifest by amino acid substitutions in polybasic motifs similar to those that influence the subcellular localization of mam-malian GTPases. These variations sug-gest that Rops could be targeted to dif-ferent locations. As a whole, the Rops form a distinct and tightly clustered branch of the Rho family tree, most closely related (~70% similar) to the Rac subfamily in animals. Only the putative proteins encoded by Arac7, Arac8 and Arac10 show any truly distinguishing features, with marked clusters of amino acid substitutions in the ‘insert region’, located between residues corresponding to amino acids 124–135 in mammalian Rac1 (Fig. 2). As this region is known to be crucial for the binding of specific effector proteins in mammalian and fungal Rho-family proteins, it is likely that these atypical GTPases have unique signalling roles within the Rop family. There is also at least one, much more distantly related, GTPase in Arabidopsis
(GenBank accession number U88402) that encodes a Rop-related protein6.
This protein has both N- and C-terminal insertions and is only ~50% identical to
trends in Cell Biology
Sp Cdc42
Cdc42 Rac
Rop
Rho
Sc Cdc42
Sp Cdc42Cdc42
Dm Cdc42
TC10
Dm RacA Dm RacB
Dm RhoA
Sc Rho
Sp Rho1
Sp Rho2 RhoG
RhoCRhoB
Rho6
Rho7
RhoA
Rac2 Rac1 Rac3
Ar ac7
Arac8
Arac10
Rop6
Rop2
Rop4
Rop3
Rop1 Arac6
Arac11 Ar
ac2
FIGURE 1
Branches of the Rho family tree. The Rho family of GTPases comprises several distinct subgroups, including Cdc42, Rac, Rho and Rop. In this diagram, phylogenetic relationships are shown of representative Rho family proteins from plants, yeasts, flies and man. The Rop group is found only in plants and is most closely related by sequence to the Racs. The Rop and Arac proteins depicted here are from Arabidopsis, but similar proteins have been found in many other plant species. Some branches of the Rho subgroup tree have been foreshortened for clarity, as indicated by slash symbols. The phenogram was constructed using the Phylip programs. Dm, Drosophila
melanogaster; Sc, Saccharomyces cerevisiae; Sp, Schizosaccharomyces pombe.
trends in Cell Biology
GTP binding Insert region
Cdc42 TQID STIEKLAKNKQK
RhoA NKKD HTRRELAKMKQE
Rac1 TKLD DTIEKLKEKKLT
Rop1 TKLD QFFIDHPGAVPI
Rop2/Arac4 TKLD QFFIDHPGAVPI
Rop3/Arac1 TKLD QFFIDHPGAVPI
Rop4/Arac5 TKLD QFFIDHPGAVPI
Rop6/Arac3 TKLD QFFAEHPGAVPI
Arac6 TKLD QFFIDHPGAVPI
Arac11 TKLD QFFIDHPGAVPI
Arac2 TKLD QFLKDHPGAASI
Arac7 TKLD GYLADHTNVITS
Arac8 TKMD HYLSDHPGLSPV
Arac10 TKLD HYLADHPGLSPV
U88402 TKLD LPDPSTGESDPV
Effector loop
NKFPSEYVPTVFDNYAVTVM
DQFPEVYVPTVFENYVADIE
NAFPGEYIPTVFDNYSANVM
NTFPTDYVPTVFDNFSANVV
NTFPTDYVPTVFDNFSANVV
NTFPTDYVPTVFDNFSANVV NTFPTDYVPTVFDNFSANVV
NKFPTDYIPTVFDNFSVNVV
NKFPTDYIPTVFDNFSVNVV
NKFPEDYVPTVFENYTSKIT NKFPTDYIPTVFDNFSANVA NTFPTDYVPTVFDNFSANVV NTFPTDYVPTVFDNFSANVV NTFPTDYVPTVFDNFSANVV NTFPTDYVPTVFDNFSANIV
FIGURE 2
the other Rop/Arac proteins. Most of the Rops and Aracs have a C-terminal isoprenylation site, as is common in Rho-family proteins in other organisms. This modification means that Rops/Aracs are likely to be present in membrane fractions in plant cells, and indeed, in both pea and Arabidopsis, various Rops localize to the plasma membrane in pollen tubes11,15. In addition, in tobacco BY-2 cells, antisera
against human Rac1 detect immunoreactive material in the membranes of intracellular organelles, possibly representing Golgi stacks and peroxisomes16.
Why this profusion of Rops? In some cases, differ-ential expression might be at play. Analysis of expression patterns shows that, although many Rops are expressed ubiquitously, Rop1 is expressed only in mature pollen and pollen tubes, whereas Arac2 is expressed exclusively in stems, roots and hypocotyls. Nevertheless, the available sequence and expression data favour the notion that at least some of the Rops have redundant functions. Perhaps this is one reason why genetic screens for loss-of-function mutants in processes likely to involve Rops have failed so far to yield genes encoding any of these proteins.
Rop regulation: Dbl or nothing?
How are Rops regulated? Rho proteins, presumably including plant Rops, are active when bound to GTP and inactive when bound to GDP or when in a nucleotide-free state. Rho GTPases are activated by GDP–GTP exchange factors (GEFs) that contain Dbl-homology (DH) domains. These proteins facilitate the exchange of GDP for GTP in cells, thereby ‘loading’ the protein with GTP, resulting in conformational changes and activation. There are two known classes of negative regulators; Rho GTPase-activating pro-teins (Rho-GAPs) that increase the intrinsic GTPase rate and thus inactivate Rho proteins, and guanine-nucleotide dissociation inhibitors (Rho-GDIs) that inhibit exchange of GDP for GTP and also block translocation of Rho proteins to the plasma mem-brane. It is interesting to note that no Dbl-type GEF sequences have yet been found in the Arabidopsis
database, even though the sequence of a substantial percentage of the genome has been deduced. As the bacterium Salmonella typhimuriumhas been shown recently to encode a protein (SopE) that acts as a GEF for Rac and Cdc42 but bears no sequence homology whatsoever to Dbl proteins17, perhaps
plants activate Rops using a completely different, and hence unrecognized, class of Rho-GEFs. As for negative regulators, two-hybrid analysis of Rop-binding proteins from Lotus japonicushas uncovered three putative GAP-like proteins that associate with the Rops LjRac1 and LjRac218. Genes encoding
simi-lar proteins are found in the Arabidopsis database. Importantly, these proteins have been shown to possess actual GAP activity on Rops, indicating that GAP proteins are likely to downregulate Rop function in plant cells. Unlike any known mammalian or yeast Rho-GAPs, these plant Rho-GAPs contain a CRIB (Cdc42/Rac interactive binding) motif, an element responsible for the binding of effector pro-teins to Rac and Cdc42 in animal cells19. Although
the requirement for the CRIB motif for binding or regulating Rops has not yet been demonstrated for-mally, it is tempting to speculate that, unlike animal or fungal Rho-GAPs, plant Rho-GAPs target their substrates (i.e. Rops) through a CRIB domain. Indeed, as all known CRIB proteins bind only to the activated forms of GTPases, such a mechanism could ensure that Rop signals are terminated effi-ciently. It is also possible that these plant Rho-GAPs play effector roles, as has been proposed for Ras GAPs in mammalian cells20,21. As for other potential
Rop regulators, two putative Rho-GDIs are found in
the Arabidopsis sequence database, although these
have not yet been shown to regulate Rops. Thus, of the three well-recognized mechanisms for Rho regulation (i.e. GEFs, GAPs and GDIs), in plants only inhibition by GAPs and probably GDIs can be inferred at present, and the overall regulation of Rops therefore remains something of an enigma.
Rop function: Rac redux?
Mammalian and fungal GTPases are well known for their ability to regulate cell morphology. Plant Rops appear to have retained this basic function. The importance of the Rop proteins in cell polarity and tip growth has been best established in pollen tubes. Pollen tubes are single cells that are charac-terized by the presence of strong polarity. Localized secretion and a steep intracellular Ca2+ gradient at
the tip of the pollen tube facilitate tip growth22–24,
allowing the cell to extend in order to bridge the considerable gap between stigma and ovule – a
trends in Cell Biology
Rop
PI-kinase
PtdIns(4,5)P2
Ca2+ Actin-binding
proteins
NADPH oxidase
H2O2
Apoptosis Cellulose
synthesis
MAPKs?
WUS inhibition CLV receptor
Cell polarity/ tip growth
Actin cytoskeleton
Pathogen defence
Meristem signalling Secondary
wall formation
FIGURE 3
Rop-regulated pathways. Rop activity has been correlated with the processes indicated in the square boxes. Parts of the signal-transduction pathways that have been elucidated to date are indicated. Arrows do not necessarily indicate direct interactions, and question mark indicates hypothetical aspect of this model. Crosstalk between pathways is expected to occur, as in, for example, the cell polarity and the actin cytoskeleton pathways. Also, a single Rop-regulated process, such as the production of reactive oxygen species, might affect both pathogen defence and secondary wall formation. CLV receptor, CLAVATA receptor; MAPKs, mitogen-activated protein kinases; PI, phosphatidylinositol; PtdIns(4,5)P2, phosphatidylinositol
(4,5)-bisphosphate; WUS, WUSCHEL protein.
distance that can span several centimetres.
Arabidopsis Rop1 and the nearly identical Arac6
(also known as At-Rac2) protein are pollen-specific Rops that are strongly localized to the plasma mem-brane at the apical domain of the pollen tube, sug-gesting a role in establishing cell polarity and tip growth11,15. Indeed, in Arabidopsis, overexpression
of a constitutively active form of this protein results in depolarized growth and isodiametric swelling of the pollen tube tip4,15. Interestingly, expression of
activated plant Rop1 in fission yeast also induces isotropic cell growth, indicating that a conserved set of effectors might be used by evolutionarily dis-parate members of the Rho family9. In pollen tubes,
overexpression of a dominant–negative mutant of Rop1, in addition to microinjection of inhibitory antibodies against Rop1, results in inhibition of tip
growth, although tip integrity is maintained4. This
effect by the dominant–negative Rop mutant is surprising, as such mutants are thought to act by sequestering endogenous Dbl-type GEFs25, of which
plants apparently have none. Although the identity of the effectors of Rops are not yet known, there are indications that, like Rac in animal cells, Rops associate with and regulate phosphatidylinositol monophosphate (PtdInsP) kinase activity11. As Rop1
is localized to the pollen tube tip, the resulting in-crease in phosphatidylinositol (4,5)-bisphosphate [PtdIns(4,5)P2] might well be involved in the regu-lation of secretion and/or actin organization through its effects on the Ca2+ gradient and on
actin-binding proteins such as gelsolin, villin and profilin. It is particularly interesting that an increase in extracellular Ca2+ partly suppresses4, whereas a
decrease enhances26, the effects of loss of Rop1
func-tion on pollen tube growth. These findings suggest that Rop1 and Ca2+ might act in a common
sig-nalling pathway that affects cell polarity; a connec-tion that has not yet been reported for Rho proteins in other organisms (Fig. 3).
Acting on actin?
So, we know that Rops affect plant cell morphology and polarity, but how do they do it? One of the best-established functions of the Rho proteins in animal and yeast cells is their involvement in regulating the actin cytoskeleton. Expression of activated forms of Rho, Rac and Cdc42 proteins results in distinctive cellular and cytoskeletal phenotypes, in large part due to changes in the rate, character and location of actin polymerization and organization27. In plant
cells, however, the evidence that Rho-like proteins are involved in regulation of the actin cytoskeleton is quite limited. Overexpression of a constitutively active mutant of Rop in tobacco pollen tubes results in an aberrant actin organization in the swollen pollen tube tip11. In normal pollen tubes, actin
bun-dles are organized parallel to the length of the tube, whereas tobacco pollen tubes transfected with acti-vated Rop develop excessively thick actin bundles coiled in a helical pattern11. In addition,
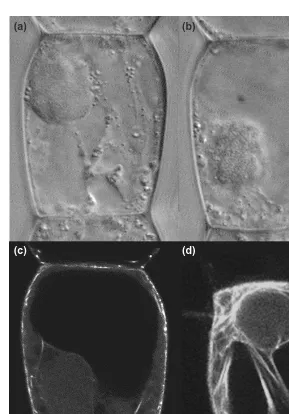
micro-injection of the Rho-inhibitor C3 exotoxin has been shown to interfere with cytoplasmic streaming, a well-established actin-based process, in pea pollen tube4. In interphase Tradescantiastamen hair cells,
C3 toxin causes cytoplasmic strands to break down and inhibits cytoplasmic streaming (Fig. 4). However, as noted previously, the C3 findings are intriguing but also peculiar, as Rho itself has not yet been found in plants and C3 does not inhibit Rop1. These results suggest that plant Rops, like their counterparts in other organisms, regulate actin dy-namics. What is missing is detailed knowledge of Rop effectors that could connect this GTPase to proteins that are involved more directly in regulating actin structure in plants. PtdInsPkinase could rep-resent one such effector, but, based on what we know about GTPases in other systems, it seems un-likely to be the only one. Biochemical purifications, two-hybrid screens and, perhaps, genetic analyses will undoubtedly help to fill in this incomplete
trends in Cell Biology
(c) (d)
FIGURE 4
Effects of a Rho inhibitor on plant cell architecture. The Rho-specific toxin C3 (1 mg ml–1) was injected into interphase Tradescantiastamen hair cells. (a)
picture. As mentioned previously, two-hybrid screens for Rop-binding partners in Lotus18 and
Arabidopsis (Z. Yang, pers. commun.) have already
revealed that proteins containing CRIB motifs are present in plants. In animal cells, several CRIB-con-taining proteins, such as p21-activated kinases28,29,
Wiskott–Aldrich protein30 and MSE55/Borg531,32,
regulate actin dynamics. Whether equivalents for these proteins will be found in plants, however, remains to be seen.
A role in defending the home turf?
Another physiological role for Rop has been estab-lished in the regulation of the elicitor-induced oxidative burst that accompanies host–pathogen interactions. This burst results in the production of reactive oxygen species (ROS) that can trigger the death of infected host cells through apoptosis33,34.
Like the oxidative burst in mammalian neutrophils, ROS in plants can be generated by an NADPH oxidase enzyme complex. In neutrophils, ROS production is governed by Rac1 and Rac2, which are required for as-sembly of the multicomponent oxidase complex that includes several so-called ‘phox’ proteins35. Although
the composition of the plant NADPH oxidase complex is not as well understood as its animal counterpart, it is known to contain p47-phox and p67-phox36, and
homologues of the essential gp91-phox subunit have also been found recently37–39. In addition, in tobacco
cells, a Rac homologue has been identified immuno-logically as a component of the NADPH enzyme com-plex associated with ROS production40. Evidence that
plant Racs regulate ROS production and cell death derives from a series of experiments in which consti-tutively active and dominant–negative mutants of OsRac1 (a Rac-like protein from rice that is most simi-lar to ArabidopsisArac7) were introduced into rice14. The
constitutively active OsRac1 induced ROS production and cell death with apoptotic characteristics, whereas the dominant–negative OsRac1 prevented ROS produc-tion and apoptosis in cells treated with the protein phosphatase inhibitor calyculin A. These results mean that certain Rops/Aracs, possibly through recruitment and assembly of an oxidase complex, are likely to be involved in pathogen defence in plants (Fig. 3).
…and in building stronger walls?
A recent report suggests another role for Rac-like proteins and ROS production in development and cell differentiation. Here, a Rac-like protein from cotton is hypothesized to be involved in the transition of primary to secondary cell wall formation. In cot-ton fibre formation, a model system for the study of secondary wall formation, Potikha et al. have estab-lished that an increase in H2O2production accom-panies the transition from primary to secondary wall formation, and that this molecule might act as a messenger in the process of inducing secondary wall formation41. Coincident with deposition of the
secondary wall and production of the H2O2, Rac13 (a protein 90% identical to ArabidopsisArac2) is highly expressed12. Indeed, when Arabidopsisor soy bean
cell cultures are transformed with the constitutively active form of the cotton Rac13, H2O2production is
stimulated, whereas transformation of the dominant– negative form of Rac13 or with antisense constructs results in decreased H2O2 levels41. These
obser-vations, together with the connection between Rops and ROS formation in pathogen defence, indicate that Rac13 is a regulator of H2O2 production and, through this mechanism, could stimulate secondary cell wall formation and/or cellulose synthesis.
Putting Rops in their place
A role for Rop in plant morphology and develop-ment has been proposed recently. A receptor-like kinase (RLK) in Arabidopsis, termed CLAVATA1 (CLV1), has been identified that is involved in meristem signalling42. This signalling pathway
regulates the balance between cell multiplication and differentiation in the meristem. Clv1 mutant plants show enlarged meristems (with up to a 1000-fold increase in undifferentiated cells) owing to a lack of cell differentiation that normally follows cell division. The CLV1 RLK is a transmembrane receptor with an extracellular leucine-rich repeat domain and an intracellular serine/threonine kinase domain. Using co-immunoprecipitation, Trotochaud et al.43 established that the CLV1
protein forms a large signalling complex that includes the CLV3 protein, KAPP (a kinase-associated protein phosphatase) and a Rop. The signalling complex is detected in two distinct forms of 185 kDa and 450 kDa. Rop is associated exclusively with the 450-kDa complex that is thought to represent the active form of the signalling complex. Although this Rop has not been shown to be regulated through its association with CLV1, nor to bind directly to this RLK, it is interesting to contemplate the possibility that Rop acts immediately downstream of a cell-sur-face receptor and, possibly, upstream of a mitogen-activated protein kinase (MAPK) cascade44.
Tantalizing, but preliminary, support for the latter aspect of this model is provided by evidence from two-hybrid screens that show that certain as-yet-uncharacterized Raf-like kinases can associate with LjRac119. In turn, the MAPK pathway is thought to
inactivate the WUSCHEL gene that is involved in normal maintenance of meristems45.
In mammalian systems, Rho GTPases can be acti-vated by G-protein-coupled receptors and by recep-tor protein tyrosine kinases. In the case of G-protein-coupled receptors, the route to some Rho GTPases might be relatively direct as two groups have re-ported that a Dbl-type GEF for RhoA binds directly to the alpha subunit of a heterotrimeric G protein46,47.
What can we expect to dig up next?
The sheer abundance and extreme conservation of the Rops argue for a crucial role in plant physiology. It is now clear that these proteins are distant cousins, rather than near siblings, of the more familiar Rho/Rac/Cdc42 groups in other eukaryotes and that they therefore could be subject to different control mechanisms and might have adopted unique signalling roles. That Rops might be regu-lated in a novel manner can be inferred from their coprecipitation with an RLK complex and also by the surprising absence (so far) in plant databases of sequences encoding the expected key regulatory proteins, such as classical Rho-GEFs. Furthermore, one group of plausible regulatory proteins we do know in plants (i.e. the GAPs) has apparently hijacked a targeting motif (the CRIB domain) and used it in a manner never seen before in any other system. The core signalling activities of this protein family – regulation of cell morphology, ROS pro-duction and, perhaps, gene transcription – do appear to be retained in plant Rops. However, nothing is known of their effector proteins. This situation is likely to be remedied soon as plant scientists gear-up their Rop pull-down assays and two-hybrid screens. Thus, we can expect to see soon just how differently the plant kingdom has decided to employ these small but powerful signalling proteins.
References
1 Mackay, D.J.G. and Hall, A. (1998) Rho GTPases. J. Biol. Chem.273, 20685–20688
2 Aspenstrom, P. (1999) Effectors for the Rho GTPases. Curr. Opin. Cell Biol.11, 95–102
3 Van Aelst, L. and D’Souza-Schorey, C. (1997) Rho GTPases and signalling networks. Genes Dev.11, 2295–2322
4 Lin, Y. and Yang, Z. (1997) Inhibition of pollen tube elongation by microinjected anti-Rop1Ps antibodies suggests a crucial role for Rho-type GTPases in the control of tip growth. Plant Cell9, 1647–1659
5 Xia, G. et al. (1996) Identification of a plant cytoskeletal, cell cycle-related and polarity-cycle-related proteins using Schizosaccharomyces pombe. Plant J.10, 761–769
6 Collins, C.C. and Johnson, D.I. (1997) An Arabidopsis thalianaexpressed sequence tag cDNA that encodes a Rac-like protein. Plant Physiol.113, 1463
7 Bischoff, F. et al. (1999) GTP-binding proteins in plants. Cell. Mol. Life Sci.55, 233–256
8 Winge, P. et al. (1997) Cloning and characterization of rac-like cDNAs from Arabidopsis thaliana. Plant Mol. Biol.35, 483–495
9 Li, H. et al. (1998) ArabidopsisRho-related GTPases: differential gene expression in pollen and polar localization in fission yeast. Plant Physiol.
18, 407–417
10 Yang, Z. and Watson, J.C. (1993) Molecular cloning and
characterization of rho, a ras-related small GTP-binding protein from garden pea. Proc. Natl. Acad. Sci. U. S. A.90, 8732–8736
11 Kost, B. et al. (1999) Rac homologues and compartmentalized phosphatidylinositol 4,5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J. Cell Biol.145, 317–330
12 Delmer, D.P. et al. (1995) Genes encoding small GTP-binding proteins analogous to mammalian rac are preferentially expressed in developing cotton fibers. Mol. Gen. Genet.248, 43–51
13 Borg, S. et al. (1997) Identification of new protein species among 33 different small GTP-binding proteins encoded by cDNAs from Lotus japonicus, and expression of corresponding mRNAs in developing root nodules. Plant J.11, 237–250
14 Kawasaki, T. et al. (1999) The small GTP-binding protein Rac is a regulator of cell death in plants. Proc. Natl. Acad. Sci. U. S. A.96, 10922–10926
15 Lin, Y. et al. (1996) Localization of a Rho GTPase implies a role in tip growth and movement of the generative cell in pollen tubes. Plant Cell
8, 293–303
16 Couchy, I. et al. (1998) Immunodetection of Rho-like plant proteins with Rac1 and Cdc42Hs antibodies. J. Exp. Bot.49, 1647–1659
17 Hardt, W.D. et al. (1998) S. typhimuriumencodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell93, 815–826
18 Borg, S. et al. (1999) Plant cell growth and differentiation may involve GAP regulation of Rac activity. FEBS Lett.453, 341–345
19 Burbelo, P.D. et al. (1995) A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J. Biol. Chem.270, 29071–29074
20 Bollag, G. and McCormick, F. (1992) GTPase activating proteins. Semin. Cancer Biol.3, 199–208
21 Tocque, B. et al. (1997) Ras-GTPase activating protein (GAP); a putative effector for Ras. Cell. Signal.9, 153–158
22 Pierson, E.S. et al. (1994) Pollen tubes growth is coupled to the extracellular calcium ion flux and the intracellular calcium gradient: effect of BAPTA-type buffers and hypertonic media. Plant Cell6, 1815–1828
23 Holdaway-Clarke, T.L. et al. (1997) Pollen tube growth and the intracellular calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell9, 1999–2010
24 Yang, Z. (1998) Signalling tip growth in plants. Curr. Opin. Plant Biol.1, 525–530
25 Feig, L.A. (1999) Tools of the trade: use of dominant-inhibitory mutants of Ras-family GTPases. Nat. Cell Biol.1, 25–27
26 Li, H. et al. (1999) Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium influx. Plant Cell
11, 1731–1742
27 Hall, A. (1998) Rho GTPases and the actin cytoskeleton. Science279, 509–514
28 Sells, M.A. and Chernoff, J. (1997) Emerging from the Pak: the p21-activated protein kinase family. Trends Cell Biol.7, 162–167
29 Bagrodia, S. and Cerione, R.A. (1999) PAK to the future. Trends Cell Biol.
9, 350–355
30 Symons, M. et al. (1996) Wiskott–Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization.
Cell84, 723–734
31 Burbelo, P.D. et al. (1999) MSE55, a Cdc42 effector protein, induces long cellular extensions in fibroblasts. Proc. Natl. Acad. Sci. U. S. A.96, 9083–9088
32 Joberty, G. et al. (1999) The Borgs, a new family of Cdc42 and TC10 GTPase-interacting proteins. Mol. Cell. Biol.19, 6585–6597
33 Levine, A. et al. (1994) H2O2from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell79, 583–593
34 Mehdy, M.C. et al. (1996) The role of activated oxygen species in plant disease resistance. Physiol. Plant.98, 365–374
35 Bokoch, G.M. (1995) Regulation of phagocyte respiratory burst by small GTP-binding proteins. Trends Cell Biol.5, 109–113
36 Dwyer, S.C. et al. (1996) Plant and human neutrophil oxidative burst complexes contain immunologically related proteins. Biochim. Biophys. Acta1289, 231–237
37 Groom, Q.J. et al. (1996) RbohA, a rice homolog of the mammalian gp91 phox respiratory burst oxidase gene. Plant J.10, 515–522
38 Keller, T. et al. (1998) A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+binding motifs. Plant Cell10, 255–266
39 Torres, M.A. et al. (1998) Six Arabidopsis thalianahomologues of the human respiratory burst oxidase (gp91phox). Plant J.14, 365–370
40 Kieffer, F. et al. (1997) Tobacco cells contain a protein, immunologically related to the neutrophil small G protein Rac2 and involved in elicitor-induced oxidative burst. FEBS Lett.403, 149–153
41 Potikha, T. et al. (1999) The involvement of hydrogen peroxide in the differentiation of secondary walls in cotton fibers. Plant Physiol.119, 849–858
42 Clark, S.E. et al. (1997) The CLAVATA1gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell
89, 575–585
43 Trotochaud, A.E. et al. (1999) The CLAVATA1 receptor-like kinase requires CLAVATA3 for its assembly into a signalling complex that includes KAPP and a Rho-related protein. Plant Cell11, 393–406
44 Kohorn, B.D. (1999) Shuffling the deck: plant signalling plays a club.
Trends Cell Biol.9, 381–383
45 Laux, T. et al. (1996) The WUSCHELgene is required for shoot and floral meristem integrity in Arabidopsis. Development122, 87–96
46 Hart, M.J. et al. (1998) Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science280, 2112–2114
47 Kozasa, T. et al. (1998) p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science280, 2109–2111