Effects of conditioned medium on activities of PAL, CHS, DAHP
synthase (DS-Co and DS-Mn) and anthocyanin production in
suspension cultures of
Fragaria ananassa
Tsukasa Mori
a,*, Miei Sakurai
b, Masaaki Sakuta
caLaboratory of Physiology,Faculty of Fisheries,Hokkaido Uni6ersity,3-1-1Minato,Hakodate,Hokkaido041,Japan
bDepartment of En6ironmental Process De6elopment,Research Institute,Ishikawajima Harima Hea6y Industries Co.Ltd.,1Shinnakahara,
Isogo-ku,Yokohama235,Japan
cDepartment of Biology,Ochanomizu Uni6ersity,2-1-1Otsuka,Tokyo112,Japan Received 4 April 2000; received in revised form 4 September 2000; accepted 26 September 2000
Abstract
A conditioned medium (CM) prepared from cell suspension cultures of strawberry stimulated anthocyanin synthesis. The effect was significantly (PB0.05) greater than that of synthetic medium (SM), with macronutrient concentrations, carbohydrate concentrations and pH adjusted to those of CM. The activity of 3-deoxy-D-arabino-heptulosonate 7-phosphate (DAHP) synthase
(EC 4.1.2.15) (DS-Mn, DS-Co), phenylalanine ammonia-lyase (PAL, EC 4.3.1.5) and chalcone synthase (CHS, EC 2.3.1.74) were monitored in the CM- and SM-cultured cells. PAL and CHS activities were found to increase significantly (PB0.05) in the CM-cultured cells. CHS transcript levels were higher in the CM-cultured cells compared to transcript abundance in SM-cultured cells. There was no significant difference in the DS-Mn and DS-Co activities of cells grown in conditioned or synthetic media. © 2001 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Anthocyanin; Strawberry; Chalcone synthase; Phenyalanine ammonia-lyase; DAHP synthase; Conditioned medium
www.elsevier.com/locate/plantsci
1. Introduction
Since the importance of cell density was re-ported for plant cell growth [1], the nursing of cell cultures has become a general method for in vitro plant cell culturing. This suggests the existence of substances, called ‘conditioning factors’ or ‘viabil-ity factors’, which may be released from in vitro cultured cells and stimulate cell proliferation [2]. Recently, sulfated peptides have been purified and identified as proliferation-stimulating factors for single mesophyll cells [3].
Anthocyanins are flavonoids with a well-studied biosynthetic pathway. PAL and CHS are key en-zymes of flavonoid synthesis and their activities have been found to increase by elicitor treatment [7], wounding [8] and UV irradiation [9]. DAHP synthase is a key enzyme in the shikimate pathway of the primary metabolism, which influences sec-ondary metabolism [10]. It has two isozymes,
known as DS-Mn activated by Mn2+ and DS-Co
activated by Co2+ [11]. These activities also differ with cell growth [12], elicitor treatment [13], nutri-ent condition [14] and light [15]. However, most studies on the regulatory mechanisms of secondary metabolism have been focused on the key enzymes PAL and CHS, with only few reports being avail-able on the role of DAHP synthase related to secondary metabolism. Furthermore, there is no report on how these enzymes of primary and secondary metabolism are involved in the produc-Abbre6iations: BA, benzylaminopurine; CHS, chalcone synthase
(EC 2.3.1.74); CM, conditioned medium; DS-Mn, DAHP synthase-Mn; DS-Co, DAHP synthase-Co; DAHP synthase, 3-deoxy-D -ara-bino-heptulosonate 7-phosphate synthase (EC 4.1.2.15); 2,4-D, 2,4-dichlorophenoxyacetic acid; FW, fresh weight; PAL, phenylala-nine ammonia-lyase (EC 4.3.1.5); SM, synthetic medium.
* Corresponding author. Fax: +81-138-435015.
E-mail address:[email protected] (T. Mori).
tion of secondary metabolites in cells grown in conditioned medium (CM).
Therefore, we studied how these key enzymes (PAL, CHS, DS-Mn, DS-Co) and CHS gene ex-pression were influenced by CM for anthocyanin synthesis and investigated the relation between stimulation of anthocyanin synthesis and changes of activities of these enzymes using strawberry suspension-cultured cells.
2. Materials and methods
2.1. Cell cultures
Cell culturing, measurement of cell growth and anthocyanin content were conducted by the method of Mori et al. [16]. Seven-day-old subcul-tured cells (1 g FW) were transferred to 100 ml of CM and SM and cultured at 8000 lux and 25°C for 1 week. The cells were harvested at 0, 6, 12, 24, 36, 48, 60, 120 and 168 h following cell transfer. SM and CM were prepared as described earlier [5].
2.2. Preparation of enzyme extracts
Cells (500 mg FW), after being harvested and frozen in liquid nitrogen and stored at −80°C, were homogenized at 1000 rpm for 15 min on ice
with a glass homogenizer, using 0.05 g
polyvinylpyrrolidone and 1.5 ml of 100 mM potas-sium-phosphate buffer (pH 8.0) containing 1.4 mM 2-mercaptoethanol. The homogenate was
cen-trifuged at 15 000×g for 15 min at 4°C. The
supernatant was purified with Sephadex G-25 pre-viously equilibrated with the same buffer. The eluate served as the enzyme extract for the assay. The concentration of protein in the enzyme extract was measured by the method of Bradford [17] using g-globulin as the standard.
2.3. Enzyme assays
PAL activity was measured by the modified method of Tanaka et al. [18]. A reaction mixture was prepared by adding 0.4 ml of 100 mM Tris – HCl buffer (pH 8.8) and 0.2 ml of 40 mM pheny-lalanine to 0.2 ml of enzyme extract. The reaction mixture was incubated for 30 min at 37°C and terminated by adding 0.2 ml 25% trichloroacetic acid (TCA). The phenylalanine was added to the
controls after incubation and the addition of TCA. The assay mixture was centrifuged at
10 000×g for 15 min at 4°C and absorbance of
the supernatant was measured at 280 nm relative to the control.
CHS (EC 2.3.1.74) activity was determined by the modified method of Kreuzaler and Hahlbrock [19]. The enzyme extract (0.08 ml) was pre-incu-bated for 1 min at 30°C and then treated with a 0.02 ml CoA-mixture and incubated for 5 min at 30°C. The CoA-mixture was prepared by mixing 50 mM p-coumaroyl-CoA with 42.5 mM [2-14
C]-malonyl-CoA (14.7 mCi/m mol) and its pH was
adjusted to 8.0 with KOH, prior to adding the enzyme extract. The reaction was terminated by adding 0.2 ml ethylacetate containing 5 mg narin-genin, mixed and then centrifuged (12 000×g, 10 min, 25°C). The organic layer (150 ml) was col-lected and evaporated. The residue was dissolved in 10 ml ethylacetate and spotted onto an Avicel-cellulose thin-layer chromatography plate. Thin-layer chromatography was conducted using 5% (v/v) acetic acid. A naringenin spot was detected under UV light, scraped off and transferred to 5 ml of a toluene solution containing 0.5% 2,5-diphenyloxaazole and 0.025%
1,4-bis[2-(4-methyl-5-phenyloxazolyl)] benzene. Radioactivity was
measured with a liquid scintillation counter, Model LS-6000-SC (Beckman Instruments, Fuller-ton, CA).
DAHP synthase activity was assayed using the modified method of Morris et al. [20]. The reac-tion mixture of DS-Mn contained 50 mM K-Epps buffer (pH 8.0), 0.5 mM dithiothreitol, 0.5 mM
MnCl2, 3 mM PEP and 0.6 mM E4P. The reaction
mixture for DS-Co contained 50 mM K-Epps buffer (pH 8.6), 10 mM MgCl2, 3 mM PEP and 3 mM E4P. The DS-Mn reaction was initiated by the addition of 20 ml enzyme extract to 180 ml of reaction mixture and the DS-Co reaction by
adding 10 ml of enzyme extract to 190 ml of
reaction mixture. This was followed by incubation of DS-Mn for 30 min and DS-Co for 20 min at 37°C, respectively. Reactions were terminated by
adding 50 ml 25% TCA. As control, 50 ml 25%
TCA was added to the mixture prior to the start of the reaction. After centrifugation (10 000×g, 15 min, 4°C), 100 ml of supernatant was collected
and 100 ml 25 mM NaIO4 containing 0.125 N
H2SO4 was added, followed by incubation for 30
N HCl and 800 ml of 0.3% thiobarbituric acid solution, was added followed by incubation for 10 min at 100°C and absorbance was measured at 549 nm.
2.4. Isolation of RNA and northern hybridization of CHS
Total RNA was extracted from 2 g fresh cells by the SDS-phenol method [21]. RNA was dissolved in TE buffer (10 mM Tris – HCl, pH 7.5, 1 mM
EDTA, 1 mM dithiothreitol and 5 U/ml
ribonu-clease inhibitor from human placenta) and 10 mg RNA samples were subjected to electrophoresis in a 1.2% formaldehyde-agarose gel and blotted onto
a nylon membrane (Hybond N+, Amersham,
Japan). Hybridization using an RNA probe of antisense CHS, was conducted overnight at 65°C
in 5×SSC, 10×Denhard’s solution, 10 mM
sodium phosphate (pH 6.5), 0.5% SDS, 50% for-mamide, 100mg/ml sonicated salmon sperm DNA. CHS cDNA was obtained from a cDNA library of strawberry suspension cells and RNA was detected with a DIG luminescent detection kit (Boehringer Mannheim).
2.5. Statistical analysis
Data were presented as means9S.E. Statistical significance was determined by the Mann – Whit-ney U-test. Statistical significance was set at PB 0.05.
3. Results and discussion
3.1. Effect of conditioned medium on PAL, CHS and DAHP synthase acti6ities
Most plant cells produce anthocyanin at the expense of cell growth, thus implying that the initiation of cell growth stagnation and an-thocyanin synthesis at the same time, influences or is influenced by various enzymatic activities. How-ever, strawberry cells accumulate anthocyanin dur-ing an early period of cell culturdur-ing in CM, while the stimulation of cell growth was not observed during this period [4,5].
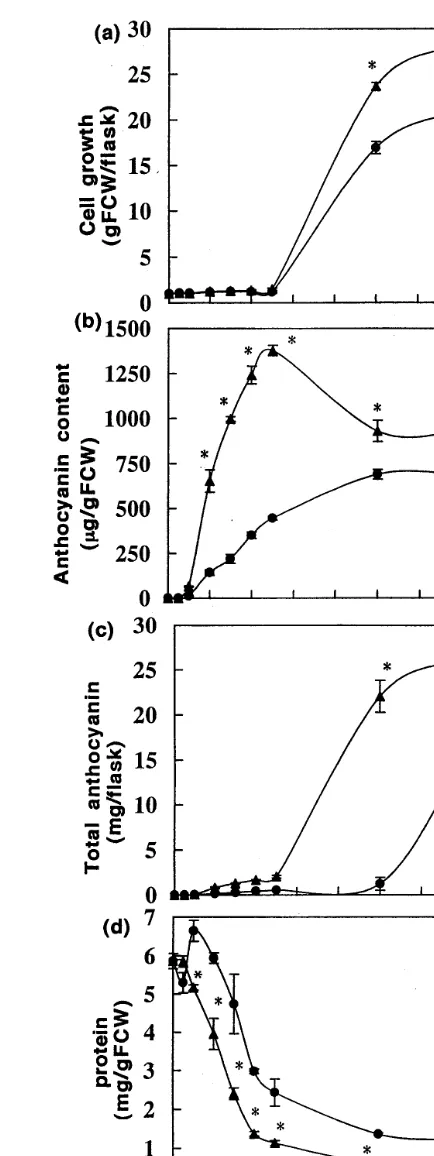
No difference in cell growth between the CM-and SM-cultured cells was observed during the first 60 h (Fig. 1a). A significant anthocyanin
Fig. 1. Changes in fresh weight and anthocyanin accumula-tion of CM- and SM-cultured strawberry cell suspensions. Cells (1 g FW) were cultured in 100 ml of CM and SM on a rotary shaker (80 rpm) under 8000 lux. Medium: SM: ; CM:. Cells were harvested at 0, 6, 12, 24, 36, 48, 60, 120
accumulation occurred during this period (Fig. 1b), while protein content in the cultured cells was drastically reduced over time, contrary to the re-sults observed for cell growth and total an-thocyanin production (Fig. 1a,c). This decrease was observed for both, CM- and SM-cultured cells, however, protein content of CM-cultured cells was significantly lower than that of SM-cul-tured cells after 12 h of culture. Since in condi-tioned culture medium used for the CM-treatment, macronutrients are decreased during the 7 days
conditioning period, the concentrations of
macronutrients (nitrate, potassium, ammonium, phosphate, carbohydrates) and pH of SM were adjusted to those of CM, as previously reported [5]. Thus, the effects of CM on anthocyanin syn-thesis were studied with no confounding effect due to pH or macronutrient concentrations.
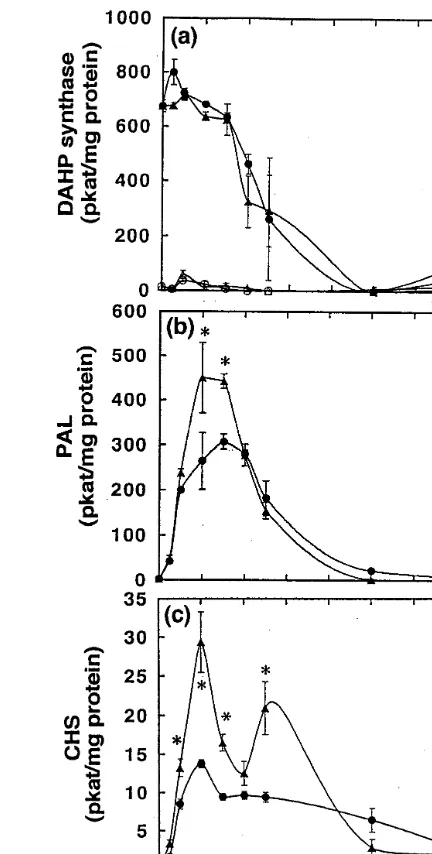
PAL activity increased after 6 h of culture in both CM- and SM-cultured cells and reached a
maximum at 24 h (450 pkat/mg protein) in the
CM-cultured cells. PAL activity was significantly (PB0.05) greater (1.8 times) in CM-cultured cells than in SM-cultured cells (Fig. 2b). CHS activity also increased after 6 h of culture and reached a
maximum at 24 h (30 pkat/mg protein) in the
CM-cultured cells, with a second small peak at
:60 h. CHS activities were significantly (PB
0.05) greater in CM-cultured cells than in SM-cul-tured cells (Fig. 2c). The activities of DS-Co in DAHP synthase showed no positive correlation with anthocyanin synthesis (Fig. 2a). Although the activity of DS-Mn was lower than that of DS-Co, it showed the reappearance of a small peak before increasing PAL activity. However, there was no significant difference between the CM-cultured and SM-cultured cells for DS-Co and DS-Mn activities. PAL and CHS activities were greater in the CM-cultured cells than in the SM-cultured cells, but no differences were observed in DAHP synthase, regardless of protein content of CM-cul-tured cells being lower than in SM-culCM-cul-tured cells. This may indicate that the conditioned medium stimulates secondary metabolic pathways in straw-berry suspension cultured cells.
3.2. Effects of conditioned medium on CHS mRNA le6els
Although PAL and CHS mRNA levels in cul-tured cells are increased through a dilution effect
Fig. 2. Changes in different enzyme activities of CM- and SM-cultured strawberry cell suspensions. (a) DAHP synthase: DS-Co: ,; DS-Mn: , ; (b) PAL; (c) CHS. Culture medium: SM: ,; CM:,. Seven-day-old subculturing
cells (1 g FW) were transferred to 100 ml of SM and CM and cultured under 8000 lux. Cells were harvested at 0, 6, 12, 24, 36, 48, 60, 120 and 168 h after transfer. Each value represents the average of three replicates and vertical lines represent the S.E. of replicates. *Mean differences significant at P=0.05 against that of the SM within the same time.
the stimulation of anthocyanin synthesis by condi-tioned medium does not seem to be due to a dilution effect.
CHS gene expression was also examined in CM-and SM-cultured cells. CHS transcript levels ini-tially increased after 6 h and reached a peak after 12 h of culture, before gradually decreasing, in both CM- and SM-cultured cells (Fig. 3a,b). As found for CHS activity (Fig. 2b,c), the CHS tran-script abundance from CM-cultured cells was higher than that from SM-cultured cells. However, the increase of CHS mRNA levels in CM-cultured cells relative to SM-cultured cells was smaller than the corresponding increase observed for CHS en-zyme activity (Fig. 2c). While, CM stimulates key enzymes of the flavonoid pathway, such as PAL and CHS and leads to an increased anthocyanin accumulation in strawberry cells, further studies are needed to determine the mechanism by which this occurs.
Acknowledgements
We thank to Dr Y. Ozeki (Tokyo University Agriculture and Technology) for his advise.
References
[1] W.H. Muir, A.C. Hildebrandt, A.J. Riker, Plant tissue cultures produced from single isolated cells, Science 119 (1954) 877 – 878.
[2] R. Stuart, H.E. Street, Studies on the growth in culture of plant cells, J. Exp. Bot. 20 (1969) 556 – 571.
[3] Y. Matsubayashi, Y. Sakagami, Phytosulfokine, sulfated peptides that induce the proliferation of single mesophyll cells of Asparagus officinalis L, Proc. Natl. Acad. Sci. USA 93 (1996) 7623 – 7627.
[4] T. Mori, M. Sakurai, M. Seki, F. Furusaki, Effects of conditioning factor on anthocyanin production in straw-berry suspension cultures, J. Sci. Food Agric. 66 (1994) 381 – 388.
[5] M. Sakurai, T. Mori, Stimulation of anthocyanin syn-thesis by conditioned medium produced by strawberry suspension cultures, J. Plant Physiol. 149 (1996) 599 – 604.
[6] T. Mori, M. Sakurai, Preparation of conditioned medium to stimulate anthocyanin production using sus-pension cultures of Fragaria ananassa cells, World J. Microbiol. Biotechnol. 15 (1999) 635 – 637.
[7] R. Lois, A. Dietrich, K. Hahlbrock, W. Schulz, A phenylalanine ammonia-lyase gene from parsley: struc-ture, regulation and identification of elicitor and light responsivecis-acting elements, EMBO J. 8 (1989) 1641 – 1648.
[8] Y. Tanaka, I. Uritani, Synthesis and turnover of pheny-lalanine ammonia-lyase in root tissue of sweet potato injured by cutting, Eur. J. Biochem. 73 (1977) 255 – 260. [9] D.N. Kuhn, J. Chappell, A. Boudet, K. Hahlbrock, Induction of phenylalanine ammonia-lyase and 4-cou-marate: CoA ligase mRNAs in cultured plant cells by UV light or fungal elicitor, Proc. Natl. Acad. Sci. USA 81 (1984) 1102 – 1106.
[10] J.M. Henstrand, K.F. McCue, K. Brink, A.K. Handa, K.M. Herrmann, E.E. Conn, Light and fungal elicitor induce 3-deoxy-D-arabino-heptulosonate 7-phosphate
synthase mRNA in suspension cultured cells of parsley (Petroselinum crispum L), Plant Physiol. 9 (1992) 761 – 763.
[11] J.L. Rubin, C.G. Gaines, R.A. Jensen, Enzymological basis for herbicidal action of glyphosate, Plant Physiol. 70 (1982) 833 – 839.
[12] R. Ganson, R.A. Jensen, Response of cytosolic-isozyme and plastid-isozyme levels of 3-deoxy-D -arabino-heptu-losonate 7-phosphate synthase to physiological state of
Nicotiana sil6estrisin suspension culture, Plant Physiol.
83 (1987) 479 – 482.
[13] K.F. McCue, E.E. Conn, Induction of 3-deoxy-D
-ara-bino-heptulosonate 7-phosphate synthase activity by fungal elicitor in cultures ofPetroselinum crispum, Proc. Natl. Acad. Sci. USA 86 (1989) 7374 – 7377.
Fig. 3. Northern hybridization analysis for CHS mRNA accumulation in CM- and SM-cultured strawberry suspension cells. (a) RNA (10mg) was loaded onto the gel and
[14] N. Suzuki, M. Sakuta, S. Shimizu, A. Komamine, Changes in the activity of 3-deoxy-D
-arabino-heptulo-sonate 7-phosphate (DAHP) synthase in suspension-cul-tured cells ofVitis, Physiol. Plant 94 (1995) 591 – 596. [15] K.F. McCue, E.E. Conn, Induction of shikimic acid
pathway enzymes by light in suspension cultured cells of parsley (Petroselinum crispum), Plant Physiol. 94 (1990) 507 – 510.
[16] T. Mori, M. Sakurai, J. Shigeta, K. Yoshida, T. Kondo, Formation of anthocyanins from cells cultured from different parts of strawberry plants, J. Food Sci. 58 (1993) 788 – 792.
[17] M.M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem. 72 (1976) 248 – 254.
[18] Y. Tanaka, M. Kojima, I. Uritani, Properties, develop-ment and cellular-localization of cinnamic acid 4-hy-droxylase in cut-injured sweet potato, Plant Cell Physiol. 15 (1974) 843 – 854.
[19] F. Kreuzaler, K. Hahlbrock, Enzymatic synthesis of an aromatic ring from acetate units. Partial purification and some properties of flavanone synthase from cell-suspen-sion cultures ofPetroselinum hortense, Eur. J. Biochem. 56 (1975) 205 – 213.
[20] P.F. Morris, R.L. Doong, R.A. Jensen, Evidence from
Solanum tuberosumin support of the dual-pathway hy-pothesis of aromatic biosynthesis, Plant Physiol. 89 (1989) 10 – 14.
[21] R.D. Palmiter, Magnesium precipitation of ribonucle-oprotein complexes expedient techniques for the isola-tion of undegraded polysomes and messenger ribonucleic acid, Biochemistry 17 (1974) 3606 – 3615.
[22] K. Kakegawa, J. Suda, M. Sugiyama, A. Komamine, Regulation of anthocyanin biosynthesis in cell suspen-sion cultures ofVitisin relation to cell division, Physiol. Plant 94 (1995) 661 – 666.
[23] E.M. Linsmaier, F. Skoog, Organic growth factor re-quirements of tobacco tissue cultures, Physiol. Plant 18 (1965) 100 – 127.