Summary Stabilized shoot cultures initiated from crown material of six adult Quercus robur L. trees and from basal epicormic shoots of a Quercus rubra L. tree showed good in vitro rooting capacity. An initial five-day dark period generally improved the rooting response but was detrimental to plantlet quality. There were clonal differences in rooting capacity. The concentration and exposure time of the indolebutyric acid (IBA) treatment were critical for root induction. In both spe-cies, best rooting efficiency was achieved by culture in medium containing 25 mg l−1 IBA for 24 h and subsequent transfer to an auxin-free medium containing 1% activated charcoal. For all clones tested, the charcoal benefited both shoot quality and root system development, the latter being enhanced by the formation of many lateral roots. Total root system area and length, measured with a digital image analyzer, were signifi-cantly greater in medium containing charcoal than in medium lacking charcoal. Because darkening the basal part of the shoots with aluminum foil during the rooting phase only caused a small increase in rooting, we conclude that the large effect of charcoal on rooting was the result of adsorption of inhibitory compounds from the medium or the explant or both, rather than of basal darkening. Other factors affecting the rooting response of Q. robur were: (a) the position on the tree of the material from which cultures were initiated (the topophysical effect); and (b) shoot quality. Recycling the same horizontally placed explant on multiplication medium allowed three successive crops of shoots to be obtained, and rootability was typically maintained from crop to crop.
Keywords: charcoal, image analysis, micropropagation, pedunculate oak, red oak.
Introduction
To exploit genetic gains in forestry species, efficient vegetative propagation techniques are needed. For the genus Quercus, the development of both macro- and micropropagation technolo-gies has been suggested (Kleinschmit 1986). However, as with many woody species, propagation from trees more than 6 to 8 years old is difficult and necessitates rejuvenation (Savill and Kanowski 1993).
The establishment and multiplication of shoot cultures de-rived from mature Quercus rubra L. (red oak) and Q. robur L. (pedunculate oak) trees have been achieved using explants isolated from shoots flushed on branch segments (Vieitez et al. 1993, Vieitez et al. 1994) and stem sections (Evers et al. 1993). The continuous culture of the same horizontal shoot explants improves the in vitro performance of cultures initiated from reinvigorated shoots induced by the forced flushing method (Vieitez et al. 1994). We have observed no decline in shoot vigor or proliferation capacity after three years of subculturing with horizontal explants.
Micropropagation requires not only stable shoot multiplica-tion, but also successful rooting of microcuttings. The rooting of oak shoots of adult origin has received little attention, probably because of the difficulty in obtaining stabilized shoot cultures, especially from the crown (Juncker and Favre 1989, Gebhardt et al. 1993). Acceptable rooting percentages have been obtained with microshoots initiated from stump sprouts (Vieitez et al. 1985, Meier-Dinkel 1987, Chalupa 1993) and from epicormic shoots taken near the base of the trunk (San-José et al. 1988, Evers et al. 1993) of adult oak trees. However, there are few reports on rooting of shoot cultures derived from the crown. Evers et al. (1993) achieved rooting rates of ≤ 20% and Vieitez et al. (1994) reported 15--46% rooting rates, but the regenerated plantlets were of poor quality. No reports have described in vitro regeneration of plantlets from red oak cul-tures of mature origin, although Vieitez et al. (1993) achieved plantlet regeneration with cultures of juvenile origin. In this study, we investigated the in vitro rooting of mature Q. robur and Q. rubra. A microcomputer based image analysis tech-nique was used to evaluate root system development.
Materials and methods
Shoot cultures of Q. robur were initiated from crown branches (collected at least 5 m from the ground) of six mature trees aged 70--300 years and designated SL1, SL3, Sainza, Tixon, Monasterio and San Esteban. Cultures established from Sainza consisted of three shoot culture lines that were derived from three sources of explants differing in position of topophysis, including from basal sprouts (collected less than 0.5 m from
Requirements for
in vitro
rooting of
Quercus robur
and
Q. rubra
shoots
derived from mature trees
M. C. SANCHEZ, M. C. SAN-JOSE, A. BALLESTER and A. M. VIEITEZ
Instituto de Investigaciones Agrobiológicas de Galicia (CSIC) Apartado 122, 15080 Santiago de Compostela, Spain
Received February 2, 1996
the ground) and from branch segments taken at 3 and 7 m above soil level. Tissue culture procedures and conditions were as previously described (Vieitez et al. 1994). Briefly, accessory or epicormic shoots were induced on branch segments (3--5 cm in diameter and 30 cm long) by placing them in a growth cabinet at 24 °C, in a 16-h photoperiod and a relative humidity of 80--85%. After 2--3 weeks, flushed shoots (2--10 cm long) were used as the source of explants for establishing cultures. Axillary shoots of the six clones were subcultured for 1--2 years before the rooting experiments began.
Quercus rubra source material consisted of epicormic shoots from the basal zone of the trunk of a 40-year-old tree designated M1. Epicormic shoots were collected in December, cut into 25-cm lengths and stored in the cold for 3 months before being forced to flush in the growth cabinet. The new growth was used as the source of initial explants.
In both species, clonal shoot multiplication was achieved by subculturing decapitated shoots placed horizontally in 500-ml glass jars containing 70 ml of either Gresshoff and Doy (1972) medium (GD) (for Q. robur) or Woody Plant Medium (WPM) (Lloyd and McCown 1980) (for Q. rubra). The multiplication medium was supplemented with 0.2 mg l−1 (0.88 µM) 6-ben-zyladenine (BA), 30 g l−1 sucrose and 6.5 g l−1 Vitroagar (Hispanlab S.A.). The pH of the medium was adjusted to 5.5--5.6 before autoclaving at 121 °C and 108 kPa for 20 min. The multiplication cycle consisted of a 4-week (Q. robur) or 5-week (Q. rubra) period, unless otherwise stated.
Root initiation
For root initiation, shoots 2--3 cm long were excised from proliferating cultures and their basal halves stripped of leaves. Rooting medium consisted of the multiplication medium with reduced macronutrients (GD reduced to one third strength and WPM reduced by half) and without BA. Unless otherwise stated, the shoots were cultured six or seven per jar in 300-ml glass jars containing 60 ml of rooting medium with or without indole-3-butyric acid (IBA) or activated charcoal (AC, Merck No. 2186).
Four series of experiments were performed. The first experi-ment evaluated the efficiency of an initial 5-day dark period and compared two treatments for initiation of rooting prior to growth in auxin-free rooting medium: (a) 7 days growth in rooting medium supplemented with 3 mg l−1 (14.7 µM) IBA, and (b) dipping the basal end of the shoots in 0.5--1 g l−1 (2.5--4.9 mM) IBA solution for 30 s (Q. robur) or 2 min (Q. rubra). In the second experiment, shoots were grown for 24 h in rooting medium with 5 to 50 mg l−1 (28.5--245 µM) IBA before transfer to auxin-free medium. The third series compared the effects of one month’s growth in auxin-free medium with or without 1% AC following a 24-h treatment with IBA at a concentration of 15 mg l−1 (73.5 µM) or 25 mg l−1 (123 µM). The fourth series evaluated in vitro rooting in a mixture of peat/vermiculite/perlite (1/1/1 by volume). Following 24 h in 25 mg l−1 IBA medium, shoots were planted in sterile glass jars containing 100 ml of substrate moistened with 70 ml of rooting medium containing 20 g l−1 sucrose. In
all of these experiments, cultures were kept in a 16-h photo-period, except during the 5-day period of darkness of the first experiment.
Basal darkening
To determine whether the effect of activated charcoal on root-ing resulted from basal darkenroot-ing or from the adsorption of inhibitory compounds by the charcoal, a set of experiments was carried out with SL1 and Monasterio shoots treated for 24 h with 25 mg l−1 or 15 mg l−1 IBA, respectively. Basal darkening was achieved either by including AC in the medium or by wrapping the culture tube in aluminum foil to the height of the agar and covering the surface with an opaque polypropylene disc with two holes for the shoots. Control shoots were grown in rooting medium without activated char-coal or screening. Test tubes, each containing two shoots in 16 ml of medium, were used. All treated shoots (AC, foil wrapped and control) were incubated in a 16-h photoperiod.
Recycling of the original explant
An experiment was designed to determine whether continuous culture (recycling) of the same horizontal explant affected the rooting response of its new shoots. Once the shoots were harvested (Cycle 1), the original explant was transferred to fresh multiplication medium for further reculture (Cycle 2). The rooting capacity of shoots taken from three consecutive culture cycles of SL1, SL3 and Monasterio donor shoots cul-tured in 2-, 3-, 4- or 6-week cycles was evaluated.
Incubation conditions
The shoot multiplication and rooting experiments were con-ducted in a growth chamber at a day/night temperature of 25/20 °C. An irradiance of 30 µmol m−2 s−1 from cool-white fluorescent lamps (OSRAM, L 40 W) was provided through-out a 16-h daily photoperiod. An initial 5-day dark period was applied in the first set of experiments.
Data collection and statistical analysis
At the end of the 1-month rooting period, the percentage of rooted shoots was assessed. Rooting characteristics of these shoots were recorded, including the average number of roots and the length of the longest root. Because shoots rooted in AC medium produced a large number of secondary and tertiary roots, an image analyzer was used to quantify the total root production in terms of projected area and total length of the root system. Shoot quality was assessed on the basis of apical necrosis, leaf drop, browning, and senescence.
Results
Quercus robur
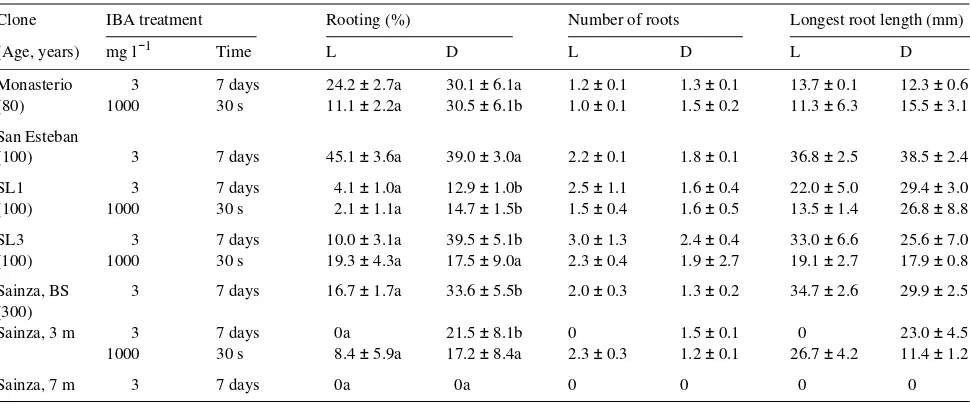
In the first series of root initiation experiments, five Q. robur clones were used (Table 1). An initial five-day dark period improved rooting frequency in six out of the 11 cases evalu-ated, but no significant differences were found for number of roots or longest root length. The two induction treatments afforded similar rooting frequencies, except for clone SL3, whose rooting percentage was doubled by combining the 7-day IBA treatment with an initial 5-7-day dark period. The quick dip method (1 g l−1 IBA for 30 s) generally produced fewer and shorter roots than the 7-day treatment. Although the quantita-tive data suggest that 3 mg l−1 IBA plus 5 days of darkness was the best treatment, the shoots thus treated exhibited browning and senescence.
Because of the poor results of the first series of experiments, 24-h IBA treatments were tested. The best rooting responses were obtained with 25 mg l−1 IBA (results not shown). Shoots treated with 50 mg l−1 IBA exhibited necrosis. The 25 mg l−1 concentration was selected for all clones except Monasterio, for which 15 mg l−1 was used because of its greater sensitivity. For all clones except Sainza 3 and Sainza 7, rooting frequen-cies with the 24-h IBA treatment were higher than those achieved with a quick dip or 7-day IBA treatment (cf. Table 2 and Table 1). An initial 5-day dark period did not improve the rooting capacity of SL1 shoots treated with 25 mg l−1 IBA (results not shown), and was not tried for other clones. In clones Sainza, San Esteban and SL1 (particularly the latter), activated charcoal (AC) significantly increased the percentage of shoots forming roots and increased the root length (Table 2). The rooting rates of Tixon and SL3 shoots were not
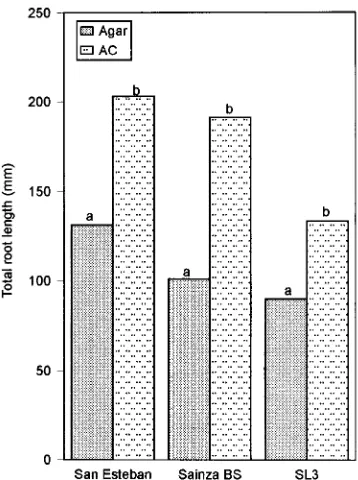
signifi-cantly affected by AC, but shoots of all clones grown on AC medium appeared healthier than those grown without AC, regardless of whether roots were produced. Activated charcoal reduced apical necrosis, leaf drop, browning and senescence and promoted the reinitiation of shoot growth. Generally, the mean number of roots per rooted shoot was not significantly altered (except for the Sainza 3 and Sainza 7 clones), but AC promoted formation of many lateral roots (Figure 1A), and this was reflected in the total root lengths (Figure 2). In the three clones evaluated, total root length was significantly greater on AC medium than on non-AC medium and it also depended on genotype. Because roots are essentially uniform cylinders, the projected area followed the same trend as the total root length (data not shown).
The rooting capacity of Sainza cultures depended on the location of the original material on the tree. On AC medium, shoots derived from basal sprouts had a significantly greater (P < 0.0001) rooting frequency than shoots derived from branch segments collected 3 or 7 m from the ground. More-over, rooted shoots of basal origin produced more roots (P < 0.005) and longer roots (P < 0.0001) than rooted shoots from higher positions.
Monasterio and SL1 shoots incubated in peat/vermicu-lite/perlite substrate had rooting rates of less than 10% because the shoots dried up after a few days despite the addition of liquid rooting medium.
In the basal darkening experiment, the rooting frequency of Monasterio shoots was unaffected by either darkening method, but that of SL1 shoots was significantly increased by both methods (Table 3). Furthermore, rooting rates of SL1 shoots were greater in AC medium than in the aluminum foil darken-ing treatment. The number of roots was not significantly
af-Table 1. Rooting ability of shoot cultures derived from five mature Q. robur trees. Sainza cultures were derived from branch segments collected at three heights on the tree: basal sprouts (BS), and 3 and 7 m from the ground. Rooting was induced either by dipping in 1 g l−1 IBA solution for 30 s or by a 7-day treatment in medium supplemented with 3 mg l−1 IBA. Shoots were cultured with (D) or without (L) an initial period of darkness. Each value represents the mean (± SE) of at least three experiments with 18 replicates per treatment per clone.
Clone IBA treatment Rooting (%) Number of roots Longest root length (mm)
(Age, years) mg l−1 Time L D L D L D
Monasterio 3 7 days 24.2 ± 2.7a 30.1 ± 6.1a 1.2 ± 0.1 1.3 ± 0.1 13.7 ± 0.1 12.3 ± 0.6 (80) 1000 30 s 11.1 ± 2.2a 30.5 ± 6.1b 1.0 ± 0.1 1.5 ± 0.2 11.3 ± 6.3 15.5 ± 3.1 San Esteban
(100) 3 7 days 45.1 ± 3.6a 39.0 ± 3.0a 2.2 ± 0.1 1.8 ± 0.1 36.8 ± 2.5 38.5 ± 2.4 SL1 3 7 days 4.1 ± 1.0a 12.9 ± 1.0b 2.5 ± 1.1 1.6 ± 0.4 22.0 ± 5.0 29.4 ± 3.0 (100) 1000 30 s 2.1 ± 1.1a 14.7 ± 1.5b 1.5 ± 0.4 1.6 ± 0.5 13.5 ± 1.4 26.8 ± 8.8 SL3 3 7 days 10.0 ± 3.1a 39.5 ± 5.1b 3.0 ± 1.3 2.4 ± 0.4 33.0 ± 6.6 25.6 ± 7.0 (100) 1000 30 s 19.3 ± 4.3a 17.5 ± 9.0a 2.3 ± 0.4 1.9 ± 2.7 19.1 ± 2.7 17.9 ± 0.8 Sainza, BS 3 7 days 16.7 ± 1.7a 33.6 ± 5.5b 2.0 ± 0.3 1.3 ± 0.2 34.7 ± 2.6 29.9 ± 2.5 (300)
Sainza, 3 m 3 7 days 0a 21.5 ± 8.1b 0 1.5 ± 0.1 0 23.0 ± 4.5
1000 30 s 8.4 ± 5.9a 17.2 ± 8.4a 2.3 ± 0.3 1.2 ± 0.1 26.7 ± 4.2 11.4 ± 1.2
Sainza, 7 m 3 7 days 0a 0a 0 0 0 0
fected by darkening in either clone, but the development of the root system (in terms of longest root, area and total root length) was significantly enhanced in AC medium (P < 0.01 for SL1; P < 0.005 for Monasterio). None of these variables was signifi-cantly improved by aluminum wrapping. The quality of both shoots and roots was also improved in charcoal medium.
Recycling the same horizontal explant increased both the number of shoots to be rooted and vigor. In all three clones, acceptable rooting rates were maintained in the second and the third cycles (Table 4). Rooting frequency among third-cycle Monasterio shoots was significantly greater than among first-cycle shoots. Within first-cycles, rooting capacity was affected by Table 2. Effect of activated charcoal (AC) on the rooting of shoot cultures derived from five mature Q. robur trees. Sainza culture origins are described in the caption to Table 1. Rooting was induced by 24 h in a medium containing 25 mg l−1 IBA and subsequent transfer to auxin-free medium with or without 1% AC for one month. Values represent the mean ± SE of four experiments with 18 replicates per treatment per clone.
Clone Rooting (%) Number of roots Longest root length (mm)
−AC +AC −AC +AC −AC +AC
Tixon 63.0 ± 1.6a 64.1 ± 8.4a 1.9 ± 0.3a 2.2 ± 0.4a 51.4 ± 1.4a 47.1 ± 4.4a Sainza, BS 51.9 ± 1.0a 72.6 ± 0.6b 2.1 ± 0.2a 2.7 ± 0.3a 37.8 ± 1.9a 51.7 ± 1.1b Sainza, 3 m 1.4 ± 1.2a 16.7 ± 2.0b 0.3 ± 0.2a 1.3 ± 0.2b 4.5 ± 3.9a 13.4 ± 2.9b
Sainza, 7 m 0a 13.0 ± 3.0b 0a 1.3 ± 0.2b 0a 9.6 ± 3.2b
SL3 51.3 ± 0.6a 53.8 ± 3.1a 2.0 ± 0.1a 1.7 ± 0.1a 39.3 ± 1.9a 39.2 ± 1.2a SL1 2.5 ± 1.3a 66.4 ± 4.8b 1.5 ± 0.4a 1.5 ± 0.1a 20.0 ± 2.1a 48.2 ± 5.5b San Esteban 63.8 ± 3.0a 85.8 ± 1.5b 2.0 ± 0.2a 2.1 ± 0.1a 42.4 ± 1.5a 59.3 ± 1.4b 1 Within each clone (row), values for a measured variable followed by the same letter are not different at P = 0.05, according to the LSD test.
shoot age, apparently through its relationship to quality. In clone SL3, in which rooting percentage was significantly greater for 6-week-old shoots than for 4-week-old shoots in the first cycle (P < 0.0001), the former were more vigorous and longer than the 4-week-old shoots. Unlike SL3 shoots, the rooting frequency of second-cycle Monasterio shoots de-creased as shoot age inde-creased.
Quercus rubra
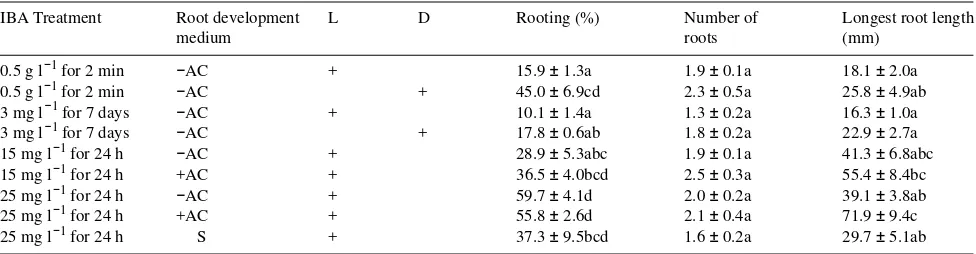
An initial 5-day dark period increased the rooting rates of shoots regardless of whether the IBA treatment used was a quick dip (Figure 1B) or a 7-day treatment in 3 mg l−1 IBA medium, although the increase was statistically significant only for the quick dip treatment (Table 5). The best rooting
percentages were induced by 24-h treatment in 25 mg l−1 IBA medium (Figure 1C). Subsequent culture on AC medium did not significantly increase rooting percentages or number of roots compared with culture on non-AC medium, but the longest root length was significantly increased. As in the Q. robur clones, promotion of root branching in the presence of AC was observed, and shoots rooted in AC medium were healthier and showed no necrosis.
The rooting capacity of red oak shoots in the mixture sub-strate did not differ significantly from that achieved in agar (with or without charcoal), although inadequate contact be-tween shoot and substrate led to shoot desiccation in some
Table 3. Effects of basal darkness, produced by activated charcoal (AC) or aluminum foil wrapping (Al), on in vitro rooting of shoots derived from crown branches of two mature oak trees. Rooting was induced by culture in IBA-containing medium for 24 h and subsequent transfer to an auxin-free medium for one month. The projected area and total root length of the root system were measured by digital image processing methods. Each value represents the mean (± SE) of four experiments with 18 replicates per treatment per clone.
Treatment IBA Rooting Number of Longest root Area Total root length
(mg l−1) (%) roots length (mm) (mm2) (mm)
Clone SL1
Control 25 11.1 ± 2.0a 1.6 ± 0.1a 30.4 ± 6.0a 55.3 ± 3.7a 49.3 ± 5.3a Al 25 23.2 ± 3.3b 1.5 ± 0.1a 25.7 ± 2.5a 68.5 ± 6.7a 59.1 ± 6.6a AC 25 55.2 ± 3.5c 1.6 ± 0.1a 48.0 ± 4.6b 135.3 ± 19.0b 116.5 ± 15.6b Clone Monasterio
Control 15 29.8 ± 4.8a 1.2 ± 0.1a 6.9 ± 1.1a 30.3 ± 3.8a 23.6 ± 1.7a Al 15 32.0 ± 3.6a 1.6 ± 0.1a 9.5 ± 0.5a 31.3 ± 2.8a 25.1 ± 1.4a AC 15 33.7 ± 3.2a 1.6 ± 0.2a 29.8 ± 3.7b 66.8 ± 4.6b 66.4 ± 3.0b 1 Within each clone and column, values followed by the same letter are not different at P = 0.05, according to the Tukey HSD test.
Figure 2. Total lengths of root systems of rooted shoots of three Q. robur clones. Within each clone there was a significant treatment effect (P = 0.05) according to the LSD test.
Table 4. Effect of triple recycling of 20 mm donor shoots on the rooting ability of three mature oak clones. In each reculture, newly developed shoots were excised, incubated for 24 h in medium containing 25 mg l−1 IBA, and then transferred to an auxin-free medium containing activated charcoal. Different reculture periods (2, 3, 4 or 6 weeks) were tested. Each value represents the mean (± SE) error of four experiments, each with 18 replicates per treatment per clone.
Reculture # Rooting (%) Number of Longest root
(Shoot age) roots length (mm)
Clone SL1
1 (4 weeks) 66.4 ± 4.8a 1.5 ± 0.1a 48.2 ± 5.5a 2 (4 weeks) 51.3 ± 3.8a 1.4 ± 0.1a 42.7 ± 1.2a 3 (4 weeks) 52.5 ± 2.4a 1.7 ± 0.0a 54.2 ± 3.8a Clone Monasterio
1 (4 weeks) 52.7 ± 1.6bc 1.5 ± 0.0a 49.8 ± 2.9b 2 (2 weeks) 57.4 ± 3.0cd 1.8 ± 0.0a 29.8 ± 2.5a 2 (3 weeks) 44.4 ± 2.3ab 1.7 ± 0.2a 32.2 ± 2.2a 2 (4 weeks) 38.5 ± 1.0a 1.7 ± 0.1a 38.6 ± 4.5ab 3 (4 weeks) 64.9 ± 2.3d 1.8 ± 0.1a 50.6 ± 3.8b Clone SL3
1 (4 weeks) 16.3 ± 2.7a 1.7 ± 0.5a 33.6 ± 6.8a 1 (6 weeks) 53.8 ± 3.1b 1.7 ± 0.1a 39.2 ± 1.2a 2 (4 weeks) 57.0 ± 4.5b 2.1 ± 0.2a 48.8 ± 1.5a 3 (4 weeks) 43.6 ± 3.8b 1.9 ± 0.2a 36.6 ± 4.6a 1 Within each clone and column, values followed by the same letter
cases. Roots developed in the mixture substrate were signifi-cantly shorter and thinner than those formed in AC medium and had a similar appearance to ex vitro formed roots.
Discussion
We have shown that stabilized shoot cultures initiated from crown material of adult Q. robur and from epicormics of Q. rubra can be rooted in vitro.
The shoots of many woody plants root better if they are incubated in darkness during the first phase of rooting and then transferred to light (McCown 1988). The beneficial effect of darkness may be ascribed to a dark-induced decrease in per-oxidase activity and an increase in endogenous phenol concen-trations during root initiation (Druart et al. 1982). Although darkness stimulates rooting in many species that are difficult to root in vitro, it generally induces shoot senescence after 7--10 days, thereby decreasing plantlet survival (Rugini et al. 1988). In this study, a 5-day dark period was detrimental to the quality of the regenerating Q. robur and Q. rubra plantlets, even when it increased rooting, because it caused shoot ne-crosis. It is possible that dark-treated shoots may be more sensitive to the phytotoxic effects of IBA. The dark response of the San Esteban clone (Table 1) supports Caboni and Damiano’s (1994) conclusion that dark treatment may not be required for certain clones or varieties. The concentration of auxin used to induce rooting and the duration of application can be critical for rooting response. In our experiments, a 24-h exposure to 25 mg l−1 IBA (with no dark period) produced the best rooting frequencies in both species, with the exception of the Sainza 3 and Sainza 7 clones. Welander (1983) showed that for apple shoots, when optimum IBA concentrations are used, the dark treatment has no effect on rooting.
Activated charcoal stimulates rooting of microcuttings in some woody species (McCown 1988). Normally, AC is in-cluded in the root development medium after root-inducing auxin treatment (Le Roux and Van Staden 1991, Dumas and Monteuuis 1995). In seedling and 8-year-old Q. robur mate-rial, the addition of activated charcoal to the IBA medium
caused a reduction in the percentage of rooted shoots, presum-ably because of adsorption of the auxin (Evers et al. 1990). Juncker and Favre (1989) used activated charcoal in the root expression medium of oak shoots; however, it is not possible to draw any conclusions about the effect of AC on rooting capacity from their study because they did not include a con-trol medium lacking AC.
In our study, AC treatment was beneficial for both root system development and shoot quality. A similar effect on the number and quality of Pinus pinaster Ait. roots was reported by Dumas and Monteuuis (1995). The stimulatory effect of charcoal may involve: (1) the reduction of light intensity at the base of the shoots, providing an environment conducive to the accumulation of auxins or cofactors, or both; and (2) the adsorption of substances such as inhibitory phenolics and any excess auxin or cytokinin carried over from previous media (McCown 1988, Bonga and von Aderkas 1992). Darkening the basal part of olive and almond shoots during the rooting phase increases their rooting capacity in the same way as an initial period of darkness (Rugini et al. 1988), and basal shoot dark-ening has been reported to be essential for in vitro root forma-tion by chestnut and walnut shoots (Rugini et al. 1993). However, excluding light at the shoot base did not improve the rooting of Camellia japonica L. (Vieitez et al. 1989) and only increased the rooting rate of one of three apple cultivars tested by Karhu and Zimmerman (1993). Nour and Thorpe (1993) suggest that the mechanism by which activated charcoal im-proves the growth and quality of cedar shoots is based on its capacity to adsorb phytohormones, phenolics and other toxins. In our study, (a) phenolic exudates were observed at the base of oak shoots when charcoal was not included in the medium and (b) basal darkening alone, by means of aluminium foil, had relatively little effect. The positive effect of AC on the in vitro rooting of oak therefore seems to be a result of the adsorption of inhibitory compounds from the medium or the removal of substances from the explants rather than a result of basal darkening. On the other hand, activated charcoal stimulates sucrose hydrolysis during autoclaving, which alters the initial carbon source of the media (Druart and De Wulf 1993). It has Table 5. Rooting performance of shoots from red oak clone M1 cultured in rooting medium for one month with (D) or without (L) an initial 5-day dark period. Rooting was induced either by dipping in 0.5 g l−1 IBA solution for 2 min or by incubation in IBA medium (3, 15 or 25 mg l−1) for 24 h or 7 days. After IBA treatment, shoots were transferred to auxin-free medium with (+AC) or without (−AC) activated charcoal, or to peat/vermiculite/perlite substrate (S). Each value represents the mean ± standard error of three experiments, each with 18 replicates per treatment.
IBA Treatment Root development L D Rooting (%) Number of Longest root length
medium roots (mm)
0.5 g l−1 for 2 min −AC + 15.9 ± 1.3a 1.9 ± 0.1a 18.1 ± 2.0a
0.5 g l−1 for 2 min −AC + 45.0 ± 6.9cd 2.3 ± 0.5a 25.8 ± 4.9ab
3 mg l−1 for 7 days −AC + 10.1 ± 1.4a 1.3 ± 0.2a 16.3 ± 1.0a
3 mg l−1 for 7 days −AC + 17.8 ± 0.6ab 1.8 ± 0.2a 22.9 ± 2.7a
15 mg l−1 for 24 h −AC + 28.9 ± 5.3abc 1.9 ± 0.1a 41.3 ± 6.8abc
15 mg l−1 for 24 h +AC + 36.5 ± 4.0bcd 2.5 ± 0.3a 55.4 ± 8.4bc
25 mg l−1 for 24 h −AC + 59.7 ± 4.1d 2.0 ± 0.2a 39.1 ± 3.8ab
25 mg l−1 for 24 h +AC + 55.8 ± 2.6d 2.1 ± 0.4a 71.9 ± 9.4c
25 mg l−1 for 24 h S + 37.3 ± 9.5bcd 1.6 ± 0.2a 29.7 ± 5.1ab
been suggested that the greater availability of glucose and fructose in an autoclaved AC medium promotes somatic em-bryogenesis in maize anther cultures (Büter et al. 1993) and may also contribute to the observed improvement in rooting of oak shoots.
The mixture substrate used in this study did not support the survival of Q. robur shoots, although it does support both Q. rubra (this work) and chestnut (unpublished results) shoots. Oak microcuttings of juvenile origin have been successfully rooted ex vitro in an 80/20 mixture of light peat and perlite (Meier-Dinkel et al. 1993), which is a more compact substrate than that used in our experiments. The difference in rooting ability in vermiculite substrate between apple and walnut has been related to the basal diameter of the shoots used for rooting (Navatel and Bourrain 1994). A similar explanation may apply to our results, because shoot base diameter increased in the following order: pedunculate oak < red oak < chestnut. Larger shoot bases are less likely to settle in the interstices between substrate particles without contacting the substrate, and there-fore are less likely to dry up.
Vieitez et al. (1994) reported that recycling the same hori-zontally placed explant several times increases multiplication efficiency and allows a shorter culture cycle. We found that the rooting ability of oak shoot crops taken from successive cul-ture cycles was maintained. However, the best shoot age for rooting depended on both clone and cycle.
A degree of rejuvenation can sometimes be obtained by in vitro procedures. Repeated subculturing for shoot multiplica-tion in vitro sometimes brings about rejuvenation, leading to increased competence for rooting (Zimmerman 1986, Bonga and von Aderkas 1992). The effect of this process may be a function of the stabilization period of shoot production. Once the stabilized state has been attained, rooting potential may be greater than in the original source tissue, although the expres-sion of this potential depends on both the quality of the micro-cuttings and the rooting environment (McCown 1988). Subculturing does not always stimulate the rooting potential of mature stock. Quercus is one of the genera that McCown and McCown (1987) characterize as difficult to stabilize and con-sequently difficult to root. The stabilization of our Quercus cultures was only achieved after elongated shoots were cul-tured horizontally on the agar surface (Vieitez et al. 1994). This procedure acts as a type of layering and stimulates the devel-opment of lateral shoots that are elongated and vigorous and probably in a better physiological condition for root formation. Thus, subculturing appears to influence rooting capacity of oak. If rooting in vitro is used as parameter of rejuvenation, then the rooting rates presented here indicate some degree of rejuvenation in our Q. robur cultures.
The shoot culture line developed from basal sprouts of the Sainza tree had higher rooting rates than crown-derived cul-tures. The position of the material of origin on the tree influ-enced rooting ability, reflecting a gradient of increasing maturity from the bottom to the top of the tree. Similar trends have been observed in chestnut trees (Sánchez and Vieitez 1991) and Sequoia sempervirens (D. Don) Endl. (Bon et al. 1994). The low rootability of Sainza 3 and Sainza 7 material suggests that in these clones, subculturing failed to achieve the
rejuvenation required for successful rooting. However, we note that Sainza was 300 years old, whereas none of the other trees was more than 100 years old; this difference in age may explain why crown material from Sainza was more recalcitrant to rejuvenation than crown material from the other trees.
Although results achieved in this work do not guarantee the viability of a large-scale micropropagation of oak, micro-propagated plants could be used as stock plants for cutting propagation. The development of an integrated system would improve the feasibility of mass propagation of selected oak clones (Chalupa 1993, Meier-Dinkel et al. 1993).
Acknowledgments
The technical assistance of M. T. Martinez and N. Vidal is acknow-ledged. This research was partially supported by the Commission of the European Communities through the ECLAIR programme, con-tract AGRE 0067 and by DGICYT, concon-tract PB 92-0017.
References
Bon, M.C., F. Riccardi and O. Monteuuis. 1994. Influence of phase change within a 90-year-old Sequoia sempervirens on its in vitro organogenic capacity and protein patterns. Trees 8:283--287. Bonga, J.M. and P. von Aderkas. 1992. In vitro culture of trees. Kluwer
Academic Publishers, Dordrecht, 236 p.
Büter, B., S.M. Pesticelli, R. Berger, J.E. Schmid and P. Stamp. 1993. Autoclaved and filter sterilized liquid media in maize anther cul-ture: significance of activated charcoal. Plant Cell Rep. 13:79--82. Caboni, E. and C. Damiano. 1994. Rooting in two almond genotypes.
Plant Sci. 96:163--165.
Chalupa, V. 1993. Vegetative propagation of oak (Quercus robur and Q.petraea) by cutting and tissue culture. Ann. Sci. For. 50, Suppl. 1:295--307.
Druart, P. and O. De Wulf. 1993. Activated charcoal catalyses sucrose hydrolysis during autoclaving. Plant Cell Tiss. Org. Cult. 32:97--99. Druart, P., C. Kevers, P. Boxus and T. Gaspar. 1982. In vitro promotion of root formation by apple shoots through darkness effect on endo-genous phenols and peroxidases. Z. Pflanzenphysiol. 108:429--436.
Dumas, E. and O. Monteuuis, 1995. In vitro rooting of micropropa-gated shoots from juvenile and mature Pinus pinaster explants: influence of activated charcoal. Plant Cell Tiss. Org. Cult. 40:231--235.
Evers, P., E. Vermeer and S. van Eeden. 1990. Genetics and breeding of oak. Final Report Project MAI BI 0044. EEC Program Wood Inducing Cork as Renewable Raw Material. Dorschkamps Re-search Institute for Forestry and Landscape Planning, Wageningen, The Netherlands.
Evers, P., E. Vermeer and S. van Eeden. 1993. Rejuvenation of Quer-cus robur. Ann. Sci. For. 50, Suppl. 1:330--335.
Gebhardt, K., U. Frühwacht-Wilms and H. Weisgerber. 1993. Micro-propagation and restricted-growth storage of adult oak genotypes. Ann. Sci. For. 50, Suppl. 1:323--329.
Gresshoff, P.M. and C.H. Doy. 1972. Development and differentiation of haploid Lycopersicon esculentum. Planta 107:161--170. Juncker, B. and J. M. Favre. 1989. Clonal effects in propagating oak
trees via in vitro culture. Plant Cell Tiss. Org. Cult. 19:267--276. Karhu, S.T. and R.H. Zimmermann. 1993. Effect of light and
Kleinschmit, J. 1986. Oak breeding in Germany: experiences and problems. In Proc. IUFRO Joint Meeting of Working Parties on Breeding Theory, Progeny Testing and Seed Orchards. Wil-liamsburg, VA, pp 250--258.
Le Roux, J.J. and J. van Staden. 1991. Micropropagation and tissue culture of Eucalyptus----a review. Tree Physiol. 9:435--477. Lloyd, G. and B. McCown. 1980. Commercially feasible
micropropa-gation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Proc. Int. Plant Prop. Soc. 30:420--427.
McCown, B.H. 1988. Adventitious rooting of tissue cultured plants. In Adventitious Root Formation in Cuttings. Eds. T.M. Davis, B.H. Haissig and N. Sankhla. Discorides Press, Portland, OR, pp 289--302.
McCown, D.D. and B.H. McCown. 1987. North American hard-woods. In Cell and Tissue Culture in Forestry, Vol. 3. Eds. J.M. Bonga and D.J. Durzan. Martinus Nijhoff Publishers, Dordrecht, pp 247--260.
Meier-Dinkel, A. 1987. In vitro Vermehrung und Weiterkultur von Stieleiche (Quercus robur L.) und Traubeneiche (Quercus petraea (Matt.) Liebl.). Allg. Forst-u. J. Ztg. 158:199--204.
Meier-Dinkel, A., B. Becker and D. Duckstein. 1993. Micropropaga-tion and ex vitro rooting of several clones of late-flushing Quercus robur L. Ann. Sci. For. 50, Suppl. 1:319--322.
Navatel, J.C. and L. Bourrain. 1994. Influence of the physical structure of the medium on in vitro rooting. Adv. Hort. Sci. 8:57--59. Nour, K.A. and T.A. Thorpe. 1993. In vitro shoot multiplication of
eastern white cedar (Thuja occidentalis). In Vitro Cell. Dev. Biol. 29P:65--71.
Rugini, E., A. Jacoboni and A. Bazzoffia. 1988. A simple in vitro method to avoid initial dark period and to increase rooting in fruit trees. Acta Hort. 227:438--440.
Rugini, E., A. Jacoboni and M. Luppino. 1993. Role of basal shoot darkening and exogenous putrescine treatments on in vitro rooting and on endogenous polyamine changes in difficult-to-root woody species. Sci. Hort. 53:63--72.
Sánchez, M.C. and A.M. Vieitez. 1991. In vitro morphogenetic com-petence of basal sprouts and crown branches of mature chestnut. Tree Physiol. 8:59--70.
San-José, M.C., A. Ballester and A.M. Vieitez. 1988. Factors affecting in vitro propagation of Quercus robur L. Tree Physiol. 4:281--290. Savill, P.S. and P.J. Kanowski. 1993. Tree improvement programs for European oaks: goals and strategies. Ann. Sci. For. 50, Suppl. 1:368--383.
Sokal. R.R. and F.J. Rohlf. 1981. Biometry. H. Freeman and Company, New York, 859 p.
Vieitez, A.M., M.C. San-José and A. Ballester. 1989. Progress towards clonal propagation of Camellia japonica cv ‘Alba Plena’ by tissue culture techniques. J. Hort. Sci. 64:605--610.
Vieitez, A.M., M.C. San-José and E. Vieitez. 1985. In vitro plantlet regeneration from juvenile and mature Quercus robur L. J. Hort. Sci. 60:99--106.
Vieitez, A.M., F. Pintos, M.C. San-José and A. Ballester. 1993. In vitro shoot proliferation determined by explant orientation of juvenile and mature Quercus rubra L. Tree Physiol. 12:107--117.
Vieitez, A.M., M.C. Sánchez, J.B. Amo-Marco and A. Ballester. 1994. Forced flushing of branch segments as a method for obtaining reactive explants of mature Quercus robur trees for micropropaga-tion. Plant Cell Tiss. Org. Cult. 37:287--295.
Welander, M. 1983. In vitro rooting of the apple rootstock M26 in adult and juvenile growth phases and acclimatization of the plantlets. Physiol. Plant. 58:231--238.