In vitro
callus induction and differentiation on Sturt’s Desert Pea
(
Swainsona formosa
)
Z. Zulkarnain
A), A. Taji
A)and N. Prakash
B)A)School of Rural Science and Agriculture, B)School of Environmental Sciences and Natural Resources Management, University of New England, Armidale NSW 2351, Australia
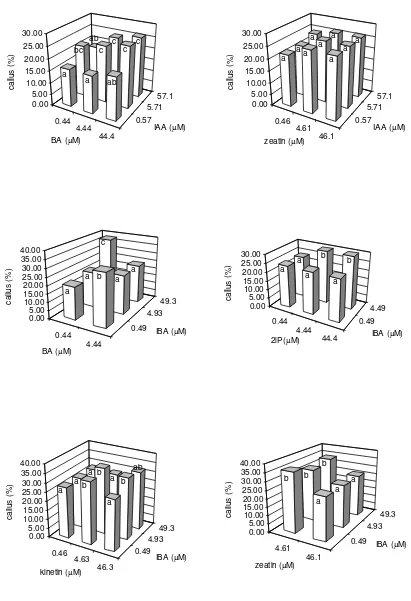
Abstract. Callus growth was induced on media containing IAA (0.57, 5.71, 57.1 µM and IBA (0.49, 4.93,
49.3 µM were combined with BA (0.44, 4.44, 44.4 µM), kinetin (0.46, 4.63, 46.3 µM), 2iP (0.49, 4.93, 49.3 µM) and zeatin (0.46, 4.61, 46.1 µM). The result indicated that callus formation was significantly affected by IAA + BA, IBA + BA, IBA + 2iP, IBA + kinetin and IBA + zeatin. Among IAA combinations, IAA at 5.71 µM or 57.1 µM in combination with 44.4 µM BA produced the highest callus formation (26%). Meanwhile, with the use of IBA the highest callus formation (38%) was obtained on 49.3 µM IBA + 0.44 µM BA, followed by 36% on 4.49 µM IBA + 44.4 µM 2iP, 4.93 µM IBA + 4.63 µM kinetin and 0.49 µM IBA + 4.61 µM zeatin, respectively. The texture and colour of callus varied widely from being compact to friable and white translucent to dark green in colour depending on the types of plant hormones used. However, green embryogenic callus was formed on media supplemented with IBA + kinetin and IBA + zeatin and, subcultured onto a new medium with similar hormones or onto a hormone-free medium. After two weeks in culture, callus grown on the hormone-free medium showed no further growth, turned chlorotic and died. Meanwhile, callus subcultured onto a medium containing IBA + kinetin produced agglomerates of green small shoots without root or roots without shoot and, callus grown on a medium supplemented with IBA + zeatin showed only further callus growth. Some of these shoots and roots, however, were found to be abnormal in appearance. Shoots were hyperhydrated, necrotic or chlorotic, and eventually died after 16 weeks in culture. Some of the roots were short and thick with no root hairs, and grew directly from within the callus.
Additional keywords: micropropagation, anther culture, breeding, auxin, cytokinin, native plant, Australia
Introduction
Sturt’s desert pea,
Swainsona formosa
(G.Don) J. Thompson) which in an Aboriginal
language is known as Marlukuru is one of Australia's most spectacular wild flowers and
is the floral emblem of South Australia. Its large flag-shaped flowers coloured bright
red (or pure white to deep purple in some wild specimens) has made this plant one of
most spectacular flowering plants in the world (Williams and Taji 1991).
The economic importance of this plant is its use as hanging basket, container or
cut flower plants (Kirby 1996a; Kirby 1996b). However, the production of large
amount of pollen grains in flowers has become an impediment in commercialisation of
Sturt’s desert pea. Pollen grains released by anther may stain the petals and therefore
reduce flower quality. In addition, self pollination during transportation may also occur
and make flowers degenerate quickly.
Haploid technology allows obtaining a homozygous generation via androgenesis
or direct plant regeneration from microspores resulting in male-sterile plants that
produce no pollen grains. To date haploid plant production has been successful in
various ornamental species such as
Anemone
,
Zantedeschia
and
Delphinium
(Custers,
Visser
et al.
2001)
. Previous attempt of Sturt’s desert pea anther culture was met with
limited success (Tade 1992). The present study was undertaken to investigate the
Materials and methods
The basal medium used was B5 (Gamborg, Millers et al. 1968) fortified with myo-inositol and vitamins,
and 2% sucrose, solidified with 8 g/L Bitek™ (Difco) agar and pH was adjusted to 5.8 ± 0.2 prior to autoclaving at 121oC (1.1 kg cm-2) for 15 min.
Indole-3-acetic acid (IAA): 0.57, 5.71, 57.1 µM and indole-3-butyric acid (IBA): 0.49, 4.93, 49.3 µM were combined with 6-benzylaminopurine (BA): 0.44, 4.44, 44.4 µM, 6-furfurylamino purine (kinetin): 0.46, 4.63, 46.3 µM, 2-isopentenyl adenine (2iP): 0.49, 4.93, 49.3 µM and zeatin: 0.46, 4.57, 45.7 µM.
Floral buds with anthers containing pollen grains at tetrad stage were surface sterilised in 70% alcohol for 10 seconds followed by rinsing in sterile water. The sepals and petals were removed to expose the anthers. Ten anthers that were obtained from a single bud were plated horizontally onto the medium in a Petri dish. Cultures were incubated at 25 ± 1oC and 16/8 hours photoperiod under cool white fluorescent lamps. Callus growth was assessed until 16 weeks after initiation.
The induction of in vitro differentiation was tried from embryogenic callus on a fresh medium containing the same growth regulators or on a growth regulator-free medium. Media preparation and environmental culture conditions were similar to callus induction. Callus growth was observed until 16 weeks of culture incubation.
Results and discussion
Callus growth
The inclusion of auxins and cytokinins in the culture medium greatly influenced callus
induction and development in Sturt’s desert pea anther culture. However, only anthers
cultured in the combination of IAA + BA or IAA + zeatin produced callus. No callus
was found in anthers cultured on either IAA + 2iP nor IAA + kinetin. IAA + BA
significantly affected callusing (
P
< 0.05) with the highest production (26%) being
obtained at 5.71 µM or 57.1 µM IAA + 44.4 µM BA. The combination of IAA +
zeatin, however, did not result in significant effect on callus formation (
P
> 0.05). In
addition, combinations of IBA with BA, 2iP, kinetin or zeatin also significantly affected
callus formation (
P
< 0.05). The highest callus formation was 38% at 49.3 µM IBA +
0.44 µM BA, followed by 36% on 4.49 µM IBA + 44.4 µM 2iP, 4.93 µM IBA + 4.63
µM kinetin and 0.49 µM IBA + 4.61 µM zeatin, respectively (Figure 1).
The comparison of callusing potential of Sturt’s desert pea anthers at different
types and concentrations of auxin and cytokinin indicated that IBA + BA was the best
one, as it showed the highest callusing capacity on 49.3 µM IBA + 0.44 µM BA. The
callus-promoting effect of auxin and cytokinin such as IBA and BA had been observed
in tissue culture of Sturt’s desert pea using anther
(Tade 1992) and hypocotyl (Taji and
Williams 1989) as culture materials.
Shoot differentiation
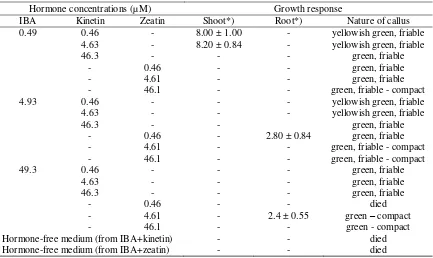
Callus from media containing IBA + kinetin and IBA + zeatin were transferred to a new
medium with or without the growth regulators. The types and concentrations of growth
regulator used were similar to those for callus initiation. Shoot differentiation was
found on callus subcultured on medium with IBA + kinetin after 4 weeks (Table 1).
The shoots, however, appeared to be hyperhydrated characterised by thick leaves
and translucent appearance and, showed a very slow growth rate. Hyperhydration is a
common phenomenon in
in vitro
systems and was also reported on shoot differentiated
from callus culture of plants such as
Chrysanthemum, Rosa
and
Vitis
(Smith 1992) and
cauliflower (Vandermoortele 1999). In addition to hyperhydration, some shoots
became chlorotic and turned yellow in colour, some others were necrotic and
completely degenerated after 16 weeks in new medium.
Root differentiation
Instead of forming shoots, morphogenic callus originating from medium containing IBA
+ zeatin showed only root formation when subcultured onto a new medium.
There was a very limited root formation (Table 1). Roots differentiated from
callus cultured on medium supplemented with 49.3 µM IBA + 0.46 µM zeatin were
found to be abnormal as indicated by their thick and short appearance without root
hairs. Meanwhile, roots regenerated from callus cultured on medium containing 4.93
µM IBA + 0.46 zeatin showed a normal appearance with lots of root hairs.
Neither shoot nor root differentiation was found on callus cultured on the
hormone-free medium. Callus cultured on this medium was found to be chlorotic and
died after 2 weeks in culture.
Anther culture of several legumes was reported by many authors with different
responses. Successful haploid regenerations were reported in
Trifolium alexandrinum
(Mokhtarzadeh and Constantin 1978),
Albizzia lebbeck
(Gharyal, Rashid
et al.
1983)
and
Trifolium pratense
(Bhojwani, Mullins
et al.
1984). In addition, some legumes
including soybean (Ivers, Palmers
et al.
1974), bean (Peters, Crocomo
et al.
1977),
pigeon pea (Bajaj, Singh
et al.
1980), peanut, alfalfa and pea (Mroginski and Kartha
1984) and winged bean (Rao, Rao
et al.
1986) were reported to produce callus but
failed to regenerate haploid plants.
Conclusions
Sturt’s desert pea is normally propagated through seeds and, little is
known about its
micropropagation. Being a diploid plant, a large number of pollen grain is produced
within the flower causing serious problem in its commercialisation as cut flowers. The
haploid production through anther culture has become a new approach in breeding
strategies of Sturt’s desert pea to produce pollenless plants. The present paper is among
Table 1. The response of callus following subculture onto medium with or without
growth regulators.
Hormone concentrations (µM) Growth response
IBA Kinetin Zeatin Shoot*) Root*) Nature of callus
0.49 0.46 - 8.00 ± 1.00 - yellowish green, friable
4.63 - 8.20 ± 0.84 - yellowish green, friable
46.3 - - - green, friable
- 0.46 - - green, friable
- 4.61 - - green, friable
- 46.1 - - green, friable - compact
4.93 0.46 - - - yellowish green, friable
4.63 - - - yellowish green, friable
46.3 - - green, friable
- 0.46 - 2.80 ± 0.84 green, friable
- 4.61 - - green, friable - compact
- 46.1 - - green, friable - compact
49.3 0.46 - - - green, friable
4.63 - - - green, friable
46.3 - - - green, friable
- 0.46 - - died
- 4.61 - 2.4 ± 0.55 green – compact
- 46.1 - - green - compact
Hormone-free medium (from IBA+kinetin) - - died
Hormone-free medium (from IBA+zeatin) - - died
*) ± Standard deviation
References
Bajaj YPS, Singh H, Gosal SS (1980) Haploid embryogenesis in anther culture of pigeon pea (Cajanus cajan). Theoretical and Applied Genetics 58, 157-159.
Bhojwani SS, Mullins K, Cohen D (1984) Intra-varietal variation for in vitro plant regeneration in the genus Trifolium. Euphytica 33, 913-921.
Custers J, Visser M, Snijder R, Litovkin K, Geest Lvd (2001) 'Model plants pave the way to haploid technology; microspore embryogenesis in ornamentals.' Plant Research International B.V., Wageningen, The Netherlands.
Gamborg OL, Millers RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Experimental Cell Research 50, 151-158.
George EF, Sherrington PD (1984) 'Plant propagation by tissue culture.' (Exegetics Limited: England)
Gharyal PK, Rashid A, Maheshwari SC (1983) Production of haploid plantlets in anther cultures of
Albizzia lebbeck L. Plant Cell Reports 2, 308-309.
Guo Y-D, Sewón P, Pulli S (1999) Improved embryogenesis from anther culture and plant regeneration in timothy. Plant Cell, Tissue and Organ Culture 57, 85-93.
Immonen S, Robinson J (2000) Stress treatment and ficoll for improving green plant regeneration in triticale anther culture. Plant Science 150, 77-84.
Kirby GC (1996a) Sturt's Desert Pea as cut flower crop. In '4th National Workshop for Australian Flower'. Perth, Australia pp. 204-209
Kirby GC (1996b) Sturt's Desert Pea for pot plant and hanging baskets. In '4th National Workshop for Australian Flower'. Perth, Australia pp. 44-48
Kristiansen K, Andersen SB (1993) Effects of donor plant temperature, photoperiod, and age on anther culture response of Capsicum annuum L. Euphytica 67, 105-109.
Mokhtarzadeh A, Constantin MJ (1978) Plant regeneration from hypocotyl- and anther-derived callus of berseem clover. Crop Science 18, 567-572.
Mroginski LA, Kartha KK (1984) Tissue culture of legume crops for crop improvement. In 'Plant Breeding Reviews'. (Ed. J Janick) pp. 215-264. (AVI Publishing Co. Inc.: New York)
Peters JA, Crocomo OJ, Sharp WR, Paddock EF, Tegenkamp I, Tegenkamp T (1977) Haploid callus cells from anthers of Phaseolus vulgaris. Phytomorphology 27, 79-85.
Rao UI, Rao IVR, Narasimham M (1986) Induction of androgenesis in the in vitro grown anthers of winged bean (Psophocarpus tetragonolobus). Phytomorphology 36, 111-116.
Smith EF (1992) The preparation of micropropagated plantlets for transplantation. British Society for Plant Growth Regulation Newsletter 1, 3-4.
Tade E (1992) Anther and ovule culture of Clianthus formosus. Master of Rural Science thesis, University of New England.
Taji AM, Williams RR (1989) In vitro propagation of Clianthus formosus (Sturt's Desert Pea) an Australian native legume. Plant Cell, Tissue and Organ Culture 16, 61-66.
Trewavas AJ (1982) Growth substance sensitivity: the limiting factor in plant development. Physiologia Plantarum 55, 60-72.
Vandermoortele J-L (1999) A procedure to prevent hyperhydricity in cauliflower axillary shoots. Plant Cell, Tissue and Organ Culture 56, 85-88.