www.elsevier.com/locate/ibmb
Isolation and endocrine regulation of an HMG–CoA synthase
cDNA from the male Jeffrey pine beetle, Dendroctonus jeffreyi
(Coleoptera: Scolytidae)
Claus Tittiger
*, Chatty O’Keeffe, Cody S. Bengoa, Lana S. Barkawi
1, Steven J.
Seybold
1, Gary J. Blomquist
Department of Biochemistry, University of Nevada, Reno, NV 89557-0014, USA
Received 31 January 2000; received in revised form 3 May 2000; accepted 8 May 2000
Abstract
We have isolated a full length 3-hydroxy-3-methylglutaryl coenzyme A synthase (HMG-S) cDNA from the male Jeffrey pine beetle, Dendroctonus jeffreyi Hopkins, and studied the effects of topical applications of juvenile hormone III (JH III) on its expression. The predicted translation product of this apparently single copy gene has 63% and 58% identity with HMG-S1 and HMG-S2 from Blattella germanica (L.), and 61% identity with Drosophila melanogaster Hmgs. HMG-S transcript levels remain uniformly low in JH III-treated and control D. jeffreyi females, but are induced approximately 2.5- to 5-fold in JH III-treated males. JH III causes a dose- and time-dependent increase in HMG-S transcripts in the male metathoracic–abdominal region. Since monoterpenoid pheromone precursor synthesis and HMG–CoA reductase expression are under the control of JH III in the metathorax of Ips bark beetles, the observed HMG-S expression pattern suggests that the isoprenoid pathway is similarly important for semioch-emical production in D. jeffreyi. 2000 Elsevier Science Ltd. All rights reserved.
Keywords: Coleoptera; Dendroctonus; cDNA; Juvenile hormone; HMG–CoA synthase; Isoprenoid; Pheromone
1. Introduction
In vertebrates, the isoprenoid pathway leads to a var-iety of sterol and nonsterol products and has been exten-sively studied in the context of controlling cholesterol biosynthesis (Goldstein and Brown, 1990). Since insects lack the ability for de novo cholesterol production (Beenakkers et al., 1985), all products from the pathway are nonsterols in these animals. The sesquiterpenoid juv-enile hormone (JH) has long been considered among the most important isoprenoid products in insects (Koyama et al., 1985; Feyereisen and Farnsworth, 1987; Couil-laud, 1991), but the importance of the timely production of dolichol, ubiquinone, and protein prenyl groups in
* Corresponding author. Tel.:+1-775-784-6480; fax: +1-775-784-1419.
E-mail address: [email protected] (C. Tittiger).
1 Present address: Departments of Entomology and Forest Resources, 219 Hodson Hall, University of Minnesota, St Paul, MN 55108-6125, USA.
0965-1748/00/$ - see front matter2000 Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 0 9 9 - 0
development and reproduction is also becoming increas-ingly clear (Havel et al., 1992; Lai et al., 1998; van Doren et al., 1998; Castillo-Gracia and Couillaud, 1999). Recently, an additional role for the isoprenoid pathway in insects, pheromone synthesis, has been demonstrated with the observation that male Ips bark beetles use it to produce monoterpenoid (C10) aggregation pheromone components (Ivarsson et al., 1993; Seybold et al., 1995; Tillman et al., 1998). Since plant monoterpenoids are produced via the DOX/P, or GAP/Pyruvate pathway (Arigoni et al., 1997), and since geranyl diphosphate (C10) generally serves as an intermediate for longer chain isoprenoids in vertebrates (Laskovics and Poulter, 1981), monoterpenoid pheromone production in the Coleoptera is a rare example of mevalonate-based monoterpenoid biosynthesis.
insect HMG-S have been done in the German cockroach,
Blattella germanica (L.). This insect has two genes
encoding apparently cytosolic enzymes (Martinez-Gon-zalez et al., 1993; Buesa et al., 1994). Their expression patterns appear coordinated in early embryos, but are complementary during larval stages. Their activity and expression are also modulated differently in different tissues during the 8 day gonadotrophic cycle, suggesting their involvement in regulating the isoprenoid pathway during egg production (Casals et al., 1996). An expressed sequence tag (EST) for Drosophila
mel-anogaster Hmgs (GenBank accession number LP4424)
was recovered during a P-element mutagenesis screen (Spradling et al., 1999), but the expression pattern has yet to be reported. The signals governing the isoprenoid pathway in insects remain mostly unknown, though in male I. paraconfusus and I. pini, JH III plays a major role in regulating HMG–CoA reductase (EC 1.1.1.34, HMG-R) transcript levels and enzyme activity (Tittiger et al., 1999; Tillman, Lu, Dwinell, Tittiger, Hall, Blomquist and Seybold, unpublished results).
Based on our studies of the endocrine regulation of de novo aggregation pheromone biosynthesis in Ips beetles, we became interested in determining if the isop-renoid pathway is similarly regulated for semiochemical production in the Jeffrey pine beetle, Dendroctonus
jef-freyi. As a first step to address this question, and to
con-tribute to the general knowledge of HMG-S in insects, we isolated an HMG-S cDNA from D. jeffreyi and stud-ied its regulation and tissue distribution following topical applications of JH III.
2. Materials and methods
2.1. Insects and treatments
Sections of D. jeffreyi-infested Pinus jeffreyi Grev. and Balf. trees from Shasta County, CA (T22N, R4E, S21, VI-19-98), and Carson City County, NV (T15N, R19E, S31, XI-4-98), were kept in darkened rearing chambers in a greenhouse (Browne, 1972). Emerging adult D. jeffreyi were collected in glass jars, sexed, and stored with damp paper towels at 4°C before use.
JH III (Sigma, St Louis, MO) was applied topically in 0.5µl acetone to abdominal venters. The insects were incubated at room temperature in small glass jars con-taining glass wool and a vial of water, and then either dissected into liquid nitrogen or frozen whole at280°C. Unless otherwise noted, the JH III dose was 5.3
µg/beetle, and the incubation time was 20 h. Control insects were treated with 0.5 µl acetone.
2.2. cDNA isolation
Polyadenylated [poly(A)+] RNA was isolated from insects that had been treated with 7.1 µg JH III or
ace-tone (controls) using the QuickPrep Micro kit (Pharmacia, Piscataway, NJ). One µg was used to pre-pare first strand template cDNA with SuperScript RNase -reverse transcriptase (Life Technologies, Rockville, MD) and T17CSX primer (Frohman et al., 1988), and diluted to a 0.5 ml final volume.
Two degenerate primers were designed based on con-served sequences of other metazoan HMG-Ss. The for-ward primer corresponded to amino acids 190–199 of
B. germanica HMG-S, and the reverse to a.a. 328–336
(Martinez-Gonzalez et al., 1993). Their sequences were: GCI TA(G/C) GA(G/C) TT(G/C) TA(G/C) AA(A/G) CC and CA(G/C) GIN GTG TAC AT(A/G) TTN CC, respectively. PCR amplifications were done in 100 µl containing 20 mM Tris–HCl (pH 8.4), 50 mM KCl, 2.5 mM MgCl2, 50 pmol each primer, 10µl template cDNA, and 2.5 U Taq DNA polymerase (Life Technologies). The cycling parameters were: 1 min at 95°C, followed by 35 cycles of 40 s at 94°C, 1 min at 52°C, 3 min at 72°C, and a 15 min extension at 72°C. Inserts were pur-ified with a Microcon 100 spin cup (Amicon, Bedford, MD), ligated into pT7Blue (Novagen, Madison, WI), and sequenced.
2.3. cDNA library construction and screening
Total RNA was isolated using a guanidinium isothi-ocyanate–CsCl centrifugation procedure (Glisin et al., 1974) from 11 male insects that had been treated with 13.3µg JH III. Poly(A)+RNA was further purified using oligo-dT push columns (Strategene, La Jolla, CA) and used to construct a library inλZAP II (Stratagene). The HMG-S cDNA fragment in pT7Blue was labeled with [32P]dCTP (NEN Dupont, Boston, MA) by PCR (Mertz and Rashtchian, 1994) using the T7 and U19 primers that flanked the insert. Replica nylon filters (Nytran+, Schleicher and Schuell, Keene, NH) containing 1.5×105 plaque forming units (pfu) of the primary library were hybridized with the32P-labeled HMG-S cDNA fragment in 50% formamide, 5×SSC, 50 mM sodium phosphate, pH 7.0, and 7% SDS at 42°C. The filters were washed twice at room temperature, 10 min/wash, with 2×SSC, 0.1% SDS, and then once at 58°C for 20 min with 0.2
×SSC, 0.1% SDS. Positive plaques were imaged using a BioRad Molecular Imager. Duplicate positive plaques were purified after a second round of screening.
2.4. Genomic Southern blotting
Genomic DNA was isolated from 10 individual adult females using a protocol based on Chia et al. (1985), digested with Eco RI, and transferred to nylon mem-brane (Hybond N+, Amersham, Piscataway, NJ) follow-ing separation on a 0.8% agarose gel. The membrane was hybridized with the32
7.0, 0.25 M NaCl, and 7% SDS at 42°C, and washed twice for 10 min at room temperature with 2 × SSC, 0.1% SDS, and once for 10 min at 45°C with 0.4×SSC, 0.1% SDS. The membrane was then hybridized separ-ately with radiolabeled λ phage DNA to visualize the marker.
2.5. Northern blotting
For the tissue distribution study, adult beetles were dissected into approximate head, thoracic, and abdomi-nal body regions. The dissections were approximate because the external morphology obscures the true bor-ders between the body regions. The tissues were frozen at 280°C until total RNA was isolated using TRIzol Reagent (Life Technologies) according to the supplier’s protocol. Two blots were prepared using dissected male and female tissues [Fig. 4(B) and (C)]. For both the time-course and JH III dose–response studies, triplicate samples of five male thoraces per sample were used. RNA was separated on glyoxal agarose gels (Sambrook et al., 1989), transferred to Hybond N membranes (Amersham), and fixed by UV cross-linking. Membranes were hybridized with the mouseβ-actin (Stratagene) or HMG-S cDNA as described above for the Southern blot. Both probes were labeled with [α32P]dCTP by PCR using the F1 and R5 primers (Fig. 1). Membranes were washed as described above for the library screens, except that the high stringency washes were for 30 min at 65°C for the HMG-S probe, and for 30 min at 42°C for β -actin. The membranes were imaged with a BioRad mol-ecular imager and densitometry was done with Molecu-lar Analyst software (BioRad, Hercules, CA).
2.6. Sequence analysis
Inserts from all recombinant plasmids and PCR pro-ducts were sequenced by primer walking using the dRhodamine dye-terminator kit (Applied Biosystems, Foster City, CA) and an ABI Prism 310 sequencer. Both strands of all clones were sequenced at least once. Sequence analysis was done using the PCGene software package (Version 6.85, IntelliGenetics, Mountain View, CA). The conceptual translation of D. melanogaster
Hmgs (FlyBase annotation FBan00043116, protein ID
number AAF577996) was obtained through FlyBase (1999). Protein sequences were aligned using CLUSTAL W (Thompson et al., 1994). The D. jeffreyi HMG-S cDNA sequence is available from Genbank, accession number AF166002.
3. Results
3.1. HMG-S cDNA
Amplification of first strand cDNA prepared from male D. jeffreyi using degenerate primers corresponding
to well conserved portions of metazoan HMG-Ss resulted in a |0.4 kb fragment whose predicted trans-lation product had over 50% identity with the corre-sponding portions of HMG-S from humans (Boukaftane and Mitchell, 1997) and B. germanica HMG-S1 and HMG-S2 (Buesa et al., 1994; data not shown). In order to determine the full length sequence, a cDNA library was constructed and hybridized with the 32P-labeled D.
jeffreyi HMG-S fragment. A screen of 7×104
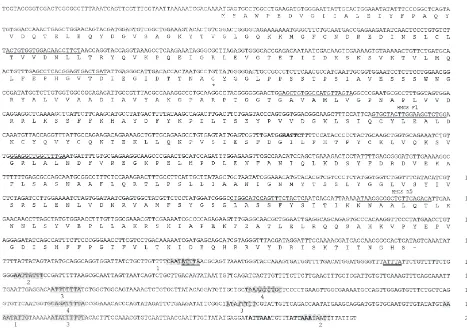
pfu of the primary library yielded six positive plaques. Five were purified after a second round of screening. Preliminary analysis showed that they were of the same length and sequence, and one was chosen for complete sequencing. The 2015 bp cDNA contains a 1371 bp open reading frame flanked by 58 bp and 584 bp 59and 39untranslated regions (utrs) (Fig. 1). The 39 utr has two polyadenyl-ation signals, located 18 and 29 bp from the 39 end. There are several 9 and 10 bp inverted repeats in the 39 utr which may form stem–loop structures. Alternatively, transcript destabilizing motifs (Wennborg et al., 1995) are also present at positions 1398 and 1542.
The open reading frame encodes a 457 amino acid (a.a.) protein with 63.1 and 58.2% identity with HMG-S1 and HMG-S2 from B. germanica, respectively, and 60.9% identity with D. melanogaster Hmgs (Fig. 2). The overall a.a. identity is 35% when aligned with insect and human cytosolic HMG-Ss, though this value rises to 63% within the putative catalytic domain (Miziorko and Behnke, 1985).
3.2. Gene copy number
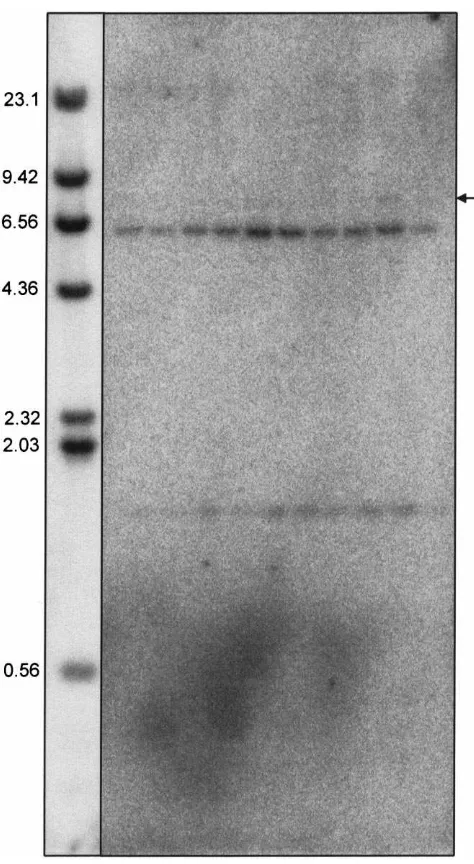
Based on the precedent set in all metazoans in which HMG-S has been studied, we expected two HMG-S genes per D. jeffreyi haploid genome. To investigate this, a Southern blot of Eco RI digested genomic DNA was prepared and hybridized with a portion of the HMG-S cDNA. The probe, which contains an internal Eco RI site (Fig. 1), hybridized to.6.3 and.1.5 kb fragments
in all samples following moderately-low stringency washes (Fig. 3). A very faint signal can also be observed in some samples at .7.9 kb.
3.3. Tissue distribution and endocrine regulation
Fig. 1. Sequence and translation of HMG–CoA synthase cDNA from D. jeffreyi. The predicted translation product is shown below the cDNA. Primer sites for sequencing are indicated by underlining; the F1 and R5 primers used for probe preparation are noted above the sequence. The
Eco RI site is noted in bold italics. The asterisk indicates the perfectly conserved cysteine. The two polyadenylation sequences are in bold, and
inverted repeat sequences are shaded and numbered. Putative transcript destabilizing motifs are double underlined.
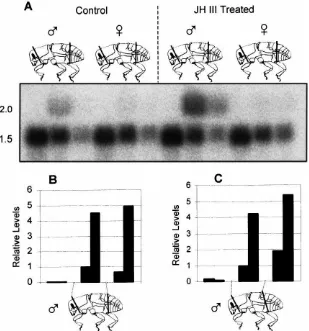
The second cut was made dorsally between the metathor-acic postnotum and abdominal tergite 1, was angled pos-teriorly, and also ended between abdominal sternites 2 and 3 [Fig. 4(C)]. Ventrally, in both cases, the meta-thorax was separated from the abdomen between the coxal base and abdominal sternite 3 (first apparent ven-tral sclerite). Terminology follows Lawrence and Britton (1991). When the perpendicular cut was performed, most of the transcript localized in the thoracic section [Fig. 4(B)]. A second blot was prepared from beetles that were dissected with the angled cut. In this case, a slightly higher proportion of message localized in the abdominal section compared to the thoracic section [Fig. 4(C)]. In both blots, control (acetone treated) insects had a uni-formly low expression level, while JH III treated males showed an approximately 4-fold induction, most of which was found in the thoracic and abdominal sections, depending on the way the bodies were dissected.
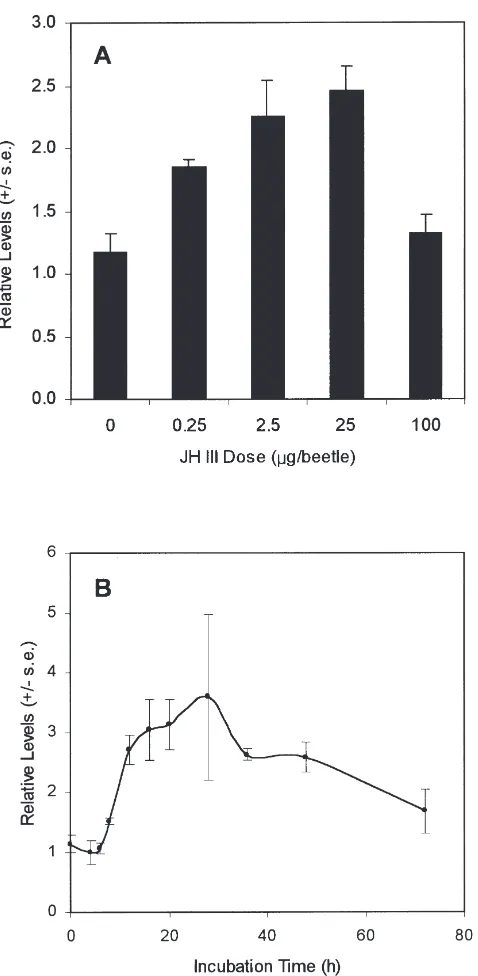
The effects of JH III dose on HMG-S transcript levels were further studied using Northern blots of total RNA from male thoraces dissected with the transverse cut [Fig. 4(C)]. HMG-S message levels increased with increasing JH III dose, to about 2.5-times uninduced
lev-els at 25µg, then declined to control levels at the highest dose tested [Fig. 5(A)]. Northern blots of RNA prepared at various times following topical application of 13.3µg JH showed a lag of about 6 h before a modest increase in HMG-S transcript levels [Fig. 5(B)]. Maximal expression (3.6-fold over controls) was reached by 15 h, followed by a gradual decline to near control levels.
4. Discussion
We used a standard combination of PCR and library screening methods to isolate a cDNA for HMG-S from male D. jeffreyi. The fact that the size of all five clones isolated from the library corresponds to the transcript size on Northern blots suggests that the clone we have characterized, designated “DJHMG-S,” is a full length cDNA.
Fig. 2. Alignment of D.jeffreyi HMG–CoA synthase cDNA predicted translation product (D. jeffreyi) with HMG-S from B. germanica (B.
german-ica 1, B. germangerman-ica 2), D. melanogaster, and humans (H. sapiens). Identgerman-ical residues are indgerman-icated by an asterisk, and similar residues by a period.
Gaps inserted in sequences to optimize the alignment are shown by hyphens. The heavy line overlies the putative HMG-S catalytic domain.
all HMG-Ss reported to date (Misra et al., 1993). Similar to B. germanica HMG-S1, a 6 a.a. gap must be inserted between residues 235–236 of the predicted
Dendroc-tonus protein in order to optimize alignments with B. germanica HMG-S2 and the human enzyme (Fig. 2). As
with the other insect sequences, the beetle sequence is shorter than the human sequence at both the N- and C-termini, and there is no recognizable amino terminal tar-geting sequence, suggesting that the bark beetle enzyme is cytosolic.
The presence of various elements in the 39utr invites speculation on their possible roles in regulating HMG-S transcript levels. In their discussion of B. germanica HMG-S, Buesa et al. (1994) noted the presence of puta-tive cytoplasmic polyadenylation signals (Fox et al., 1989) close (|180 nt) to the 39 terminus to address the observed delay between HMG-S transcript levels and enzyme activity. Two such “UUUUUAU” signals are also present in the DJHMG-S 39 utr, but at positions 1439 and 1695 — hundreds of bases upstream of the polyadenylation signals. Secondly, the 9 and 10 bp inverted repeats may affect transcript stability by for-ming stem–loop structures, as has been hypothesized for
mitochondrial HMG-S transcripts in rats (Ayte´ et al., 1993). Alternatively, transcript destabilizing motifs that may be recognized by specific RNases (Wennborg et al., 1995) are also present at positions 1488 and 1542. The presence of these motifs in the D. jeffreyi HMG-S 39utr suggests that transcript stability may be modulated, as observed for HMG-S in B. germanica; however, the possible role of any of these sequences in D. jeffreyi must await assays of HMG-S transcript stability, protein levels, and activity.
Fig. 3. HMG-S gene copy number. Southern blot of genomic DNA from individual female D. jeffreyi hybridized with HMG-S cDNA and washed under moderately low stringency.λDNA marker is shown to the left, with fragment sizes noted in kb. The arrow to the right indi-cates the faint|7.9 kb signal.
HMG-S locus in metazoans is unusual, but not unpre-cedented. A survey of the recently completed D.
mel-anogaster genome database revealed a single HMG-S
locus at 53C1 on chromosome 2 (FlyBase, 1999). Here, two alternatively spliced transcripts are predicted to encode HMG-S; alternate first exons encoding the 59utrs splice just upstream of a common second exon, which contains the coding region. One of these, CG4311, has been confirmed during a P-element search (Spradling et al., 1999). A single copy HMG-S gene raises questions about the enzyme’s role in insect metabolism. In ver-tebrates, one gene encodes a mitochondrial HMG-S for ketone body production, while a second gene encodes a cytosolic form for isoprenoid biosynthesis. It is
interest-ing that all insect HMG-S sequences identified thus far are predicted to be cytosolic, even though insects have ketone body synthetic enzymes such as HMG–CoA lyase (CG10399; FlyBase, 1999), and can metabolize ketone bodies (Bailey et al., 1972; Beis et al., 1980). This suggests that a mitochondrial HMG-S should be present in insects; and calls for studies of the sub-cellular localization of HMG-S in various insect tissues.
HMG-S showed a modest response to topically applied JH III in a dose- and time-dependent manner in male D. jeffreyi. It is surprising that no increase in HMG-S transcript levels was observed in JH III-treated female insects since JH stimulates oogenesis in insects (Riddiford, 1994), and choriogenesis is thought to require synthesis of prenylated proteins. In cockroaches, the two HMG-S genes are coordinately regulated in the ovary during the gonadotrophic cycle, but not in the fat body (Casals et al., 1996). The absence of detectable amounts of HMG-S transcript in female D. jeffreyi may reflect the time point and/or JH III dose chosen for analysis. Alternatively, if the precedent set in cock-roaches and other Metazoa holds true in bark beetles, a second, undetected HMG-S gene may be activated in female D. jeffreyi.
The JH III-mediated regulation of HMG-S, likely con-trolling the isoprenoid pathway, suggests that semioch-emical production in D. jeffreyi involves the isoprenoid pathway and is regulated in a manner similar to that in
Ips bark beetles. In male Ips spp., the isoprenoid
path-way is important for the production of monoterpenoid pheromone components (Ivarsson et al., 1993; Seybold et al., 1995; Tillman et al., 1998). The effect of JH III on HMG-R transcript levels in male I. paraconfusus and
I. pini (Tittiger et al., 1999; Tillman, Lu, Dwinell,
Tit-tiger, Hall, Blomquist and Seybold, unpublished results) is analogous to that observed for HMG-S in D. jeffreyi, and HMG-R transcript levels in male D. jeffreyi also fol-low a very similar JH III-regulated expression pattern (Tittiger, Bengoa, Barkawi, Blomquist and Seybold, unpublished results). Furthermore, almost all of the detectable HMG-S transcript localizes in the metathorax and abdomen (Fig. 4), with the slight differences in tissue isolation suggesting that expression localizes near the metathoracic–abdominal border. This body region also has relatively high HMG-R mRNA levels (Hall, Mastick, Tittiger, Seybold and Blomquist, unpublished results), and is the same general area where pheromone precursors are synthesized in I. paraconfusus (Ivarsson et al., 1998). Together, these data suggest that the isop-renoid pathway is involved in semiochemical biosynth-esis in male D. jeffreyi.
hypotheti-Fig. 4. Tissue distribution of HMG-S mRNA in male and female D. jeffreyi. (A) Representative Northern blot of RNA isolated from male and female insects that had been treated with acetone (Control) or JH III and hybridized with HMG-S andβ-actin. The approximate sizes of transcripts in kb are given on the left. (B) Quantitative presentation of signal strengths for male insects from panel (A). (C) Quantitative representation of data from a second Northern blot (not shown). Grey bars represent control males; black bars represent JH III-treated males. Insects were dissected as diagrammed below the graphs. Expression levels are shown relative to control “thoraces”, which were arbitrarily set at one.
cal precursor to frontalin is 6-methyl-6-hepten-2-one (6-MHO), which may arise via the isoprenoid pathway or via the fatty acid pathway from carbon salvaged from leucine, or a combination of both isoprenoid and fatty acid biosynthetic pathways (Barkawi, Bengoa, Blomqu-ist, Francke and Seybold, unpublished results). Prelimi-nary biochemical studies suggest at least some isop-renoid steps are involved in frontalin synthesis, and JH III induces frontalin synthesis in a dose- and time-depen-dent manner in male D. jeffreyi (Barkawi, Blomquist, and Seybold, unpublished results) similar to the pulse of HMG-S transcript (Fig. 5). We are continuing our stud-ies on the regulation of Jeffrey pine beetle pheromone biosynthesis by isolating and investigating cDNAs for other mevalonate pathway enzymes and localizing their expression by in situ hybridizations, and through additional biochemical characterizations of pheromone component synthesis.
Acknowledgements
We thank F. Lu, G. Hall, A.J. Blomquist and B. Strom for assistance in collecting beetles, Dr. W. Francke (Universita¨t Hamburg), and Dr. S.J. Weller (University of Minnesota) for helpful discussions. CO and CSB were supported by the Howard Hughes Undergraduate Scho-lar Program, University of Nevada. This work was funded by USDA grant 98-35302-6792 to GJB, SJS, and CT, NSF grant IBN-9906530 to SJS and GJB, and a joint Nevada Agricultural Experiment Station and Coop-erative Extension Grant to GJB and SJS, and is a contri-bution of the Nevada Agricultural Experiment Station.
References
Fig. 5. Endocrine regulation of HMG-S transcript levels in male D.
jeffreyi. Triplicate samples containing thoracic tissues isolated using
transverse cuts [Fig. 4(A)] from five beetles per sample were used for each point. (A) HMG-S mRNA levels vs JH III dose at 20 h following JH III application. (B) HMG-S mRNA levels vs. time following topical application of 13.3 µg JH III. In both graphs, transcript levels are shown relative to acetone treated (control) male tissue, which was arbi-trarily set at one.
higher plants by intramolecular skeletal rearrangement. Proc. Natl. Acad. Sci. USA 94, 10600–10605.
Ayte´, J., Gil-Gomez, G., Hegardt, F.G., 1993. Structural characteriz-ation of the 39noncoding region of the gene encoding rat mitochon-drial 3-hydroxy-3-methylglutaryl coenzyme A synthase. Gene 123, 267–270.
Bailey, E., Horne, J.A., Izatt, M.E.G., Hill, L., 1972. The role of ketone
bodies in the metabolism of the adult desert locust. Biochem. J. 128, 79.
Beenakkers, A.M., Van der Horst, D.J., van Marrewijk, W.J., 1985. Insect lipids and lipoproteins, and their role in physiological pro-cesses. Prog. Lipid. Res. 24, 19–67.
Beis, A., Zammit, V.A., Newsholme, E.A., 1980. Activities of 3-hyd-roxybutyrate dehydrogenase, 3-oxoacid CoA–transferase and ace-toacetyl–CoA thiolase in relation to ketone body utilisation in muscles from vertebrates and invertebrates. Eur. J. Biochem. 104, 209–215.
Boukaftane, Y., Mitchell, G.A., 1997. Cloning and characterization of the human mitochondrial 3-hydroxy-3-methylglutaryl CoA syn-thase gene. Gene 195, 121–126.
Browne, L.E., 1972. An emergence cage and refrigerated collector for wood-boring insects and their associates. J. Econ. Entomol. 65, 1499–1501.
Buesa, C., Martinez-Gonzalez, J., Casals, N., Haro, D., Piulachs, M.-D., Belles, X., Hegardt, F.G., 1994. Blattella germanica has two HMG–CoA synthase genes: both are regulated in the ovary during the gonadotropic cycle. J. Biol. Chem. 269, 11707–11713. Casals, N., Buesa, C., Piulachs, M.-D., Caban˜o´, J., Marrero, P.F.,
Belle`s, X., Hegardt, F.G., 1996. Coordinated expression and activity of 3-hydroxy-3-methylglutaryl coenzyme A synthase and reductase in the fat body of Blattella germanica (L.) during vitel-logenesis. Insect Biochem. Molec. Biol. 26, 837–843.
Castillo-Gracia, M., Couillaud, F., 1999. Molecular cloning and tissue expression of an insect farnesyl diphosphate synthase. Eur. J. Biochem. 262, 365–370.
Chia, W., Karp, R., McGill, S., Ashburner, M., 1985. Molecular analy-sis of the Adh region of the genome of Drosophila melanogaster. J. Molec. Biol. 186, 689–706.
Couillaud, F., 1991. Evidence for regulation of juvenile hormone biosynthesis operating before mevalonate in locust corpora allata. Mol. Cell. Endocrinol. 77, 159–166.
Feyereisen, R., Farnsworth, D.E., 1987. Characterization and regu-lation of HMG–CoA reductase during a cycle of juvenile hormone synthesis. Mol. Cell. Endocrinol. 53, 227–238.
FlyBase, 1999. The FlyBase database of the Drosophila genome pro-jects and community literature. Http://flybase.bio.indiana.edu/. Nucl. Acid Res. 27, 85–88.
Fox, C.A., Sheets, M.D., Wickens, M.P., 1989. Poly(A) addition dur-ing maturation of frog oocytes: distinct nuclear and cytoplasmic activities and regulation by the sequence UUUUUAU. Genes Dev. 3, 2151–2162.
Frohman, M.A., Dush, M.K., Martin, G.R., 1988. Rapid production of full length cDNAs from rare transcripts: amplification using a sin-gle gene-specific oligonucleotide primer. Proc. Natl. Acad. Sci. USA 85, 8998–9002.
Glisin, V., Crkvenjakov, R., Byus, C., 1974. Ribonucleic acid isolation by cesium chloride centrifugation. Biochemistry 13, 2633–2637. Goldstein, J.L., Brown, M.S., 1990. Regulation of the mevalonate
path-way. Nature 343, 425–430.
Havel, C.M., Fisher, P., Watson, J.A., 1992. Isopentenoid synthesis in embryonic Drosophila cells: prenylated protein profile and prenyl group usage. Arch. Biochem. Biophys. 295, 410–420.
Ivarsson, P., Schlyter, F., Birgersson, G., 1993. Demonstration of de novo pheromone biosynthesis in Ips duplicatus (Coleoptera: Scolytidae): inhibition of ipsdienol and E-myrcenol production by compactin. Insect Biochem. Molec. Biol. 23, 655–662.
Ivarsson, P., Tittiger, C., Blomquist, C., Borgeson, C.E., Seybold, S.J., Blomquist, G.J., Ho¨gberg, H.-E., 1998. Pheromone precursor syn-thesis is localized in the metathorax of Ips paraconfusus Lanier (Coleoptera: Scolytidae). Naturwissenschaften 85, 507–511. Kinzer, G.W., Fentiman, A.F. Jr., Page, T.E. Jr., Foltz, R.L., Vite´,
J.P., Pitman, G.B., 1969. Bark beetle attractants: indentification, synthesis, and field bioassay of a new compound isolated from
Koyama, T., Matsubara, M., Ogura, K., 1985. Isoprenoid enzyme sys-tems of silkworm II. Formation of the juvenile hormone skeletons by farnesyl pyrophosphate synthetase II. J. Biochem. (Tokyo) 98, 457–463.
Lai, C., McMahon, R., Young, C., Mackay, T.F., Langley, C.H., 1998.
quemao, a Drosophila bristle locus, encodes geranylgeranyl
pyro-phosphate synthase. Genetics 149, 1051–1061.
Lawrence, J.F., Britton, E.B., 1991. Coleoptera. In: Insects of Aus-tralia. Cornell University Press, Ithaca, NY, pp. 543–683. Laskovics, F.M., Poulter, C.D., 1981. Prenyltransferase: determination
of the binding mechanism and individual kinetic constants for far-nesyl pyrophosphate synthase by rapid quench and isotope par-titioning experiments. Biochemistry 20, 1893–1901.
Martinez-Gonzalez, J., Buesa, C., Piulachs, M.-D., Belles, X., Hegardt, F.G., 1993. 3-Hydroxy-3-methylglutaryl–coenzyme A synthase from Blattella germanica. Cloning, expression, developmental pat-tern, and tissue expression. Eur. J. Biochem. 217, 691–699. Mertz, L.M., Rashtchian, A., 1994. Nucleotide imbalance and
poly-merase chain reaction: effects on DNA amplification and synthesis of high specific activity radiolabeled DNA probes. Anal. Biochem. 221, 160–165.
Misra, I., Narasimhan, C., Miziorko, H.M., 1993. Avian 3-hydroxy-3-methylglutaryl–CoA synthase. Characterization of a recombinant cholesterogenic isozyme and demonstration of the requirement for a sulfhydryl functionality in formation of the acetyl–enzyme reac-tion intermediate. J. Biol. Chem. 268, 12129–12135.
Miziorko, H.M., Behnke, C.E., 1985. Amino acid sequence of an active site peptide of avian liver mitochondrial 3-hydroxy-3-methylgluta-ryl–CoA synthase. J. Biol. Chem. 260, 13513–13516.
Paine, T.D., Millar, J.G., Hanlon, C.C., Hwang, J.-S., 1999. Identifi-cation of semiochemicals associated with Jeffrey pine beetle,
Den-droctonus jeffreyi. J. Chem. Ecol. 25, 433–453.
Renwick, J.A.A., Pitman, G.B., 1979. An attractant isolated from the female Jeffrey pine beetle, Dendroctonus jeffreyi. Environ. Ento-mol. 8, 40–41.
Riddiford, L.M., 1994. Cellular and molecular actions of juvenile
hor-mone I. General considerations and premetamorphic actions. Adv. Insect Phys. 24, 213–273.
Sambrook, J., Fritsch, E.F., Maniatis, T., 1989. Molecular Cloning, A Laboratory Manual. Cold Spring Harbor Laboratory Press, New York.
Seybold, S.J., Quilici, D.R., Tillman, J.A., Vanderwel, D., Wood, D.L., Blomquist, G.J., 1995. De novo biosynthesis of the aggregation pheromone components ipsenol and ipsdienol by the pine bark beetles Ips paraconfusus Lanier and Ips pini (Say) (Coleoptera: Scolytidae). Proc. Natl. Acad. Sci. USA 92, 8393–8397. Spradling, A.C., Stern, D., Beaton, A., Rhem, E.J., Laverty, T.,
Mozden, N., Misra, S., Rubin, G., 1999. The Berkely Drosophila Genome Project gene disruption project: single P-element inser-tions mutating 25% of vital Drosophila genes. Genetics 153, 135–177.
Thompson, J.D., Higgins, D.G., Gibson, T.J., 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence align-ment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl. Acids Res. 22, 4673–4680. Tillman, J.A., Holbrook, G.L., Dallara, P.L., Schal, C., Wood, D.L.,
Blomquist, G.J., Seybold, S.J., 1998. Endocrine regulation of de novo aggregation pheromone biosynthesis in the pine engraver, Ips
pini (Say) (Coleoptera: Scolytidae). Insect Biochem. Molec. Biol.
28, 705–715.
Tittiger, C., Blomquist, G.J., Ivarsson, P., Borgeson, C.E., Seybold, S.J., 1999. Juvenile hormone regulation of HMG-R gene expression in the bark beetle Ips paraconfusus (Coleoptera: Scolytidae): impli-cations for male aggregation pheromone biosynthesis. Cell. Molec. Life Sci. 55, 121–127.
van Doren, M., Broihier, H.T., Moore, L.A., Lehmann, R., 1998. HMG–CoA reductase guides migrating primordial germ cells. Nat-ure 396, 466–469.