L
Journal of Experimental Marine Biology and Ecology 245 (2000) 69–81
www.elsevier.nl / locate / jembe
Egestion rates of the estuarine mysid Neomysis integer
(Peracarida: Mysidacea) in relation to a variable environment
a b a ,*
S.D. Roast , J. Widdows , M.B. Jones
a
Plymouth Environmental Research Centre(Department of Biological Sciences), University of Plymouth,
Plymouth, Devon PL4 8AA, UK
b
Centre for Coastal and Marine Sciences, Plymouth Marine Laboratory, Prospect Place, West Hoe,
Plymouth, Devon PL1 3DH, UK
Received 21 August 1998; received in revised form 23 March 1999; accepted 7 October 1999
Abstract
The hyperbenthic, estuarine mysid Neomysis integer (Crustacea: Mysidacea) is exposed to wide fluctuations of temperature and salinity on tidal and seasonal cycles. Using sieved sediment as an environmentally relevant food source and egestion rates as a measure of ingestion, the feeding rates of N. integer have been quantified at temperatures (5, 10 and 158C) and salinities (1, 10, 20
21
and 30‰) experienced in the field. Egestion rates (0.017–0.049 mg faeces mg dry wt. mysid 21
h ) increased with increasing temperature (Q10 values ranged from ¯1.9–2.4) and with
increasing salinity. There was a significant interaction between temperature and salinity such that egestion rates were suppressed at high temperature ($108C) in combination with high salinity (30‰). Male egestion rates were not significantly different from those of females at any temperature / salinity combination. Absorption efficiency (¯0.35) was unaffected by temperature
or salinity, confirming that egestion rates are representative of energy acquisition by N. integer. In the estuarine environment, mysid feeding rates are predicted to be low for much of the tidal cycle as the sites occupied by N. integer are dominated by low salinity, cold river water. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Estuarine mysids; Sediment; Egestion rates; Feeding; Temperature; Salinity
1. Introduction
Mysids (Crustacea: Peracarida) contribute significantly to the secondary production of estuaries. The hyperbenthic mysid Neomysis integer dominates the upper regions of
*Corresponding author. Tel.:144-175-223-2911; fax:144-175-223-2970.
E-mail address: [email protected] (M.B. Jones)
22
European estuaries and has an estimated productivity of 300 mg ash-free dry weight m
21
year in the Westerschelde Estuary (Netherlands) (Mees et al., 1994). As many mysids are hyperbenthic, they are thought to provide a significant link in the exchange of organic matter between the benthic and pelagic systems of estuaries, however, published data on the contribution of mysids to such food fluxes are limited (Moffat, 1996; Mees and Jones, 1998; Roast et al., 1998a). While it is well established that the feeding rates of many crustaceans are influenced by various factors including temperature, salinity, weight, gender and food density (Kinne, 1970, 1971; Newell and Branch, 1980; Toda et al., 1987; Guerin and Stickle, 1995), few of these factors have been investigated for mysids. Previous investigations of mysid feeding have concentrated on filter feeding and predatory feeding (Cooper and Goldman, 1982; Fulton, 1982; Webb et al., 1987; Chigbu and Sibley, 1994). In laboratory feeding experiments, mysids are generally fed brine shrimp (Artemia sp.) nauplii (Astthorsson, 1980; Collins et al., 1991) or Daphnia magna (Irvine et al., 1993), food items not representative of their normal diet. Stomach content analyses have indicated that mysids feed on a wide variety of foods including detritus (Mauchline, 1980). For N. integer, amorphous material from sediment flocs has been identified as an important food item (Fockedey and Mees, 1999).
The aims of the present study were to establish the effects of temperature and salinity on the feeding rates of Neomysis integer using an environmentally relevant food source, and to interpret the implications of these laboratory findings to mysids in the natural environment. To achieve the latter, mysids were collected from the East Looe River Estuary, Cornwall (UK), where details of seasonal and tidal fluctuations of water temperature, salinity and current velocity are available (Roast et al., 1998b; 1999).
2. Materials and methods
2.1. Animal collection and maintenance
During spring 1996, adult Neomysis integer were collected from Terras Bridge, East Looe River Estuary, Cornwall, UK (National grid reference SX 532256) by sweeping a Freshwater Biological Association (FBA) dip net (1 mm mesh) along the water’s edge at low tide. Mysids were returned to the laboratory in habitat water (salinity¯1‰), placed
in holding tanks (1061‰, 15618C, ambient lighting from fluorescent lights) and fed ad libitum on ,48 h old Artemia sp. (Great Lakes, Utah) hatched from cysts in the laboratory.
2.2. Measurement of egestion rate
the more readily quantified rate of egestion was used as a surrogate measure of mysid feeding rate. Although gut residence times of crustaceans are variable (Murtaugh, 1984), egestion rates have been used previously to calculate feeding rates of crustaceans (Gaudy, 1974; Reeve et al., 1977) including mysids (Gaudy et al., 1991). For mysids in particular, egestion rates are highly positively correlated with ingestion rates (Murtaugh, 1984), validating their use as a measure of feeding rate.
Sediment was collected from the intertidal region at Terras Bridge, where mysids swarmed, by scraping off the top 10 mm of surface sediment. Granulometric analysis showed the sediment in this part of the estuary consisted mainly of mud [particles ,100
mm accounted for more than 75% by weight of the sediment (Roast et al., 1998b)]. The sediment was returned to the laboratory in water of ¯1‰, stored in the dark in a
refrigerator (¯28C) and used within 7 days. Immediately prior to each experiment, the
sediment was passed through a 63mm sieve into a plastic aquarium, using water of 10‰ to rinse the sediment through the sieve. After standing for 1 h, when most sediment particles had dropped out of suspension, the supernatant was decanted off to leave a concentrated slurry of sediment (,63 mm diameter size). The slurry was mixed vigorously to ensure a homogenous sediment suspension immediately prior to injecting approximately 100 ml of slurry into 500 ml plastic containers (110 mm diameter) using a 50 ml plastic syringe. The containers were left for 1 h to consolidate the sediment. Exposure water was decanted carefully into each vessel so that the sediment was undisturbed and a single mysid was placed in each vessel. After feeding for 16 h, each mysid was removed, freeze-dried and weighed (60.01 mg) using a Sartorius R200-D balance. Following mysid removal, the water in each test chamber was shaken gently to re-suspend the sediment and the resultant slurry was sieved through a 128mm sieve (the larger sieve being used to allow sediment flocs, which formed during the course of the experiment, to pass through the sieve). Neomysis integer faecal material (¯1.5 mm long
and cylindrical) was retained on the sieve while the loose sediment passed through. The former was washed gently with distilled water and collected onto pre-ashed, weighed Whatman GF / F filter papers. Filter papers and faeces were freeze-dried and weighed (60.01 mg). Egestion rates were calculated as mg dry weight of faecal material
21 21
mg mysid dry weight h .
2.3. Measurement of food absorption efficiency
correction for any residual weight change. Absorption efficiency was calculated using the equation:
A5(F2E )4[(12E )3F ]
where: A5absorption efficiency, F5ash-free fraction of food source, and E5ash-free fraction of faeces (Conover, 1966).
2.4. Experimental protocol
Egestion rates and absorption efficiencies were investigated at salinities (1, 10, 20 and 30‰) and temperatures (5, 10 and 158C) within the range experienced by N. integer in the estuarine environment (Roast et al., 1998b). Salinities were prepared by diluting filtered (10 mm) seawater with tap water (de-chlorinated by aeration for 24 h). All experiments were carried out in a Sanyo MLR-350HT growth cabinet with pro-grammable temperature (60.18C) and photoperiod. Test vessels were placed in the cabinet 2 h prior to the addition of mysids to allow the water temperature to equilibrate with cabinet temperature. Experimental vessels were aerated constantly with filtered, compressed air. Experiments were run for 16 h (overnight), with the cabinet lighting programmed to synchronize with the natural photoperiod [16 h light / 8 h dark with dawn and dusk sequence (i.e. gradual increase and decrease of light intensity)]. During the experiment, mysids were, therefore, exposed to 4 h light / 8 h dark / 4 h light. All mysids were adults of similar size (1261 mm from the anterior margin of the rostrum to the tip of the telson); ovigerous females were excluded. A total of 18 experiments was run at each temperature / salinity combination (using nine mysids of each gender).
2.5. Statistical treatment of results
Two-way analysis of variance (ANOVA) was applied to egestion rate data to determine the significance of temperature, salinity or gender effects and to establish factor interactions. Multiple linear regression analysis determined how egestion rates differed with temperature, salinity and gender.
3. Results
3.1. Effect of temperature on egestion rates
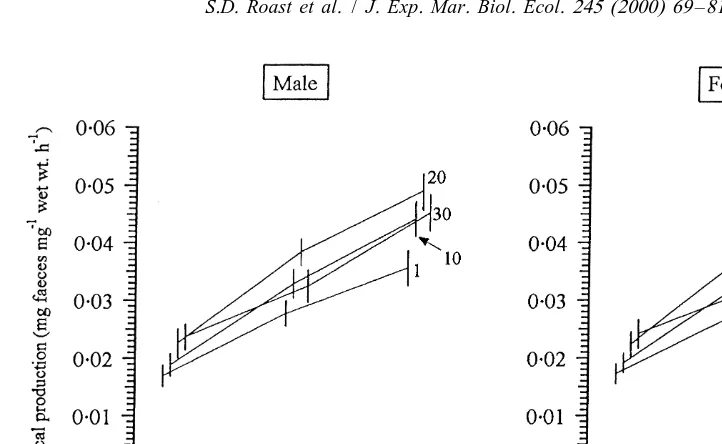
Fig. 1. Effect of temperature on the egestion rate of Neomysis integer at various salinities (numbers correspond to salinity, ‰). n59 for each temperature / salinity combination. Data are means with 95% confidence intervals.
ture sensitivities for egestion rates over a wide salinity range or between males and females (Table 1).
3.2. Effect of salinity
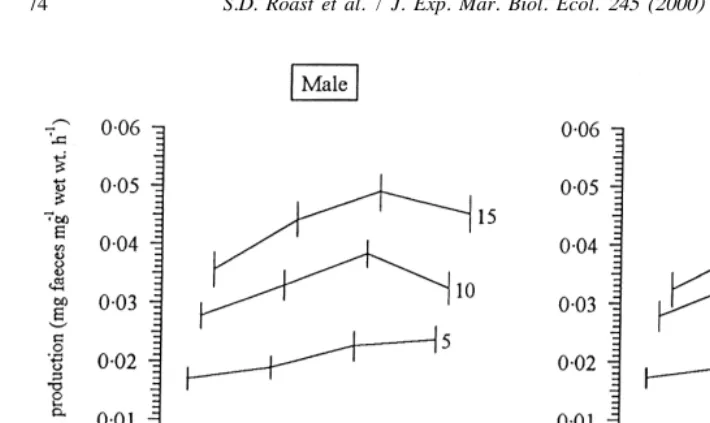
Generally, faecal production increased significantly with increasing salinity [ANOVA, f539.3 (male) and 47.9 (female), d.f.53, p,0.01] (Fig. 2). As salinity interacted significantly with temperature (see previous section), the relationship between these two variables was complex. At 58C, faecal production increased as salinity was increased from 20 to 30‰, but at 10 and 158C, faecal production decreased at 30‰; this effect
Table 1
Temperature coefficients (Q ) for the faecal production of male and female Neomysis integer at various10
salinities (n59 for each temperature / salinity combination)
Gender Salinity (‰) Q10value
Male 1 2.10
10 2.35
20 2.17
30 1.91
Female 1 1.88
10 2.06
20 2.09
Fig. 2. Effect of salinity on the egestion rate of Neomysis integer at various temperatures (numbers correspond to temperature,8C). n59 for each temperature / salinity combination. Data are means with 95% confidence intervals.
was significant only at 108C (Fig. 2) (95% confidence intervals, p,0.05). Thus, egestion rates at 10 and 30‰ were not significantly different at 108C (or 158C for males) (95% confidence intervals, p.0.05), whereas they were significantly different at 58C (95% confidence intervals, p,0.05).
3.3. Effect of gender
At each temperature / salinity combination, there was no significant difference between male and female egestion rates (Figs. 1 and 2) (95% confidence intervals, p.0.05), and no significant interaction between gender and either temperature (ANOVA, f51.45, d.f.52, p.0.05) or salinity (ANOVA, f50.09, d.f.53, p .0.05).
3.4. Combined effects of temperature, salinity and gender
Multiple linear regression analysis revealed that temperature, salinity and gender affected the egestion rate of N. integer according to the equation:
E50.006110.0022T10.0003S20.0005G
21 21
Table 2
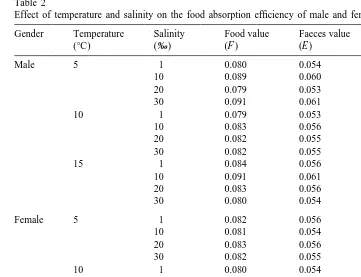
a
Effect of temperature and salinity on the food absorption efficiency of male and female Neomysis integer
Gender Temperature Salinity Food value Faeces value Absorption
(8C) (‰) (F ) (E ) efficiency
Male 5 1 0.080 0.054 0.344
10 0.089 0.060 0.347
20 0.079 0.053 0.343
30 0.091 0.061 0.346
10 1 0.079 0.053 0.347
10 0.083 0.056 0.341
20 0.082 0.055 0.344
30 0.082 0.055 0.349
15 1 0.084 0.056 0.349
10 0.091 0.061 0.352
20 0.083 0.056 0.347
30 0.080 0.054 0.350
Female 5 1 0.082 0.056 0.338
10 0.081 0.054 0.347
20 0.083 0.056 0.341
30 0.082 0.055 0.349
10 1 0.080 0.054 0.344
10 0.079 0.053 0.347
20 0.082 0.055 0.349
30 0.082 0.055 0.344
15 1 0.083 0.056 0.347
10 0.082 0.055 0.349
20 0.081 0.055 0.340
30 0.083 0.056 0.347
a
Data for each temperature / salinity combination are pooled from nine replicates. F5ash-free fraction of sediment, E5ash-free fraction of faeces.
for gender implies that male egestion rates were faster than those of females, however, this was not significant (multiple linear regression, t5 20.91, d.f.51, p.0.05).
3.5. Absorption efficiencies
There were no obvious increases in absorption efficiency with increased temperature or salinity (Table 2). Absorption efficiency of females appeared to be more variable than males (Table 2), but this could not be confirmed due to pooled data preventing statistical analyses.
4. Discussion
mysid feeding. The use of sediment as an environmentally relevant food source is supported by field observations in the Westerschelde Estuary (Netherlands), where large quantities of detritus were found in the stomachs of adult Neomysis integer (Fockedey and Mees, 1999). The majority of detrital particles in the stomachs of N. integer from the Westerschelde were of a similar size (,65mm) to the sediment used in the present study, however, absence of meiobenthic fauna and micro-organisms (bacteria, fungi, etc.), normally associated with sediment detrital particles in mysid stomachs, led Fockedey and Mees (1999) to conclude that N. integer fed only from the water column and did not forage in the substratum. In the laboratory, N. integer has been observed to sink to the substratum and collect aggregations of sediment for processing prior to ingestion (Roast, 1997), supporting the use of surface sediment in its diet. Further work is required to establish the source of the sediment used as a food by N. integer. The relatively low organic content of the sediment used in the present study (¯8–9%), and
the refractory nature of this material, was chosen to be representative of British estuaries (e.g. Widdows et al., 1979, 1998), and helps to explain the lower absorption efficiency for N. integer feeding from sediment (¯0.35, present study), compared with feeding on
zooplankton (0.7–0.9) or phytoplankton (0.6–0.9) (Astthorsson, 1980). Comparatively low absorption efficiencies (¯0.15) have been recorded for the amphipod Hyalella
azteca feeding on sediment (Hargrave, 1970). In general, if material of high nutritional value forms only a small proportion of the total organic fraction of the sediment (i.e. there is a high proportion of refractory material), the overall absorption will be low, even if the nutritional fraction is absorbed efficiently (Grahame, 1983). The data imply, therefore, that N. integer needs to consume sediment at a much faster rate than when feeding on phyto- or zooplankton to obtain the same amount of energy. The present study demonstrates that N. integer feeds rapidly on sediment, with mysids passing their own dry weight in faecal material within 24 h. Thus, while it may be more beneficial energetically for N. integer to feed on meiofauna [e.g. calanoid copepods (Fockedey and Mees, 1999)], sediment may be an important food resource in certain estuarine areas when other food types are absent.
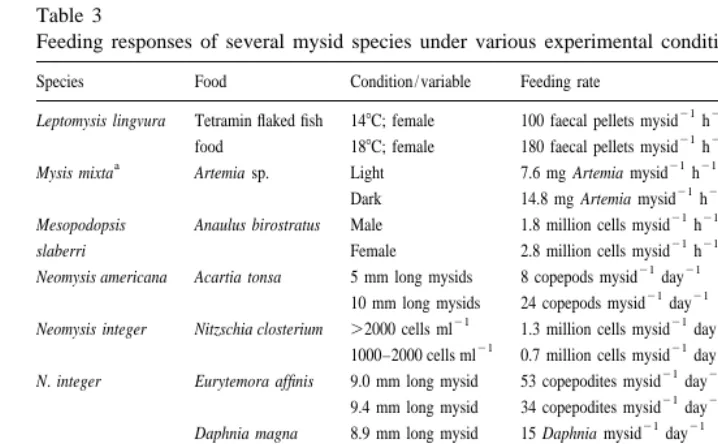
Table 3
Feeding responses of several mysid species under various experimental conditions
Species Food Condition / variable Feeding rate Reference
21 21
Leptomysis lingvura Tetramin flaked fish 148C; female 100 faecal pellets mysid h Gaudy et al. (1991) 21 21
food 188C; female 180 faecal pellets mysid h
a 21 21
Mysis mixta Artemia sp. Light 7.6 mg Artemia mysid h Gorokhova and Hansson (1997) 21 21
Dark 14.8 mg Artemia mysid h 21 21
Mesopodopsis Anaulus birostratus Male 1.8 million cells mysid h 21 21
slaberri Female 2.8 million cells mysid h
21 21
Neomysis americana Acartia tonsa 5 mm long mysids 8 copepods mysid day Fulton (1982) 21 21
10 mm long mysids 24 copepods mysid day
21 21 21
Neomysis integer Nitzschia closterium .2000 cells ml 1.3 million cells mysid day Lucas (1936)
21 21 21
1000–2000 cells ml 0.7 million cells mysid day 21 21
N. integer Eurytemora affinis 9.0 mm long mysid 53 copepodites mysid day Irvine et al. (1993) 21 21
9.4 mm long mysid 34 copepodites mysid day 21 21
Daphnia magna 8.9 mm long mysid 15 Daphnia mysid day 21 21
9.5 mm long mysid 4.4 Daphnia mysid day
b 21 21
N. integer Sediment 58C (10‰, males) 0.057 mg faeces mg dry wt. h This study 21 21
Neomysis mercedis Daphnia magna 108C 29 Daphnia mysid 12 h Chigbu and Sibley (1994) 21 21
148C 30 Daphnia mysid 12 h
a 21
Original data expressed g mysid. Representative dry weight of Mysis mixta taken as 4.0 mg (based on length data from Mauchline and Murano, 1977).
b
Calculated assuming mean dry weight of N. integer53.0 mg (own data).
available for growth is also highest at 20‰ (Roast, 1997). However, although oxygen consumption by N. integer decreases with increasing salinity, the stronger influence of temperature on mysid oxygen consumption suggests that oxygen consumption is greatest at high tide (Roast et al., 1999). The increased feeding rates predicted for N. integer at high tide may, therefore, relate to this increased rate of oxygen consumption and associated energetic costs.
The availability of detailed descriptions of tidal and seasonal fluctuations of temperature and salinity at Terras Bridge (Roast et al., 1998b) enables discussion of the environmental implications of these feeding rate data to N. integer. Terras Bridge is close to the tidal limit and is dominated by freshwater flow. The site is flooded with seawater for ¯2 h either side of coastal high tide, with low salinity (or fresh) water
present for the remainder of the tidal cycle. Temperature varies tidally (the fresh river water is¯5-78C cooler than the seawater) and seasonally (summer high tide maxima are ¯158C, compared with winter high tide maxima of ¯88C). If temperature effects are
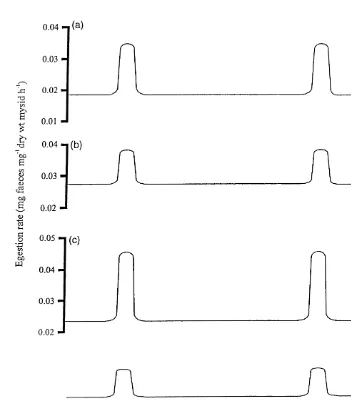
considered in isolation, feeding rates of N. integer are predicted to be comparatively low for much of the tidal cycle due to the lower temperature of the freshwater flow, and rise as the site floods with warmer, incoming seawater (Fig. 3a). Similarly, if the effects of salinity on mysid feeding rates are considered in isolation, feeding rates are predicted to be comparatively low for most of the tidal cycle when the site is dominated by freshwater (Fig. 3b). As the salinity increases with the flood tide, feeding rates are predicted to increase to a maximum at 20–30‰ (Fig. 3b). When the effects of temperature and salinity are considered together, changes in mysid feeding rates are generally predicted to be even more pronounced than for either factor acting alone (Fig. 3c). At high tide, however, feeding rates are predicted to decrease slightly due to the interaction between temperature and salinity. Although increases in both temperature and salinity elevate mysid feeding rates, changes in temperature appear to have a more pronounced effect than do changes in salinity (Fig. 3a and b). At Terras Bridge, changes in these two variables are reciprocal, such that temperature and salinity increase and decrease together (Roast et al., 1998b, 1999). Seasonal temperature change is also predicted to cause seasonal changes in the feeding rate of N. integer, with increased rates of feeding occurring during the warmer summer months, the main reproductive period (Mauchline, 1971; Roast, 1997).
In conclusion, the present study provides quantitative data on the sediment ingested by a dominant member of the hyperbenthic fauna of the upper reaches of European estuaries under different salinity and temperature conditions. Such data, together with details of seasonal population dynamics (Moffat and Jones, 1992), are required to identify the role played by hyperbenthic mysids in the detrital-based food fluxes of estuaries.
Acknowledgements
Fig. 3. Predicted effects of tidally-based temperature and salinity changes on the egestion rate of Neomysis
integer. (a) Effect of temperature only, (b) effect of salinity only, and (c) effect of temperature and salinity
acting in concert on mysid metabolism. H.W.5time of high water. Temperature and salinity data taken from Roast (1997).
References
Astthorsson, O.S., 1980. The life history and ecological energetics of Neomysis integer (Leach) (Crustacea, Mysidacea). PhD thesis, University of Aberdeen, UK, 165 pp. [SS]
Cannon, H.G., Manton, S.M., 1927. On the feeding mechanism of a Mysid Crustacean, Hemimysis lamornae. Trans. R. Soc. Edinburgh 55, 219–253.
Chigbu, P., Sibley, T.H., 1994. Predation by Neomysis mercedis: effects of temperature, Daphnia magna size and prey density on ingestion rate and size selectivity. Freshwater Biol. 32, 39–48.
Collins, G.B., Bengston, D.A., Moore, J.C., 1991. Characterization of reference Artemia III for marine toxicological studies. In: Mayes, M.A., Barron, M.G. (Eds.), Aquatic Toxicology and Risk Assessment, vol. 14, American Society for Testing and Materials, Philadelphia, PA, pp. 315–323.
Cooper, S.D., Goldman, C.R., 1982. Environmental factors affecting predation rates of Mysis relicta. Can. J. Fish. Aquat. Sci. 39, 203–208.
Conover, R.J., 1966. Assimilation of organic matter by zooplankton. Limnol. Oceanog. 11, 338–345. Fockedey, N., Mees, J., 1999. Feeding of the hyperbenthic mysid Neomysis integer in the maximum turbidity
zone of the Elbe, Westerschelde and Gironde estuaries. J. Mar. Syst., 619.
Fulton, III R.S., 1982. Predatory feeding of two marine mysids. Mar. Biol. 72, 183–191.
Gaudy, R., 1974. Feeding of four species of pelagic copepods under experimental conditions. Mar. Biol. 25, 125–141.
´
Gaudy, R., Guerin, J.P., Kerambrun, P., 1991. Sublethal effects of cadmium on respiratory metabolism, nutrition, excretion and hydrolyse activity in Leptomysis lingvura (Crustacea: Mysidacea). Mar. Biol. 109, 493–501.
Gorokhova, E., Hansson, S., 1997. Effects of experimental conditions on the feeding rate of Mysis mixta (Crustacea, Mysidacea). Hydrobiologia 355, 167–172.
Grahame, J., 1983. Adaptive aspects of feeding mechanisms. In: Vernberg, J., Vernberg, W.B. (Eds.), The Biology of Crustacea, Environmental Adaptations, Vol. 8, Academic Press, London, pp. 65–108, ch. 3. ´
Guerin, J.P., Stickle, W.B., 1995. Effects of cadmium on survival, osmoregulatory ability and bioenergetics of juvenile blue crabs Callinectes sapidus at different salinities. Mar. Environ. Res. 40, 227–246.
Hargrave, B.T., 1970. The utilization of benthic microflora by Hyalella azeteca (Amphipoda). J. Anim. Ecol. 39, 427–437.
Irvine, K., Moss, B., Bales, M., Snook, D., 1993. The changing ecosystem of a shallow, brackish water lake, Hickling Broad, Norfolk, UK. 1: Trophic relationships with special reference to the role of Neomysis
integer. Freshwater Biol. 29, 119–139.
Kinne, O., 1970. The effects of temperature and salinity on marine and brackish water animals. I. Temperature. In: Kinne, O. (Ed.), Marine Ecology, Vol. 1, Wiley–Interscience, New York, pp. 407–514, part 1. Kinne, O., 1971. The effects of temperature and salinity on marine and brackish water animals. II. Salinity and
temperature salinity combinations. In: Kinne, O. (Ed.), Marine Ecology, Vol. 1, Wiley–Interscience, New York, pp. 821–995, part 2.
Lucas, C.E., 1936. On certain inter-relations between phytoplankton and zooplankton under experimental conditions. J. Cons. Int. Explor. Mer. 11, 343–361.
Mauchline, J., 1971. The biology of Neomysis integer (Crustacea, Mysidacea). J. Mar. Biol. Assoc. UK 51, 347–354.
Mauchline, J., 1980. The biology of mysids. Adv. Mar. Biol. 18, 1–369.
Mauchline, J., Murano, M., 1977. World list of the Mysidacea, Crustacea. J. Tokyo Univ. Fish. 64, 39–88. Mees, J., Jones, M.B., 1998. The hyperbenthos. Oceanog. Mar. Biol. Annu. Rev. 35, 221–255.
Mees, J., Abdulkerim, Z., Hamerlynck, O., 1994. Life history, growth and production of Neomysis integer in the Westerschelde estuary (S.W. Netherlands). Mar. Ecol. Prog. Ser. 109, 43–57.
Moffat, A.M., 1996. Ecophysiology of mysids (Crustacea: Peracarida) in the River Tamar Estuary. PhD thesis, University of Plymouth, 172 pp.
Moffat, A.M., Jones, M.B., 1992. Bionomics of Mesopodopsis slabberi and Neomysis integer (Crustacea: Mysidacea) in the Tamar Estuary. In: Kohn, V.J., Jones, M.B., Moffat, A.M. (Eds.), Taxonomy, Biology and Ecology of (Baltic) Mysids (Mysidacea: Crustacea), Rostock University Press, Rostock, pp. 109–119. Murtaugh, P.A., 1984. Variable gut residence time: problems in inferring feeding rate from stomach fullness of
Newell, R.C., Branch, G.M., 1980. The influence of temperature on the maintenance of metabolic energy balance in marine invertebrates. Adv. Mar. Biol. 17, 329–396.
Reeve, M.R., Walter, M.A., Darcy, K., Ikeda, T., 1977. Evaluation of potential indicators of sublethal toxic stress on marine zooplankton (feeding, fecundity, respiration and excretion). Bull. Mar. Sci. 27, 105–113. Roast, S.D., 1997. The ecophysiology of Neomysis integer (Peracarida: Mysidacea). PhD thesis, University of
Plymouth, Plymouth, UK, 232 pp.
Roast, S.D., Thompson, R.S., Widdows, J., Jones, M.B., 1998a. Mysids and environmental monitoring: a case for their use in estuaries. Mar. Freshwater Res. 49, 319–326.
Roast, S.D., Widdows, J., Jones, M.B., 1998b. The position maintenance behaviour of Neomysis integer (Peracarida: Mysidacea) in response to current velocity, substratum and salinity. J. Exp. Mar. Biol. Ecol. 220, 25–45.
Roast, S.D., Widdows, J., Jones, M.B., 1999. Respiratory responses of the estuarine mysid Neomysis integer (Peracarida: Mysidacea) in relation to a variable environment. Mar. Biol. 133, 643–649.
Schmidt-Nielsen, K. (Ed.), 1997. Animal Physiology (Adaptation and Environment), 5th ed, Cambridge University Press, Cambridge, UK, 607 pp.
Tattersall, W.M., Tattersall, O.S. (Eds.), 1951. The British Mysidacea, Bartholomew Press, Dorking, 460 pp., The Ray Society Series no. 136.
Toda, H., Arima, T., Takahashi, M., Ichimura, S., 1987. Physiological evaluation on the growth process of the mysid Neomysis intermedia Czerniawsky. J. Plank. Res. 9, 51–63.
Webb, P., Perissonotto, R., Wooldridge, T.H., 1987. Diet and feeding of Gastrosaccus psammodytes (Crustacea, Mysidacea) with special reference to the surf diatom Anaulus birostratus. Mar. Ecol. Prog. Ser. 45, 255–261.
Widdows, J., 1993. Marine and estuarine toxicity tests. In: Calow, P. (Ed.), Handbook of Ecotoxicology, Blackwell, Oxford, pp. 145–166.
Widdows, J., Salkeld, P.N., 1993. Practical procedures for the measurement of scope for growth. MAP Tech. Rep. Ser 71, 147–172, (UNEP Athens).
Widdows, J., Fieth, P., Worrall, C.M., 1979. Relationships between seston, available food and feeding activity in the common mussel Mytilus edulis. Mar. Biol. 50, 195–207.