Summary We measured needle pigment content and photo-synthetic rates of 1-year-old western larch (Larix occidentalis Nutt.) during autumn foliar senescence. Chlorophyll (Chl) and carotenoid (xanthophyll + β-carotene) contents of needles declined 11 and 17%, respectively, before CO2 assimilation rate
began to decline. Chlorophyll a/b ratio, Chl/carotenoid ratio, photochemical efficiency (Fv/Fm), and photochemical
quench-ing did not begin to decline until late in senescence. Internal CO2/ambient CO2 did not change during needle yellowing. In
seedlings in warmed soil (average 3 °C above natural condi-tions), the decline in needle chlorophyll content was delayed by 10 days and the decline in CO2 assimilation rate was delayed
by 5 days, compared with seedlings in soil at ambient tempera-ture. In seedlings exposed to an extended 16-h photoperiod, the decline in needle chlorophyll content was delayed by 32 days, and the decline in CO2 assimilation rate was delayed by
21 days, compared with seedlings exposed to natural day lengths. In addition to delaying the onset of needle senescence, the treatments affected the sequence of events during senes-cence. Differences among treatment groups provide evidence that the onset of pigment loss and photosynthetic decline and the sequence of events during needle senescence are affected by soil temperature and day length.
Keywords: chlorophyll fluorescence, gas exchange, leaf senes-cence, photoperiod, soil temperature.
Introduction
Autumn foliar color change in deciduous perennials is trig-gered both by leaf-specific maturation processes and environ-mental conditions. The precise effects of individual environmental conditions on leaf senescence have proved dif-ficult to identify because several factors, such as air tempera-ture, day length and rainfall, often change at the same time. In addition, some cellular components deteriorate during senes-cence as a direct result of the environment, whereas other components are only indirectly affected by the environment. Previous studies on autumn senescing tissue in deciduous trees examined pigment degradation (Goodwin 1958, Sanger 1971),
chloroplast ultrastructure (Cunninghame et al. 1982), gas ex-change (Matyssek et al. 1991), chloroplast efficiency (Adams et al. 1990), respiratory enzymes (Dean et al. 1993), fluores-cence emission spectra (Lang and Lichtenthaler 1991), and amounts of plant hormones (Osborne 1973). However, in these studies no attempt was made to relate the findings with changes in autumn weather. Other studies relating weather conditions to the timing of senescence were not accompanied by detailed physiological measurements (Addicott and Lyon 1973, Kozlowski et al. 1991, Worrall 1993).
Environmental conditions affect the onset of leaf senescence as well as the process of senescence, i.e., the sequence and rate of changes (Smart 1994). One hypothesis is that autumn weather and cumulative stress imposed on leaves trigger senes-cence-related genes that coordinate a preprogrammed set of events. This leads to the prediction that the onset of autumn foliar senescence will vary with environmental conditions, but that senescence processes will be relatively insensitive to autumn conditions. An alternative hypothesis is that leaf com-ponents are affected differently by autumn conditions, in which case both the onset and process of senescence will vary according to environmental conditions. For example, in a year when air temperature begins to decline before irradiance de-clines, carbon reduction may decline before light harvesting capacity. In contrast,when autumn temperatures remain high, a series of phytochrome-mediated events could initiate senes-cence. The existence of different patterns of senescence has been documented for several systems (Pell and Dann 1991), but not for the interrelated events occurring during autumn foliar senescence.
We collected data on the photosynthetic apparatus of 1-year-old western larch (Larix occidentalis Nutt.) to quantify the influence of autumn weather on foliar senescence. We com-pared outdoor seedlings under natural conditions to outdoor seedlings with soil heating or supplemental light to extend the photoperiod. The experimental design allowed us to quantify the effect of soil temperature and photoperiod regime on foliar senescence. The environmental manipulations affected both the onset and pattern of decline in photosynthetic function.
Photosynthetic decline and pigment loss during autumn foliar
senescence in western larch (
Larix occidentalis
)
SELMA I. ROSENTHAL
1,2and EDITH L. CAMM
31
Department of Botany, University of British Columbia, BC V6T 1Z4, Canada
2 Present address: Department of Plant Pathology, Physiology and Weed Science, Virginia Tech, Blacksburg, VA 24061-0330, USA
3 University College of the Fraser Valley, Abbotsford, BC V2S 4N2, Canada
Received May 15, 1996
Materials and methods
Experimental design
One-year-old western larch (Larix occidentalis) seedlings, ob-tained from Seedlot 5266, Thompson Okanagan Dry Seed Planning Zone, BC (49° N, 119° W, elevation 1150 m) were grown outdoors in 15-cm diameter pots from March until August and watered regularly at the experimental site in Van-couver, BC (49° N, 123° W, elevation 90 m). In late August (Julian date 240), potted trees were divided into three treat-ment groups: seedlings in natural conditions (control) (n = 26), seedlings in warmed soil (n = 34), and seedlings receiving extended photoperiod (n = 31). All of the seedlings were placed in gravel-bottomed cold frames under a translucent plastic roof. The frame for seedlings in warmed soil was heated with cables under the gravel, which raised soil temperature an average of 3 °C above ambient during the experiment. Soil temperature probes were placed at a depth of 3 cm in the 15-cm diameter pots. For seedlings receiving an extended day length, a 16-h photoperiod was provided by means of supplemental fluorescent lights (100 µmol m−2 s−1). A CR10 data logger (Campbell Scientific, Edmonton, AB) recorded air tempera-ture, soil temperature and relative humidity. Photosynthetic photon flux (PPF) was measured with an LI-190SA quantum sensor (Li-Cor, Inc., Lincoln, NE).
Our choice of a deciduous conifer allowed sequential har-vesting of same age needles. On 37 days over a 15-week period beginning on Julian day 247 (September 4), needle samples (three per treatment) each consisting of one needle from a side branch of each seedling were collected randomly from all treatments between 0730 and 0930 h to insure three inde-pendent and representative samples from each treatment group on each measurement day. Sampling was stopped when CO2
assimilation rates (see below) became negative. Because of a shortage of material, we did not collect from seedlings in the extended photoperiod treatment between days 320 and 338 (November 15 and December 3). Larix occidentalis is decidu-ous, though a few acropetal needles may remain green throughout the winter. Because these wintergreen needles (Richards and Bliss 1985) occur on the shoot tips, they were not in our sampling area.
Measurements of pigment contents and photosynthetic rates
Chlorophyll and carotenoid (xanthophyll + β-carotene) con-tents were determined after acetone extraction (Lichtenthaler and Wellburn 1983). Carbon assimilation rate at a PPF of 1000 µmol m−2 s−1 was measured at 20--25 °C with an ADC-LCA2 (Analytical Development Company, Hoddesdon, UK) infrared gas analyzer (IRGA) equipped with a narrow leaf chamber. Leaf areas of individual needles were determined from length and width measurements, and adjusted for needle morphology (Vance and Running 1985). Stomatal conduc-tance and internal CO2 concentrations were measured with the
IRGA.
Photosynthetic oxygen evolution was measured with a leaf disc oxygen electrode (Hansatech, Kings Lynn, U.K.) equipped with a red light emitting diode (660 nm). A disc with
a composite flat leaf surface area of 10 cm2 was made by placing 30 needles side by side, trimming the apex and base from the needles, and holding them together by four narrow pieces of tape. The tape was placed at the edge of the leaf disc: stomata occurred mostly on the adaxial leaf surface. To main-tain chamber CO2 concentration, the mat in the leaf disc
chamber contained 1.0 M NaHCO3 adjusted to pH 9 with
1.0 M Na2CO3, and the chamber was flushed with 50 ml l−1
CO2 from a 1.0 M NaHCO3 solution (Walker 1987). Oxygen
measurements were corrected for respiration, which was meas-ured both after 10 min in the dark and after 5--10 min of light-saturating illumination. Respiration was assumed to re-main constant as the actinic light increased.
Chlorophyll a fluorescence
Photochemical efficiency (Fv/Fm) and photochemical
quench-ing (qQ, Kooten and Snell 1990) were measured with a pulse modulated PAM 101/103 fluorometer (H. Walz, Effeltrich, Germany) equipped with a Schott lamp (Schott, Mainz, Ger-many). Needles were dark-adapted for 10 min. The pulse intensity to achieve close to saturation in a dark-adapted leaf was determined by measuring Fv/Fm for green needles taken in
October from trees grown in cold frames at a PPF of 600 µmol m−2 s−1 (n = 8 per light intensity) over a range of PPFs: 1300 µmol m−2 s−1 = 0.780 (0.023 SE); 2000 µmol m−2 s−1 = 0.781 (0.022 SE); 4000 µmol m−2 s−1 = 0.785 (0.020 SE); 7000 µmol m−2 s−1 = 0.782 (0.021 SE); and 13,000 µmol m−2 s−1 = 0.793 (0.013 SE). We used a saturating pulse of 2000 µmol m−2 s−1, which was three to 10 times higher than the irradiance that the plants were grown in. The method suggested by Mark-graf and Berry (1990), which estimates the ‘‘true’’ height of a saturating pulse, was used to correct for potential problems caused by subsaturating pulses at higher actinic irradiances. At low irradiances, L waves were visible (Larcher and Neuner 1989); however, calculating photochemical quenching with or without L waves did not change the interpretation of the data.
Determination of break points and the onset of senescence
Results
Environmental conditions and pigment loss
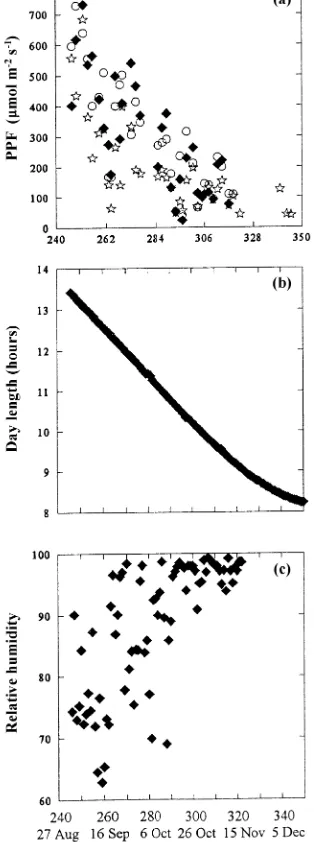
At the start of the experiment on Julian day 240 (August 27), mean air temperature at needle harvest was 23 °C (Figure 1a) and declined to a mean of 12 °C by Julian day 285 (Octo-ber 11), when chlorophyll content (see below) began to de-cline. Soil temperature in the control treatment averaged 11.3 ± 2.5 °C SD (n = 72) from September to December, with a minimum of 5.9 °C and a maximum of 16.7 °C. In the warmed soil treatment, soil temperature averaged 14.2 ± 2.1 °C SD (n = 72, Figure 1b) with a minimum of 9.3 °C and a maximum of 18.6 °C. For all seedlings, photosynthetic photon flux (PPF), measured in the morning at needle harvest, declined from 600 µmol m−2 s−1 at the start of the experiment to 200 µmol m−2 s−1 by Julian day 285 (Figure 2a). Day length (Atmospheric Environmental Services, Vancouver, BC) de-clined from 13.5 to 8.5 h (Figure 2b). Day length was 11 h when leaf chlorophyll content began to decline. Relative hu-midity increased from 60% in late summer and remained consistently above 90% after Julian day 285 (Figure 2c).
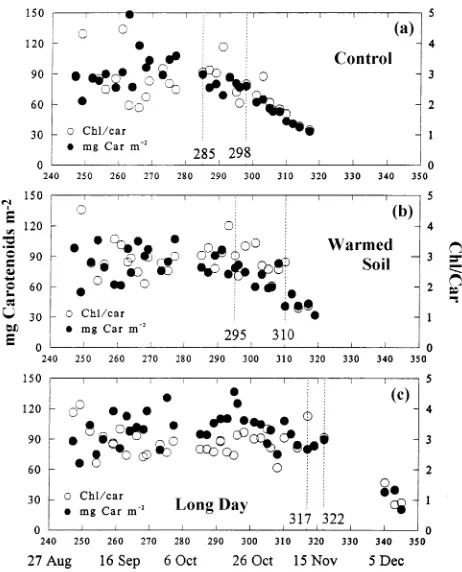
Figures 3 and 4 show the time courses of chlorophyll and carotenoid loss in seedlings in the three treatments. Among the treatments, mean chlorophyll contents of green needles,
meas-ured before chlorophyll content began to decline, were not significantly different (P > 0.10) (Table 1). The onset of the decline in chlorophyll content, determined from piecewise linear regressions, was Julian day 285 for control seedlings, Julian day 295 for warmed-soil seedlings, and Julian day 317 for long-day seedlings. For each treatment group, the sum-of-squared residual value for chlorophyll (Figure 5) had clearly begun to rise above its minimum before the decline in CO2
assimilation rate began (see below). For example, among con-trol seedlings, chlorophyll content declined 11%, from 276 to 245 mg Chl m−2, before the rate of CO2 assimilation began to
decline. We conclude, therefore, that the decline in chlorophyll content preceded the decline in CO2 assimilation rate.
Figure 1. (a) Air temperature (°C) at needle harvest for control seedlings (r), seedlings with warmed soil (s), and seedlings receiving 16-h photoperiods (q). (b) Minimum soil temperature for warmed-soil seed-lings (dashed line) averaged 3 °C above soil temperatures for control seedlings and seedlings receiving 16-h photoperiods (solid line). Maxi-mum and miniMaxi-mum values for control seedlings were 16.6 and 5.9 °C, respectively, and for warmed-soil seedlings, 18.6 and 9.3 °C.
Both Chl a and Chl b contents began to decline at approxi-mately the same time as total chlorophyll content. The decline in total chlorophyll content in control, warmed-soil and long-day seedlings began on Julian long-days 285, 295 and 317, respec-tively. The corresponding Julian days for the decline in Chl a content were 285, 298 and 317, and for the decline in Chl b content they were 275, 298 and 317, respectively. Two findings need to be considered when interpreting these results. First, the start of the decline in Chl a content and total chlorophyll content differed, as indicated by the pronounced V-shaped pattern to the plot of sum-of-squared residuals from the various piecewise linear regressions. Second, the sum-of-squared re-siduals for Chl b content of control seedlings showed little variation between Julian days 264 and 285. Thus, although the global minimum in sum-of-squared residuals for Chl b content occurred on Julian day 275, that value differed little from the sum-of-squared residuals associated with Julian day 264 or 285. Accordingly, we see little evidence to suggest that Chl a, Chl b or total chlorophyll start to decline on different dates.
The Chl a/b ratio started to decrease 3 weeks after the onset of the decline in total chlorophyll content in control (Julian day 306) and warmed-soil seedlings (Julian day 314; Figure 3). Based on the different onset dates of decline for chlorophyll Figure 3. Date of decline of chlorophyll and chlorophyll a/b ratio for (a) control, (b) warmed-soil and (c) long-day seedlings. Each point is the mean of three measurements. Dates of decline were determined by a piecewise linear regression estimated for each measurement day (see text and Figure 5). We defined the non-senescing (green) period by constant Chl levels, early senescence by declining Chl contents but constant Chl a/b ratio, and late senescence by declining Chl contents and Chl a/b ratio.
Figure 4. Date of decline of carotenoid and Chl/carotenoid ratio for (a) control, (b) warmed-soil and (c) long-day seedlings. Each point is the mean of three measurements. Dates of decline were determined by a piecewise linear regression estimated for each measurement day (see text and Figure 5). See Figure 3 for definition of green, early and late senescence.
Table 1. Chlorophyll content and chlorophyll a/b ratio in Larix occi-dentalis needles at different stages of needle senescence (see Figure 3 for definition of green, early and late senescence). Values are means ± 95% confidence interval (sample size in parenthesis).
Green Early Late
senescence1 senescence2
Chlorophyll (mg m−2)
Control 276 ± 26 (43) 205 ± 24 (24) 68 ± 17 (12) Warmed soil3 256 ± 16 (58) 185 ± 25 (25) 56 ± 28 (4) Long day3 294 ± 18 (86) 119 ± 64 (14)
Chlorophyll a/b ratio
Control 3.17 ± 0.18 (43) 3.1 ± 0.19 (29) 2.19 ± 0.35 (15) Warmed soil3 3.24 ± 0.11 (58) 3.3 ± 0.29 (27) 1.62 ± 0.78 (6) Long day3 3.26 ± 0.09 (88) 2.06 ± 0.48 (15)
1
Chlorophyll content is different (P < 0.05) from that of green tissue, whereas Chl a/b ratio is not different (P > 0.25) from that of green tissue.
2 Both Chl content and Chl a/b ratio are significantly different (P <
0.05) from values for green tissue.
3 Warmed-soil and long-day treatment groups are not significantly
content and Chl a/b ratio, we defined early senescence as the period when the chlorophyll content declined but the Chl a/b ratio did not change (Figure 3). This stage occurred between Julian days 285 and 306 (October 11--November 1) in control seedlings and between Julian days 295 and 314 (October 21--November 9) in seedlings in the warmed soil treatment, whereas in seedlings receiving an extended photoperiod, early senescence did not begin until Julian day 317 (November 12). Late senescence was defined as the time after the Chl a/b ratio started to decline (Figure 3). Our definitions of early and late senescence do not describe senescence in all tree species (Bortlik et al. 1987, Adams et al. 1990, Dean et al. 1993) but are useful in the interpretation of our data.
Dates for the start of the decline in total needle carotenoids (Figure 4), which were the same as those for total chlorophyll, were Julian days 285, 295 and 317 for control, warmed-soil and long-day seedlings, respectively. Carotenoid content de-clined about 50% during autumn, from a mean over all seed-lings of 94 mg m−2 in green tissue to 46 mg m−2 (Table 2) in late senescing tissue. The Chl/carotenoid ratio also fell by about 50% during the sampling period (Table 2), with the onset of decline occurring on Julian days 298, 310 and 322, for control, warmed-soil and long-day seedlings, respectively.
Gas exchange
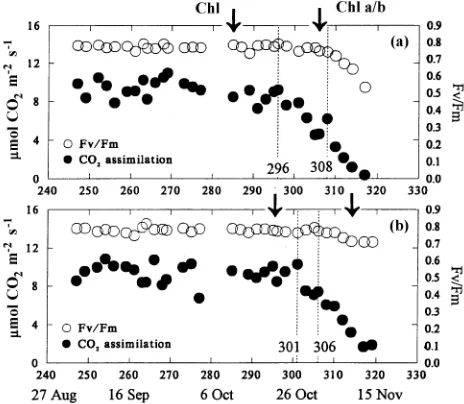
Carbon dioxide assimilation per unit area began to decline approximately 1 week after the onset of the decline in chloro-phyll content (Figure 6). The decline in photosynthetic rate
began on Julian day 296 in control seedlings (Figure 6a), Julian day 301 in seedlings in the warmed-soil treatment (Figure 6b), and Julian day 317 in long-day seedlings. Mean stomatal Figure 5. Determination of date of decline for chlorophyll contents,
CO2 assimilation (see Figure 6) and chlorophyll a/b ratio for control
seedlings based on piecewise linear regressions. Sum-of-squared re-siduals are plotted from separate piecewise linear regressions where each regression corresponded to a different break point (see text). The date associated with the minimum sum-of-squared residuals (arrows) was chosen as the best estimate of the break point.
Table 2. Carotenoid (xanthophylls (x) + carotenoids (c)) and chloro-phyll/carotenoid ratio ((Chl a + Chl b)/(x + c)) in Larix occidentalis
needles at different stages of senescence (see Figure 3 for definition of green, early and late senescence). Values are means ± 95% confidence interval (sample size in parenthesis).
Green Early Late
senescence1 senescence2
Carotenoid (mg m−2)
Control 96.90 ± 10.66 74.06 ± 5.43 42.67 ± 5.83
(43) (29) (15)
Warmed soil3 86.01 ± 6.28 65.90 ± 7.60 38.67 ± 7.88
(58) (27) (6)
Long day3 100.86 ± 5.46 55.20 ± 16.20
(88) (15)
Chlorophyll/carotenoid ratio
Control 3.04 ± 0.41 2.76 ± 0.27 1.53 ± 0.27
(43) (29) (12)
Warmed soil3 3.22 ± 0.41 2.83 ± 0.32 1.30 ± 0.39
(58) (24) (4)
Long day3 2.90 ± 0.14 1.84 ± 0.56
(85) (14)
1 Carotenoid contents are less than (P < 0.001) those from green tissue
and Chl/Car ratios are not different (P > 0.10) from green tissue.
2 Both carotenoid contents and Chl/Car ratios are significantly
differ-ent (P < 0.001) from green tissue.
3 Carotenoid contents for warmed-soil and long-day treatment groups
are not significantly different (P > 0.05) from control seedlingsand Chl/carotenoid ratios are not significantly different (P > 0.10) than control seedlings.
Figure 6. Carbon dioxide assimilation and Fv/Fm for seedlings under
conductance (Figure 7) values for all seedlings decreased from 300 mmol m−2 s−1 when needles were green to less than 50 mmol m−2 s−1 on Julian day 303 (October 29). The similar onset date of decline for all seedlings suggests that the date of decline in stomatal conductance was not affected by photope-riod or a small increase in soil temperature. We observed no significant increase in the ratio of intercellular to ambient CO2
concentration in late compared to early autumn needles (Ta-ble 3).
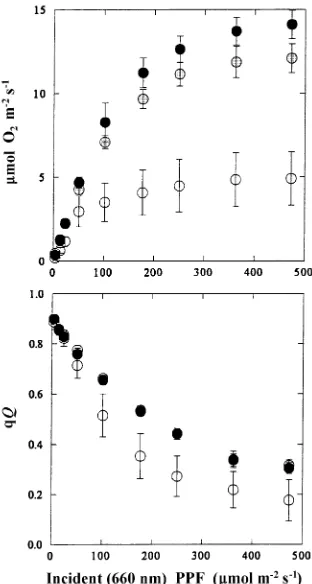
Oxygen evolution per mg Chl increased during early senes-cence and then declined during late senessenes-cence (Figure 8). The light-saturated rate of oxygen evolution was lower in senescing tissue than in green tissue (Figure 9). Dark respiration was constant during autumn and had a mean of 0.5 µmol O2 m−2 s−1
when needles were pre-illuminated at 100 µmol m−2 s−1 and then dark-adapted for 10 min (data not shown). When dark respiration was measured immediately after illumination at 470 µmol m−2 s−1, the mean rate was 1.5 µmol O2 m−2 s−1,
which is consistent with reports (Walker 1992) that respiration is greater after exposure to high irradiances.
Chlorophyll a fluorescence
We measured photochemical efficiency of photosystem II (PSII) (Fv/Fm) in green and senescing tissues to determine if
there was a correlation between a decline in PSII and declining CO2 assimilation rates. Photochemical efficiency of dark
adapted green tissue (Fv/Fm) was 0.773 ± 0.017 SD (n = 46),
0.779 ± 0.020 SD (n = 62) and 0.787 ± 0.023 SD (n = 99) in Figure 7. Stomatal conductance (mmol m−2 s−1) in naturally
senesc-ing seedlsenesc-ings (r) on Julian day 303 (October 29), and in warmed-soil (s) and long-day (q) seedlings on Julian day 305. Each point is the mean of three measurements.
Figure 8. Oxygen evolution per mg Chl for control seedlings. Each point is the mean of three measurements. Line drawn using LOWESS smoothing function in Systat (Wilkinson 1990).
Table 3. Internal/ambient CO2 concentration in Larix occidentalis
needles at different stages of senescence (see Figure 3 for definition of green, early and late senescence). Ratios are means ± 95% confidence interval (sample size in parenthesis).
Green Early Late
senescence1 senescence2
Control 0.847 ± 0.032 0.868 ± 0.010 0.892 ± 0.032
(42) (26) (15)
Warmed soil3 0.849 ± 0.024 0.856 ± 0.008 0.875 ± 0.093
(55) (27) (6)
Long day3 0.846 ± 0.014 0.883 ± 0.039
(85) (14)
1 Early senescence needles not significantly different (P > 0.10) from
green needles.
2 Late senescence needles not significantly different (P > 0.05) from
green needles.
3 Warmed-soil and long-day treatment groups not significantly
differ-ent (P > 0.10) from control needles.
Figure 9. (a) Oxygen evolution per leaf area (20 °C, 50 ml l−1 CO2,
control, warmed-soil and long-day needles, respectively, and declined to 0.5 in senescent needles (Figure 6a). Photochemi-cal efficiency (Fv/Fm) began to decline after the decline in CO2
assimilation rate in all seedlings. The decline in Fv/Fm began
at the same time in control and warmed-soil seedlings (Julian days 308 and day 306, respectively, Figures 6a and 6b) but was delayed in long-day seedlings (Julian day 322).
Photochemical quenching (qQ, Kooten and Snell 1990) in-dicates the proportion of oxidized QA, the quinone acceptor of
photosystem II. To plot qQ as a function of light intensity, data were grouped into green, early senescing and late senescing phases as defined by chlorophyll loss (Figure 3). For all seed-lings, photochemical quenching was less in late senescing tissue than in green tissue at irradiances greater than 25 µmol m−2 s−1 (Figure 9b).
Discussion
The sequence of events during autumn foliar senescence in western larch seedlings was similar to that in other deciduous species (Sanger 1971, Lichtenthaler 1987, Adams et al. 1990) in that chloroplasts remained efficient until the late stages of senescence. In addition, the order of events----a decline in chlorophyll content followed by declines in CO2 assimilation
rate, and, finally, Chl a/b ratio----is the same as that found in western larch needles senescing in controlled environment chambers at autumn temperatures and with a short photoperiod (Rosenthal and Camm 1996). The similarity suggests that this pattern of needle degradation is typical of a variety of autumn-like conditions. In the present study, the conditions at the time chlorophyll content began to decline included: a decrease in mean air temperature from 23 to 12 °C (Figure 1a), a decline in irradiance to below 350 µmol m−2 s−1 (Figure 2a), a day length of less than 11 h (Figure 2b), and a relative humidity above 85% (Figure 2c).
The first measured parameter to decline was needle pigment content (chlorophyll and carotenoids). During the early phase of senescence when the amount of chlorophyll per leaf area declined, chloroplast function did not decline. The stable Chl a/b ratio during early senescence (Figure 3) suggests that Chl a and Chl a/b-containing complexes were degraded at the same rate. The constant Fv/Fm ratio indicates that the thylakoids
sampled by the fluorescence technique (i.e., those near the leaf surface) remained functional. Furthermore, photosynthetic rate per mg Chl increased during early senescence (Figure 8). During late senescence, there was loss in function of nearly all of the photosynthetic components. A decline in the Chl a/b ratio and the Chl/carotenoid ratio ((Chl a + Chl b)/(xantho-phyll + β-carotene)) suggested preferential loss of Chl a-con-taining proteins closely associated with the reaction centers over loss of light harvesting proteins (Lichtenthaler 1987). The subsequent decline in qQ, which was not apparent under light limitation (Figure 9b), is consistent with a loss in PSII reaction centers or a decrease in carboxylation, or both.
The ratio of internal to ambient CO2 concentration did not
increase significantly (Table 3) during late senescence suggest-ing that changes in photosynthetic function and carbon fixation
compensated for changes in stomatal conductance in yellow leaves. A constant ratio of internal to ambient CO2
concentra-tion during senescence has been reported in some species (e.g., Woolhouse and Jenkins 1983) but not in others (e.g., Makino 1984, Thomas 1991). The variations in stomatal conductance during senescence that have been observed in some species may be associated with stomatal patchiness (Thomas 1991) and the history of the leaf (i.e., the age of the leaf or the conditions inducing senescence, or both). In our study, the late senescent tissue contained on average between 50 and 120 mg Chl m−2 (Table 1) and had positive photosynthetic rates (Fig-ure 6). Thus, these needles may contain less senescent tissue than needles for which an increase in internal to ambient CO2
concentrations is reported.
The warmed-soil treatment delayed the onset of needle senescence but, once senescence began, it progressed more rapidly in warmed-soil seedlings than in control seedlings. For example, the 3 °C increase in soil temperature (Figure 1b) delayed needle yellowing by 10 days compared with control seedlings (Figure 3). Other pigment-related parameters (initia-tion of decline in carotenoids, and in Chl/carotenoids and Chl a/b ratios) were delayed by approximately the same period (8--14 days). However, other processes were not delayed to the same extent. For example, carbon assimilation and Fv/Fm
be-gan to decline in warmed-soil seedlings only slightly (2--5 days) later than in naturally senescing seedlings. As a result, the interval between the onset of the decline in pigment content and the onset of the decline in CO2 assimilation rate was
shorter for seedlings in warmed soil than for naturally senesc-ing seedlsenesc-ings. Needle senescence in western larch seedlsenesc-ings in controlled environment chambers showed a similar pattern: CO2 assimilation rate began to decline approximately 1 week
after the onset of the decline in chlorophyll content in seed-lings maintained at an air temperature of 15 °C, but the interval was approximately 2 weeks when seedlings were maintained at 8 °C (Rosenthal and Camm 1996).
The extended photoperiod treatment delayed the onset of decline of most parameters. Delayed senescence has been observed in deciduous trees growing near street lights (Olm-sted 1951), though previous studies have not determined whether the delay was the result of photoperiod extension or a higher air temperature, or both. We have shown that extended photoperiod alone can delay needle yellowing in larch. Fur-thermore, we have demonstrated that, during this extended green period, the needles are functional, in contrast to some stay-green mutants in which assimilation rates decline even though the chlorophyll content is maintained (Smart 1994). Rosenthal and Camm (1996) observed that senescing needles of western larch maintained in a controlled environment growth chamber set to a 16-h photoperiod and a temperature of 8 °C showed a similar pattern of decline in CO2 assimilation
rate relative to the decline in chlorophyll content. Similarly, we found that chlorophyll content and CO2 assimilation rate
controlled for in this experiment might have triggered the decline. For example, stomatal conductance in larch has been shown to be correlated with irradiance (Benecke et al. 1981, Matyssek and Schulze 1988) and to leaf-to-air vapor pressure difference (Benecke et al. 1981, Sandford and Jarvis 1986, Matyssek and Schulze 1988). Although a drop in soil tempera-ture has been shown to lower stomatal conductance in other conifers (e.g., Day et al. 1991), the 3 °C difference between the soil temperature in our treatments was not large enough to affect the onset of decline in stomatal conductance.
Our photoperiod treatment did not affect the onset of decline in stomatal conductance. The extended photoperiod delayed the onset of decline in assimilation rate and chlorophyll loss, and thus might have been expected to also delay stomatal conductance. However, the large changes in photosynthetic photon flux density, air temperature and relative humidity appear to have had a greater influence on the timing of stomatal conductance changes than changes in assimilation rates and Chl content.
We were able to study selected senescence-related events in larch needles, even though such events are among the physi-ological and developmental changes experienced by the whole plant. Development of the abscission layer (Neger and Fuchs 1915) and bud development induced by shortened days (Mei-jer and van der Veen 1957, Romberger 1963) may affect translocation and assimilate partitioning. It is possible that our environmental manipulations affected abscission layer devel-opment and bud formation thereby influencing the timing of needle senescence indirectly as well as directly.
We were able to quantify the effects of changes in soil temperature and photoperiod on the onset of different degrada-tive events associated with foliar senescence in western larch. Further, we found that because degradative events are differ-ently affected by the changing environment, the course of breakdown of the photosynthetic apparatus during senescence can vary. We have demonstrated that the response of the plant to yearly variation in environmental conditions can be bal-anced between assimilation and autumn shutdown.
Acknowledgments
This research was in part funded by a grant from the Natural Sciences and Engineering Research Council of Canada and a GREAT award from the Science Council of British Columbia and Weyerhaeuser Canada. We also thank Drs. B. Green and I. Taylor for helpful discus-sions during preparation of this manuscript.
References
Adams, W.W., K. Winter, U. Schreiber and P. Schramel. 1990. Photo-synthesis and chlorophyll fluorescence characteristics in relation-ship to changes in pigment and element composition of leaves of
Platanus occidentalis L. during autumnal senescence. Plant Physiol. 93:1184--1190.
Addicott, F.T. and J.L. Lyon. 1973. Physiological ecology of abscis-sion. In Shedding of Plant Parts. Ed. T.T. Kozlowski. Academic Press, New York, pp 85--124.
Benecke, U., E.-D. Schulze, R. Matyssek and W.M. Havranek. 1981. Environmental control of CO2-assimilation and leaf conductance in Larix decidua Mill. Oecologia 50:54--61.
Bortlik, K., H. Gut and P. Matile. 1987. Yellowing and non-yellowing trees: a comparison of protein and chlorophyll loss in senescent leaves. Bot. Helv. 97:323--328.
Cunninghame, M.E., J.R. Hillman and B.G. Bowes. 1982. Ultrastruc-tural changes in mesophyll cells of Larix decidua ×kaempferi
during leaf maturation and senescence. Flora 172:161--172. Day, T.A., S.A. Heckathorn and E.H. DeLucia. 1991. Limitation of
photosynthesis in Pinus taeda L. (loblolly pine) at low soil tempera-tures. Plant Physiol. 96:1246--1254.
Dean, M.A., C.A. Letner and J.H. Eley. 1993. Effect of autumn foliar senescence on chlorophyll a:b ratio and respiratory enzymes of
Populus tremuloides. Bull. TorreyBot. Club 120:269--274. Goodwin, T.W. 1958. Studies in carotenogenesis. The changes in
carotenoid and chlorophyll pigments in the leaves of deciduous trees during autumn necrosis. Biochem. J. 68:503--511.
Kooten, O. and J.F. Snell. 1990. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth. Res. 25:147--150.
Kozlowski, T.T., P.J. Kramer and S.G. Pallardy. 1991. Extent and significance of shedding of plant parts. In The Physiological Ecol-ogy of Woody Plants. Ed. T.T. Kozlowski. Academic Press, New York, pp 1--34.
Lang, M. and H.K. Lichtenthaler. 1991. Changes in the blue-green and red fluorescence-emission spectra of beech leaves during the autumnal chlorophyll breakdown. J. Plant Physiol. 138:550--553. Larcher, W. and G. Neuner. 1989. Cold induced sudden reversible
lowering of in vivo chlorophyll fluorescence after saturating light pulses. Plant Physiol. 89:740--742.
Lichtenthaler, H.K. 1987. Chlorophyll fluorescence signatures of leaves during the autumnal chlorophyll breakdown. J. Plant Physiol. 131:101--110.
Lichtenthaler, H.K. and A.R. Wellburn. 1983. Determination of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 11:591--592.
Makino, A., T. Mae and K. Ohira. 1984. Changes in photosynthetic capacity in rice leaves from emergence through senescence. Analy-sis from ribulose-1,5-bisphosphate carboxylase and leaf conduc-tance. Plant Cell Physiol. 25:511--521.
Markgraf, T. and J.A. Berry. 1990. Measurement of photochemical and non-photochemical quenching: correction for turnover of PS2 during steady-state photosynthesis. In Current Research in Photo-synthesis. Vol. IV. Ed. M. Baltscheffsky. Kluwer Academic Publish-ers, Dordrecht, The Netherlands, pp 270--282.
Matyssek, R. and E.-D. Schulze. 1988. Carbon uptake and respiration in above-ground parts of a Larix decidua × leptolepis tree. Trees 2:233--241.
Matyssek, R., M.S. Günthardt-Goerg, T. Keller and C. Scheidegger. 1991. Impairment of gas exchange and structure in birch leaves (Betula pendula) caused by low ozone concentrations. Trees 5:5--13.
Meijer, G. and R. van der Veen. 1957. Wavelength dependence on photoperiodic responses.Acta Bot. Neerl. 6:429--433.
Neger, F.W. and J. Fuchs. 1915. Untersuchungen über den Nadelfall der Koniferen. Jahrb. Wissensch. Bot. 55:608--660.
Neter, J., W. Wasserman and M. Kutner. 1990. Applied linear statisti-cal models, 3rd Edn. Richard D. Irwin, Inc., Homewood, IL, pp 370--374.
Olmsted, C.E. 1951. Experiments on photoperiodism, dormancy and leaf age and abscission in sugar maple. Bot. Gaz. 112:365--393. Osborne, D.J. 1973. Internal factors regulating abscission. In
Pell, E.J. and M.S. Dann. 1991. Multiple stress-induced foliar senes-cence and implications for whole plant longevity. In Response of Plants to Multiple Stresses. Eds. H.A. Mooney, W.E. Winner and E.J. Pell. Academic Press, San Diego, CA, pp 189--204.
Richards, J.H. and L.C. Bliss. 1985. Winter water relations of a deciduous timberline conifer, Larix lyalli. Oecologia 69:16--24. Romberger, J.A. 1963. Meristems, growth and development in woody
plants. USDA Forest Service, Tech. Bull. No. 1293, Washington, D.C., pp 46--47.
Rosenthal, S.I. and E.L. Camm. 1996. Effects of air temperature, photoperiod, and leaf age on foliar senescence of western larch (Larix occidentalis Nutt.) in environmentally controlled chambers. Plant Cell Environ. 19:1057--1065.
Sandford, A.P. and P.G. Jarvis. 1986. Stomatal responses to humidity in selected conifers. Tree Physiol. 2:89--103.
Sanger, J.E. 1971. Quantitative investigation of leaf pigments from their inception in buds through autumn coloration to decomposition in falling leaves. Ecology 52:1075--1089.
Smart, C.M. 1994. Tansley Review No. 64. Gene expression during leaf senescence. New Phytol. 126:419--448.
Thomas, C., S.D. Davis and G. Tallman. 1991. Responses of stomata of senescing and nonsenescing leaves of Nicotiana glauca to changes in intercellular concentrations of leaf carbon dioxide. Plant Cell Environ. 14:971--978.
Vance, N.C. and S.W. Running. 1985. Light reduction and moisture stress: effects on growth and water relations of western larch seed-lings. Can. J. For. Res. 15:72--77.
Walker, D.A. 1987. The use of the oxygen electrode and fluorescence probes in simple measurements of photosynthesis. Oxygraphics Ltd., University of Sheffield, Kings Lynn, U.K., pp 1--16. Walker, D.A. 1992. Excited leaves. New Phytol. 121:325--345. Wilkinson, L. 1990. Systat: The system for statistics. Systat, Inc.,
Evanston, IL, pp 304--305.
Woolhouse, H.W. and G.I. Jenkins. 1983. Physiological responses, metabolic changes and regulation during leaf senescence. In
Growth and Functioning of Leaves. Eds. J.E. Dale and F.L. Milthorpe. Cambridge, NY, pp 449--487.