Plant Science 149 (1999) 115 – 123
Changes to the content of sugars, sugar alcohols, myo-inositol,
carboxylic acids and inorganic anions in developing grains from
different varieties of Robusta (
Coffea canephora
) and Arabica (
C
.
arabica
) coffees
William John Rogers *, Ste´phane Michaux, Maryse Bastin, Peter Bucheli
Department of Plant Science,Nestle´ Research Centre,101A6enue Gusta6e Eiffel,Notre Dame D’Oe´,BP9716,37097Tours Cedex2,France
Received 4 March 1999; received in revised form 2 July 1999; accepted 20 July 1999
Abstract
Changes in concentration of mono- and oligosaccharides, sugar alcohols, myo-inositol, carboxylic acids and inorganic anions in coffee grains were analysed during grain development in three cultivars ofCoffea arabicaL (Arabica) and two ofC.canephora L var. Robusta (Robusta) by high performance anion exchange chromatography coupled to pulsed electrochemical detection (HPAE-PED). The majority of the components analysed either decreased in concentration during the first half of the development period or accumulated steadily during the latter half of the period. The profiles are taken to indicate relationships between the perisperm, the principal tissue in the young grain and the endosperm during maturation. While most of the free sugar in the mature grain is accounted for by sucrose, fructose and glucose are both at higher concentrations in the perisperm. Considerable amounts of myo-inositol (3 – 4% dry weight (DW)) are found in young grains, while only the phosphorylated form phytic acid occurs in mature grains (0.3 – 0.6% DW). Quinic acid, which is present in very low amounts in mature endosperm, represents between 6 and 16% DW in young grains, this possibly being the major precursor pool for the high amounts of chlorogenic acids (5 – 10% DW) which are a characteristic of mature coffee grains. Of the other organic acids analysed, citric and malic acids are dominant in the mature grain, with higher concentrations in Arabica than Robusta. The results are discussed with respect to the potential implications for transport mechanisms in developing coffee grains and also the importance of the compounds analysed for industrial quality and flavour. © 1999 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Coffea arabica;Coffea canephora; Seed development; Endosperm; Perisperm
www.elsevier.com/locate/plantsci
1. Introduction
Analysis of the levels of some of the principal free components occurring in mature coffee grains are available in the literature [1 – 7]. The content and forms of chlorogenic acids, which occur at high concentrations in the grains, have received
particular attention [8]. The great majority of the studies have considered the components in mature grains from an industrial perspective as precursors of coffee beverage flavour and aroma. Information regarding the evolution and accumulation of com-pounds during coffee grain maturation is more scarce. Some exceptions to this include the work done on caffeine biosynthesis and transport [9 – 12], attempts to correlate grain maturity to the generation of components in the final beverage [13,14] and some measurements of enzyme activi-ties during fruit ripening [15]. Such developmental
Abbre6iations:WAF, weeks after flowering.
* Corresponding author: Tel.: +33-2-47628383; fax: + 33-2-47491414.
E-mail address:[email protected] (W.J. Rogers)
information is, however, an essential biochemical base for the elucidation of mechanisms of trans-port and metabolism in the grains and should provide targets for the improvement of the technological and agronomic character of the species.
In this study we describe changes in concentra-tions during grain maturation of a limited number of components (free sugars, sugar alcohols, car-boxylic acids and inorganic anions) likely to be implicated in final beverage quality in the two main commercial coffee species, Coffea arabica
(Arabica) and C. canephora (Robusta). Sucrose, while being significantly degraded during roasting, remains in roasted grains at concentrations of 0.4 – 2.8% dry weight (DW) and is likely to con-tribute to beverage sweetness [16]. It is also the main contributor of reducing sugars which are implicated in Maillard reactions occurring during roasting. Inorganic, chlorogenic and carboxylic acids contribute to the final beverage acidity [3], acidity being associated with better flavour and aroma [17].
The principal interest of this study is the rela-tionship between concentration profiles in the young grain and the more mature stages in an attempt to begin to identify biochemical mecha-nisms of transport and accumulation for a range of components. The grain of Coffea species is dominated by a well developed maternal perisperm tissue up to approximately the halfway stage of maturation (from the ‘pinhead’ stage until approximately 15 weeks after flowering (WAF)), following which the locular space is progressively filled with endosperm up to full grain maturity at between 20 and 30 WAF.
The mature coffee grain has been convincingly identified as endosperm [18] and not perisperm as suggested previously [19]. This identification has been supported by more recent studies [20 – 22]. Wormer [23] also observed the role of the perisperm, during the expansion occurring during the first half of the maturation period, in defining the final size of the locular space. Although virtu-ally nothing is known about the characteristics of metabolism and transport in the fruits and grains of coffee species, the relatively large size of the two principal tissues and the duration of the matura-tion period provide an interesting model for the study of relationships between them during grain development.
2. Materials and methods
2.1. Plant material
Fruit from varieties of Robusta (Dormilon and Rom) and varieties of Arabica (CRM, Caturra Commercial and Caturra 2308) were obtained from trees cultivated on the experimental farm of Nestle´ R&D Centre Quito, Ecuador. Trees were cultivated on the same site at an altitude of ap-proximately 80 m above sea level (MAS). Fruit were harvested during 1996 at defined stages fol-lowing flowering, frozen immediately in liquid ni-trogen and packaged in dry ice for transport. Samples normally consisted of ten fruit (20 grains).
2.2. Extraction
Grain tissues were separated from pericarp and hulls (locules). For a detailed analysis of free sugars in some cases the perisperm in the young fruit was separated entirely from the endosperm, in other cases the entire contents of the grain were used without separation. The tissue was homoge-nized in a mortar with liquid nitrogen and the powder obtained was lyophilized for 24 h (Lyolab bII, Secfroid). The sample was stored at −20°C where necessary before being weighed and sus-pended in 70 ml of double-distilled water previ-ously pre-heated to 70°C, shaken vigorprevi-ously and incubated for 30 min at 70°C. After cooling to room temperature, the sample was made to 100 ml by adding double-distilled water and then filtered on paper (Schleicher and Schuell filter paper 597.5). An aliquot (3 ml) of the filtrate was filtered a second time on a C18 cartridge (SEP PAK)
which had been equilibrated beforehand with 3 ml methanol followed by 3 ml of water.
2.3. Separation by HPAE-PED
isocrati-W.J.Rogers et al./Plant Science149 (1999) 115 – 123 117
cally with 81 mM NaOH and 480 mM NaOH, respectively. Carboxylic acids and inorganic an-ions were separated by a gradient of 0.5 – 38.25 mM NaOH for 18 min. Phytic acid was eluted with a gradient of 23 – 60 mM NaOH for 1 min. The injected sample volume was 20ml and the flow rate usually 1 ml/min. Standards of sugars, oligosaccharides, sugar alcohols, carboxylic acids and inorganic anions were from Sigma (St. Louis, MO) except stachyose tetrahydrate which was from Extrasynthe`se (Genay, France). All results represent the mean of three repetitions.
3. Results
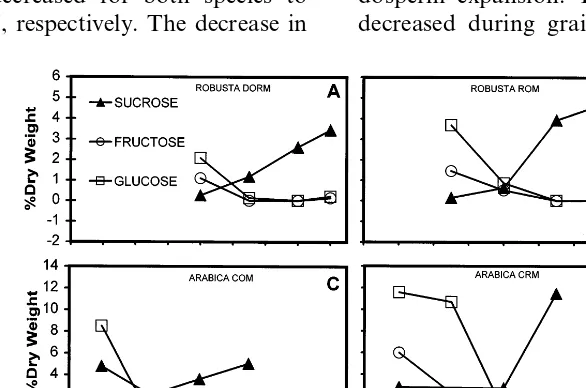
Concentrations of glucose, fructose and sucrose evolved in a very similar way during maturation for both Arabica and Robusta grains (Fig. 1). At the earliest stages of maturity examined (Arabicas: 12 WAF; Robustas: 18 and 24 WAF) until ap-proximately the halfway stage of maturation, glu-cose and fructose were usually the major free sugars of young coffee grains, glucose being con-sistently approximately twice the concentration of fructose. Levels of glucose were higher in the Arabica varieties studied (between 8 and 12% DW) than in Robusta (2 to 4% DW). By the end of grain development (30 WAF for Arabica, 36 to 40 WAF for Robusta) concentrations of glucose and fructose had decreased for both species to 0.03 and 0.04% DW, respectively. The decrease in
these two sugars was accompanied by an increase in sucrose, which approached 100% of total free sugars in mature grains, again being higher on average in Arabica (from 5 to 12% DW) than Robusta (4 – 5% DW). A more refined analysis of free sugars in one variety of Arabica (Caturra 2308), in which maternal perisperm tissues were dissected from endosperm and analysed sepa-rately, explains the maturation profile of these components (Fig. 2). Higher concentrations of glu-cose and fructose (the former being approximately twice that of the latter), compared to sucrose, are specifically associated with perisperm tissue. Su-crose is always the dominant sugar in endosperm tissue even at the earliest stages of maturation of this tissue.
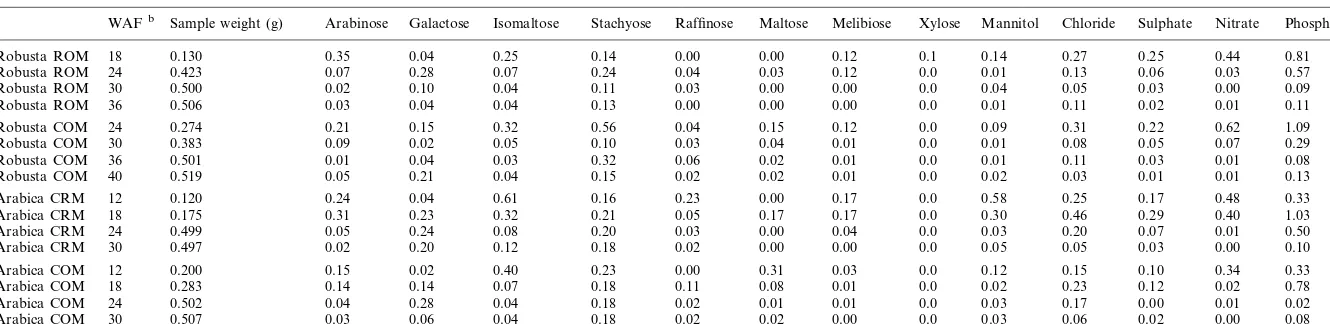
The results of the analysis of other sugars and sugar alcohols are presented in Table 1. Stachyose content was normally within the range 0.1 – 0.25% DW, although for one variety (Robusta COM) higher levels of 0.32 and 0.56% were observed for two of the four stages. Despite the variability of levels noted for this one variety, no overall ten-dency indicating a fundamental modification of the metabolism of this sugar during maturation was discernible. Concentrations of galactose simi-larly tended to be low at all levels, although a transient increase may be noted to levels of 0.2 – 0.3% DW at the halfway stage of development, this corresponding to the period of rapid en-dosperm expansion. The levels of free arabinose decreased during grain maturation from 0.10 to
W
.
J
.
Rogers
et
al
.
/
Plant
Science
149
(1999)
115
–
123
Table 1
Content of free sugars, sugar alcohols and inorganic anions (% g/g DW) in whole grains of two varieties of Robusta and two varieties of Arabica during grain maturationa
Stachyose
WAFb Sample weight (g) Arabinose Galactose Isomaltose Raffinose Maltose Melibiose Xylose Mannitol Chloride Sulphate Nitrate Phosphate
0.14 0.00 0.00 0.12 0.1 0.14 0.27
0.04 0.25 0.25 0.44 0.81
0.35 0.130
18 Robusta ROM
0.24 0.04 0.03 0.12 0.0 0.01 0.13 0.06
Robusta ROM 24 0.423 0.07 0.28 0.07 0.03 0.57
0.11 0.03 0.00 0.00 0.0 0.04 0.05
0.04 0.03
0.500 0.02 0.10 0.00 0.09
Robusta ROM 30
0.04
36 0.506 0.03 0.04 0.13 0.00 0.00 0.00 0.0 0.01 0.11 0.02 0.01 0.11
Robusta ROM
24 0.274 0.21 0.32 0.56 0.04 0.15 0.12 0.0 0.09 0.31 0.22 0.62 1.09
Robusta COM 0.15
0.10 0.03 0.04 0.01 0.0 0.01 0.08
0.05 0.05
Robusta COM 30 0.383 0.09 0.02 0.07 0.29
0.32 0.06 0.02 0.01 0.0 0.01 0.11 0.03 0.01 0.08
Robusta COM 36 0.501 0.01 0.04 0.03
0.15 0.02 0.02 0.01 0.0 0.02 0.03 0.01
0.04 0.01
40 0.519 0.13
Robusta COM 0.05 0.21
12 0.120 0.24 0.61 0.16 0.23 0.00 0.17 0.0 0.58 0.25 0.17 0.48 0.33
Arabica CRM 0.04
18 0.175 0.31 0.32 0.21 0.05 0.17 0.17 0.0 0.30 0.46 0.29 0.40 1.03
Arabica CRM 0.23
0.20 0.03 0.00 0.04 0.0 0.03 0.20
0.08 0.07
Arabica CRM 24 0.499 0.05 0.24 0.01 0.50
0.18 0.02 0.00 0.00 0.0 0.05 0.05 0.03 0.00
Arabica CRM 30 0.497 0.02 0.20 0.12 0.10
0.23 0.00 0.31 0.03 0.0 0.12 0.15
0.02 0.10
0.200 0.15 0.40 0.34 0.33
Arabica COM 12
18 0.283 0.14 0.07 0.18 0.11 0.08 0.01 0.0 0.02 0.23 0.12 0.02 0.78
Arabica COM 0.14
Arabica COM 24 0.502 0.04 0.28 0.04 0.18 0.02 0.01 0.01 0.0 0.03 0.17 0.00 0.01 0.02
0.18 0.02 0.02 0.00 0.0 0.03 0.06 0.02 0.00
0.04 0.08
Arabica COM 30 0.507 0.03 0.06
a
Results are means of triplicate experiments. b
W.J.Rogers et al./Plant Science149 (1999) 115 – 123 119
Fig. 2. Changes in concentrations of free sugars in grains of Arabica variety Caturra 2308 during maturation. After being separated from pericarp and locules, grains were divided into perisperm (mainly in the young grains up to 20 WAF) and endosperm tissues for analysis.
0.30% DW in young grains to trace levels (0.02 – 0.04% DW) between 30 and 36 WAF. Mannitol decreased towards the end of grain maturity to levels of between 0.01 (Robusta ROM) and 0.05% DW (Arabica CRM). No evidence for the pres-ence of free arabitol, sorbitol, dulcitol, xylitol or scyllo-inositol was found (data not shown).
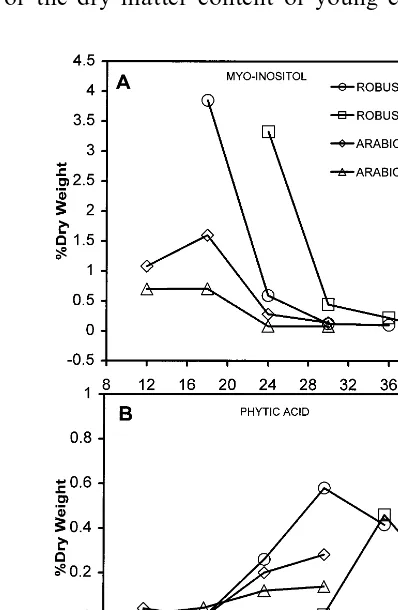
The concentration of myo-inositol decreased during grain development (Fig. 3) from between 3 and 4% (Robustas), 0.7 and 1.08% DW (Arabicas) in the perisperm-dominated stages to approxi-mately 0.05% for the mature Robustas and be-tween 0.08 and 0.14% DW for mature Arabicas. In contrast, phytic acid increased during grain maturation, reaching higher levels in mature Ro-busta (0.4 – 0.6% DW) than in Arabica (0.1 – 0.3% DW).
Data for the free carboxylic acids analysed that were above trace levels are given in Fig. 4. The content of citric acid was very low at the begin-ning of grain development (0.04 – 0.19% DW) but increased substantially during the development of the grain, reaching levels of between 1.28 and 1.58% DW at the latest maturity stages tested. Malic acid content was either maintained rela-tively high or tended to increase during the first half of development, before declining to lower levels (0.4 – 0.5% DW) in the mature grains. How-ever, malic acid was consistently the second most abundant carboxylic acid following citric acid. During grain development, concentrations of ox-alic and acetic acid decreased and were very low
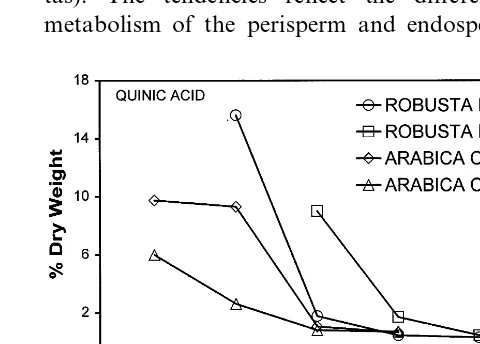
towards the end of maturity (0 – 0.03% DW). Formic acid remained constantly at a trace level. Quinic acid was found to form a significant part of the dry matter content of young coffee grains
Fig. 4. Concentrations of organic acids during maturation of whole grains of two varieties of Robusta and two varieties of Arabica. Grains were separated from pericarp and locules before analysis.
(Fig. 5). At the earliest developmental stages ex-amined, its content varied between 6 and 16% DW, respectively, for the Arabica and Robusta coffees. Towards the end of grain development levels decreased to much lower levels (0.34% DW for Robusta and 0.67% DW for Arabica).
Concentrations of chloride, sulphate and nitrate ions decreased during grain maturation (Table 1). The contents of sulphate and nitrate were between undetectable and 0.03% DW at the end of grain development. Phosphate concentrations were rela-tively high (between 0.7 and 1.1% DW) in young grains in both species until the halfway stage of development before decreasing to concentrations of around 0.10% DW at the end of grain development.
4. Discussion
The five varieties, two of Robusta and three of Arabica, used for these studies were grown either at the same site or, in the case of Robusta Dormilon, at a neighbouring site very close by, at
80 MAS. The climatic conditions and the
alti-tude were those associated with Robusta cultiva-tion, Arabica normally being cultivated at above 1000 MAS. Such conditions would not be consid-ered as favourable for the production of good quality Arabica coffee grains. However, the choice of a single climatic condition allows us to elimi-nate considerations of this major environmental influence. The duration of fruit maturation for the
Robusta varieties studied was between 6 and 8 weeks longer than for the Arabicas, this difference being characteristic for the two species [25]. This being taken into account, the concentration profi-les of the components analysed are very consistent for the two species.
The two principal tendencies observed are a diminution for some components from an initial higher level in the young grains and steady accu-mulation for others from a very low level. Regard-ing the second tendency, the low level is maintained for approximately the first 10 to 15 WAF of maturation (up to 20 WAF for Robus-tas). The tendencies reflect the differences in metabolism of the perisperm and endosperm
W.J.Rogers et al./Plant Science149 (1999) 115 – 123 121
sues. For one series of analysis of sugars the two tissues were dissected and analysed separately. However, our own observations have indicated that for the initial ten to fifteen weeks of matura-tion the perisperm dominates the interlocular space, and for much of this time the embryo sac remains tiny. The fact that the concentration curves are essentially the same for dissected and non-dissected tissues supports the assumption that concentrations in the whole young grains are es-sentially those characteristic of the perisperm tis-sue. It is clear that the concentrations measured represent equilibria between the processes of im-port, biosynthesis, metabolism and export for each of the components. Inferences regarding mecha-nisms of transport and precursor-product relations must, therefore, be cautious.
The dominant free sugars in coffee grains were consistently glucose, fructose and sucrose, all oth-ers being at very low levels. Sucrose represents almost the totality of free sugar in the mature grains. Higher levels of sucrose in mature Arabica than in Robusta endosperm are correlated with higher levels of glucose and fructose in perisperm tissue for the two species. The low levels of glucose and fructose measured in mature grains are in agreement with other reports [5,26], as are the higher levels of sucrose for Arabica compared to Robusta ([1] and Refs. therein, [5]). The occur-rence in dried commercial coffee grains of elevated concentrations of reducing sugars which is occa-sionally noted is likely to be a result of degrada-tion during storage [27,28]. Assuming that sucrose is the major carbohydrate transported in the phloem (which has yet to be ascertained for coffee) then it is clear that the levels observed of glucose and fructose in the perisperm represent enhanced sucrose catabolism in this tissue. The catabolism of sucrose in the perisperm is consistent with the requirement by this tissue of an increase of os-motic pressure to enable both the initial expansion within the locular space and a sink function. Fur-thermore, the fact that the embryo sac is entirely surrounded by perisperm during the first 10 – 15 weeks of development (personal observations) would suggest that during this first phase of fruit growth at least there may be resynthesis of sucrose for early endosperm requirements, as has been described for other plants, e.g. maize [29]. The imbalance consistently observed in the perisperm between glucose and fructose, the latter being
consistently half the concentration of the former, suggests that fructose is also diverted towards other pathways following sucrose breakdown or that all the glucose in the perisperm is not be directly related to sucrose catabolism.
The two oligosaccharides stachyose and raffinose were both in low concentrations in the grains. Stachyose was the highest normally at around 0.2% and its content was constant throughout grain development. Raffinose was in lower and also constant concentrations. The low concentrations in mature grains are in agreement with other results [30,1]. There is no evidence, therefore, for an important change in the role of the raffinose series in coffee grain development. Both myo-inositol and phytic acid were higher in Robustas than in Arabicas. The accumulation of phytic acid, the hexaphosphoric form of inositol, as a phosphate store is typical of many seed storage tissues, the phosphate being released by the action of phytase during germination. Perisperm myo-inositol is possibly the precursor of this endosperm storage compound. The pools are not necessarily linked, however. Free myo-inositol is known to be involved in many plant metabolic pathways, being a precursor of inositol phos-phates, phosphoinositides, cell wall polysaccha-rides via the myo-inositol oxidation pathway [31,32] and IAA conjugates and also being impli-cated in signal transduction pathways.
The pH of Arabica coffee beverages is normally reported to be lower than for Robustas [1,17]. Arabicas are valued for the impact on taste of their higher acidity, while tasting notes for Robus-tas usually do not include this parameter. Among the carboxylic acids analyzed, the predominance of citric acid was confirmed and shown to be part of a steady process of accumulation during matu-ration. In the four varieties examined, malic acid was consistently observed to be approximately one third of the concentration of citric acid. The higher concentrations of these organic acids in the Arabica compared with the Robusta coffees is in agreement with other results [3]. However, it is important to consider the fate of components fol-lowing the different processing steps, especially roasting and high temperature extraction. Despite lower concentrations in coffee grains (0.10% DW), some authors [1] consider that phosphoric acid, with a pKa of 1.97, may contribute more to
reported that, while citric acid is reduced and malic acid remains constant or degrades slightly during roasting, phosphoric, acetic and quinic acids in-crease [3,6].
The family of esters formed between quinic and cinnamic acids, termed chlorogenic acids, which were not analysed in the present report, occur in high concentrations in coffee grains (5 – 10% DW). Of all the phytochemical components analysed these have perhaps received the most attention [33,8]. In con-trast to the situation for caffeine metabolism [9], very little is known about the biosynthesis, transport and metabolism of these compounds within the plant. It has been reported that they are the major source of phenolic compounds for the developing cotyledons, following germination, for the synthesis of lignified structures [34]. However, they are also likely to be implicated in other processes, e.g. dormancy [35]. Coffee represents the highest dietary source of at least one chlorogenic acid (feruloylquinic acid). These compounds are likely to make a significant contribution to the final beverage flavour and it has been shown that caffeic, ferulic, quinic and coumaric acids are all degraded to a range of phenolic compounds during roasting [36]. The high quinic acid content found in the perisperm, which reached more than 15% DW in certain cases, may again point to a role for this tissue in the supply of the ester form and/or import of the mature chlorogenic acids into the endosperm. While it is assumed that the precur-sors of chlorogenic acids are synthesized in the photosynthetic tissues, very little is known about their transport within the plant. Quinic acid itself is always in low abundance in coffee beverages (concentrations being observed from 0.5 to 0.91% DW) [8].
In conclusion, this purely analytical study pro-vides data, not previously available, on the evolution of sugars, sugar alcohols, myo-inositol, carboxylic acids and inorganic anions during coffee grain development. It is hoped that the results will provide pointers for future investigation at the molecular and biochemical levels regarding the transport and metabolism of these compounds in the grain and also regarding the roles of the different tissues within the coffee grain during maturation.
Acknowledgements
The authors thank M. Alvarez (Nestle´ R&D Centre Quito, Ecuador) for providing coffee fruit samples throughout the season for this work.
References
[1] M.N. Clifford, Chemical and physical aspects of green coffee and coffee products, in: M.N. Clifford, K.C. Wilson (Eds.), Coffee: Botany, Biochemistry and Produc-tion of Beans and Beverage, Croom Helm, London, 1985, pp. 305 – 375.
[2] C.A.B. De Maria, L.C. Trugo, R.F.A. Moreira, C.C. Werneck, Composition of green coffee fractions and their contribution to the volatile profile formed during roasting, Food Chem. 50 (1994) 141 – 145.
[3] H.G. Maier, Les acides du cafe´, Cafe´ Cacao The´ 31 (1) (1987) 49 – 58.
[4] J. Poisson, Aspects chimiques et biologiques de la com-position du cafe´ vert, Proceedings of the 8th Interna-tional Scientific Colloquium on Coffee, Ivory Coast, International Scientific Association on Coffee, Paris, 1977, pp. 33 – 57.
[5] R. Silwar, C. Lu¨llmann, The determination of mono-and disaccharides in green Arabica mono-and Robusta coffees using high performance liquid chromatography, Cafe´ Cacao The´ 32 (4) (1988) 319 – 322.
[6] M. Weers, H. Balzer, A. Bradbury, O.G. Vitzhum, Anal-ysis of acids in coffee by capillary electrophoresis, Pro-ceedings of the 16th International Scientific Colloquium on Coffee, Japan, International Scientific Association on Coffee, Paris, 1995, pp. 218 – 219.
[7] R. Wo¨hrmann, B. Hojabr-Kalali, H.G. Maier, Volatile minor acids in coffee. I. Contents of green and roasted coffee, Dtsch. Lebensmittel-Rundsch. 93 (1997) 191 – 194. [8] M.N. Clifford, The nature of chlorogenic acids. Are they advantageous compounds in coffee? Proceedings of the 17th International Scientific Colloquium on Coffee, Kenya, International Scientific Association on Coffee, Paris, 1997, pp. 79 – 91.
[9] A. Crozier, T.W. Baumann, H. Ashihara, T. Suzuki, G.R. Waller, Pathways involved in the biosynthesis and catabolism of caffeine in Coffea and Camellia, Proceed-ings of the 17th International Scientific Colloquium on Coffee, Kenya, International Scientific Association on Coffee, Paris, 1997, pp. 106 – 113.
[10] E. Looser, T.W. Baumann, H. Wanner, The biosynthesis of caffeine in the coffee plant, Phytochemistry 13 (1974) 2515 – 2518.
[11] S.S. Mo¨sli Waldhauser, J.A. Kretschmar, T.W. Bau-mann,N-Methyltransferase activities in caffeine biosyn-thesis: biochemical characterization and time course during leaf development of Coffea arabica, Phytochem-istry 44 (1997) 853 – 859.
[12] G.R. Waller, T. Suzuki, Caffeine metabolism by Coffea arabica L. fruit, Proceedings of the 13th International Scientific Colloquium on Coffee, Colombia, Interna-tional Scientific Association on Coffee, Paris, 1989, pp. 351 – 361.
W.J.Rogers et al./Plant Science149 (1999) 115 – 123 123 [14] B. Guyot, E. Petnga, R. Lotode, J.C. Vincent, Analyse
qualitative d’un cafe´, Coffea canephora var Robusta en fonction de la maturite´. 2. Application de l’analyse statis-tique multidimensionnelle, Cafe´ Cacao The´ 32 (1988) 229 – 242.
[15] K.D. Golden, M.E. John, E.A. Kean, Beta-galactosidase fromCoffea arabicaand its role in fruit ripening, Phyto-chemistry 34 (1993) 355 – 360.
[16] R. Viani, Coffee, in: Ullmann’s Encyclopaedia of Indus-trial Chemistry, vol. A7, VCH Verlag, Weinheim, 1986, pp. 315 – 339.
[17] M. Sivetz, How acidity affects coffee flavour, Food Technol. 5 (1972) 70 – 77.
[18] A.J.T. Mendes, Cytological observations in Coffea. VI. Embryo and endosperm development in Coffea arabica L., Am. J. Bot. 28 (1941) 784 – 789.
[19] W.G. Houk, Endosperm and perisperm of coffee with notes on the morphology of the ovule and seed develop-ment, Am. J. Bot. 25 (1938) 56 – 60.
[20] J.F. Giorgini, E. Comoli, Effect of embryo and exoge-nous GA3 on endospermic endo-b-mannanase activity of Coffea arabica L during germination and early seedling growth, R. Bras. Fisiol. Veg. 8 (1) (1996) 43 – 49. [21] P.K. Ramaiah, N. Vasudeva, Observations on the
growth of coffee berries in South India, Turrialba 19 (4) (1969) 455 – 464.
[22] W.J. Rogers, G. Be´zard, A. Deshayes, I. Meyer, V. Pe´tiard, P. Marraccini, Biochemical and molecular char-acterization and expression of the 11S-type storage protein from Coffea arabica endosperm, Plant Physiol. Biochem. 37 (4) (1999) 261 – 272.
[23] T.M. Wormer, The growth of the coffee berry, Ann. Bot. 28 (1) (1964) 47 – 55.
[24] R. Locher, P. Bucheli, Comparison of soluble sugar degradation in soybean seed under simulated tropical storage conditions, Crop Sci. 38 (1998) 1229 – 1235. [25] R. Coste, Cafe´iers et cafe´s, Maisonneuve et Larose,
Paris, 1989.
[26] M.L. Wolfrom, R.A. Plunkett, M.L. Laver, Carbohy-drates of the coffee bean, Agric. Food Chem. 8 (1960) 58 – 65.
[27] P. Bucheli, I. Meyer, M. Pasquier, R. Locher, Determi-nation of soluble sugars by high performance anion exchange chromatography (HPAE) and pulsed electro-chemical detection (PED) in coffee beans upon acceler-ated storage, Plant Physiol. Biochem. Special Issue 10th FESPP Congress, Florence, Italy, Sept 9 – 13, 1996. L-12 p. 325.
[28] P. Bucheli, I. Meyer, A. Pittet, G. Vuataz, R. Viani, Industrial storage of green Robusta coffee under tropical conditions and its impact on raw material quality and ochratoxin A content, J. Agric. Food Chem. 46 (11) (1998) 4507 – 4511.
[29] B.G. Cobb, L.C. Hannah, Sugar utilization by develop-ing wild and shrunken-2 maize seeds, Plant Physiol. 80 (1986) 609 – 611.
[30] M. Shadaksharaswamy, G. Ramachandra, Changes in the oligosaccharides and the a-galactosidase content of coffee seeds during soaking and germination, Phyto-chemistry 7 (1968) 715 – 719.
[31] M.W. Loewus, F.A. Loewus, The C-5 hydrogen isotope-effect in 1L-myo-inositol 1-phosphate synthase as
evi-dence for the myo-inositol oxidation pathway, Carbohydr. Res. 82 (1980) 333 – 342.
[32] C.J. Smith, Carbohydrate chemistry, in: P.J. Lea, R.C. Leegood (Eds.), Plant Biochemistry and Molecular Biol-ogy, Wiley, New York, 1993, pp. 73 – 111.
[33] K.J. Balyaya, M.N. Clifford, Individual chlorogenic acids and caffeine contents in commercial grades of wet and dry processed Indian green Robusta coffee, J. Food Sci. Tech. 104 – 32 (1995) 108.
[34] R.J. Aerts, T.W. Baumann, Distribution and utilization of chlorogenic acid in Coffeaseedlings, J. Exp. Bot. 45 (273) (1994) 497 – 503.
[35] J.D. Bewley, M. Black, Seeds — Physiology of Develop-ment and Germination, Plenum, New York, 1994. [36] V. Leloup, A. Louvrier, R. Liardon, Degradation
mecha-nisms of chlorogenic acids during roasting, Proceedings of the 16th International Scientific Colloquium on fee, Japan, International Scientific Association on Cof-fee, Paris, 1995, pp. 192 – 198.