Cost-Effectiveness of Apixaban vs. Other New Oral Anticoagulants for the
Prevention of Stroke: An Analysis on Patients with Non-Valvular Atrial
Fibrillation in the Greek Healthcare...
Article in Clinical Drug Investigation · September 2015 DOI: 10.1007/s40261-015-0321-7
CITATIONS
0
5 authors, including:
Some of the authors of this publication are also working on these related projects:
Human resources in the health and social welfare sectorView project
Secondary cervical cancer prevention in GreeceView project Kostas Athanasakis
Εθνική Σχολή Δημόσιας Υγείας
115PUBLICATIONS 275CITATIONS
SEE PROFILE
Eleftheria Karampli
National School of Public Health, Athens, Greece (ESDY)
60PUBLICATIONS 98CITATIONS
SEE PROFILE
Aikaterini Bilitou
Novartis Global Services Centre
12PUBLICATIONS 120CITATIONS
SEE PROFILE
John Kyriopoulos
National School of Public Health (EDSY)
397PUBLICATIONS 1,290CITATIONS
SEE PROFILE
All content following this page was uploaded by Aikaterini Bilitou on 26 October 2015.
O R I G I N A L R E S E A R C H A R T I C L E
Cost-Effectiveness of Apixaban vs. Other New Oral
Anticoagulants for the Prevention of Stroke: An Analysis
on Patients with Non-Valvular Atrial Fibrillation in the Greek
Healthcare Setting
Kostas Athanasakis1•Eleftheria Karampli1•Dimitrios Tsounis2•
Aikaterini Bilitou2•John Kyriopoulos1
ÓSpringer International Publishing Switzerland 2015
Abstract
Background and Objectives Three new oral anticoagu-lants (NOACs) are currently approved for stroke preven-tion and systemic embolism in patients with non-valvular atrial fibrillation (NVAF). The objective of this analysis was to assess the cost effectiveness of apixaban against other NOACs for the prevention of stroke in patients with NVAF in Greece.
Methods A Markov model that evaluated clinical events, quality-adjusted life expectancy, and costs for patients treated with apixaban or other NOACs formed the basis of the analysis. Clinical events were modeled for a lifetime horizon, based on clinical efficacy data from an indirect comparison, using the ARISTOTLE, ROCKET-AF, and RE-LY clinical trials. Resource use associated with patient monitoring was elicited via a panel of experts (cardiolo-gists and internists). Cost calculations reflect the local clinical setting and followed a third-party payer perspective (Euros, discounted at 3 %).
Results Apixaban was projected to reduce the occurrence of clinical events and increase quality-adjusted life expectancy and incremental costs of treatment compared with other NOACs. Taking into account costs of medica-tions, patient monitoring, and management of events, the incremental cost-effectiveness ratios for apixaban 5 mg
twice daily vs. dabigatran 110 mg twice daily, dabigatran 150 mg twice daily, and rivaroxaban 20 mg once daily were estimated at €9907/quality-adjusted life-year (QALY), €13,727/QALY, and €6936/QALY gained, respectively. Extensive sensitivity analyses indicated that results were robust over a wide range of inputs.
Conclusions Based on the results of this analysis, apixa-ban can be a cost-effective alternative to other NOACs for the prevention of stroke in patients with NVAF in Greece.
Key Points
New oral anticoagulants (NOACs) are the preferred option to vitamin K antagonists in the majority of patients with non-valvular atrial fibrillation (NVAF). Apixaban is approved for the prevention of stroke and systemic embolism in adult patients with NVAF, with one or more risk factors.
Patients treated with apixaban were predicted to experience fewer major cardiovascular events and a lower number of cardiovascular-related deaths and hospitalizations compared with those taking the comparator NOACs.
The projected treatment cost of apixaban over a lifetime was higher than for other NOACs; however, it was mostly offset by the reduction in the
occurrence of clinical events
The incremental cost-effectiveness ratio of apixaban relative to other NOACs ranged between€5300 and €12,900 per quality-adjusted life-year gained, constituting apixaban as a cost-effective therapeutic option for the target population
& Eleftheria Karampli
1 Department of Health Economics, National School of Public
Health, Athens, Greece
1 Introduction
Atrial fibrillation (AF) is the most common sustained car-diac arrhythmia and a significant cause of cardiovascular morbidity and mortality [1]. The overall prevalence of AF is estimated at 0.9–1.2 %, rising to 3–5 % in people aged 65–80 years and to 10 % in those aged older than 80 years [1–4]. Population aging, improved survival rates for patients with cardiovascular diseases, and prolonged exposure to predisposing cardiovascular risk factors will contribute to a considerable increase in AF prevalence in the future [5]. AF is an independent risk factor for stroke [6]; furthermore, strokes related to AF are typically more severe and result in higher disability and mortality [7]. Apart from its clinical consequences, AF poses a significant financial burden on the healthcare system, driven mostly by hospitalizations [8–10]. Evidence from the Berlin Acute Stroke Study also suggests that acute hospitalization costs are higher for patients experiencing an AF-related stroke [11].
Prevention of thromboembolism is one of the key goals in AF management [12]. Until a few years back, vitamin K antagonists (VKAs) (e.g., warfarin, acenocoumarol) were recommended for patients with non-valvular AF (NVAF) and moderate-to-high risk of stroke, whereas antiplatelet therapy (aspirin plus clopidogrel or aspirin monotherapy) was con-sidered an option in patients at low risk when oral anticoag-ulation was contraindicated or refused [13, 14]. Although VKAs have been proven effective, in both randomized trials and large ‘‘real-world’’ studies [15], their use presents important limitations associated with their narrow therapeutic range, interactions with drugs and foods, high risk of adverse events including hemorrhages, and frequent monitoring requirements [14, 16]. These limitations, together with patient, physician, and healthcare system factors have been connected to the underuse of anticoagulation therapy and poor adherence to guidelines in clinical practice [17].
The need for novel anticoagulants that address these limitations has led to the development of two classes of new oral anticoagulants (NOACs): the oral direct thrombin inhi-bitors (e.g., dabigatran) and oral direct factor Xa inhiinhi-bitors (e.g., rivaroxaban, apixaban, edoxaban) [18]. In clinical tri-als, NOACs have demonstrated non-inferiority compared with VKAs and an improved safety profile regarding bleed-ing complications [18]. Furthermore, NOACs are used in fixed doses with no need for regular laboratory monitoring [12]. On this basis, the updated 2012 guidelines from the European Society of Cardiology recommend NOACs as preferable to VKAs in the majority of patients with NVAF, and suggest that antiplatelet therapy be limited to patients who refuse any form of oral anticoagulant [18].
Apixaban, has been approved by the European Medicines Agency for the prevention of stroke and systemic embolism in adult patients with NVAF, with one or more risk factors, such as having had a previous stroke, high blood pressure, diabetes mellitus, heart failure, or beingC75 years old [19]. In two randomized clinical trials for stroke prevention in AF [20, 21], apixaban was superior to warfarin and aspirin among those patients who were unsuitable or refused to receive warfarin [12].
In Greece, the prevalence of cardioembolic strokes is higher than in other European countries; in addition, a significant proportion of patients with a first-ever car-dioembolic stroke have experienced AF [22–24]. Future trends such as population aging and increasing prevalence of obesity and diabetes are expected to increase AF prevalence and the risk for cardiovascular disease [23], thus leading to higher demand for anticoagulation ther-apy. Currently, all three NOACs are included in the positive list of medicines reimbursed by social insurance. From the decision-makers’ perspective, it is important to evaluate both costs and outcomes related to the intro-duction of each of the NOACs in clinical practice. Cost-effectiveness analysis (CEA) assesses the (incremental) cost and (incremental) benefit (in terms of relevant health outcomes) of a health technology. CEA is increasingly used internationally to inform drug reimbursement deci-sions and development of guidelines on cost-effective prescribing [25]. A plethora of published CEAs have compared dabigatran, rivaroxaban, and apixaban to VKAs (mainly warfarin). All three NOACs have consistently presented favorable cost-effectiveness ratios as alterna-tives to VKAs for stroke prevention in AF [26,27], thus gaining ground as treatment options. Apart from the cost-effectiveness evidence, NOACs are expected to be widely used in the near future, following their rapid current uptake, as documented by recent studies [28,29]. In this light, cost-effectiveness analyses among the newer alter-natives are of value, to inform current and future deci-sions on the costs and outcomes for each of the available NOACs for the treatment of NVAF patients that follow anticoagulation. This evaluation of the impact of NOACs from a clinical, organizational, and cost perspective on the Greek healthcare system is necessary to inform decision making, especially under the current pressure of financial constraints.
2 Methods
2.1 Health Economics Model
A previously developed Markov model that simulated clinical events, quality-adjusted life years (QALYs), and costs for a cohort of patients with NVAF subsequent to treatment with apixaban or other NOACs formed the basis of the analysis [30] (Fig.1). The model was adapted to reflect resource use and cost data from a Greek healthcare perspective.
Patients in the Markov model are assumed to start in the NVAF health state (Fig.1). As the simulation progresses, patients either remain at the NVAF state or move to other health states based on specific transition probabilities, i.e., patients may experience disease episodes, such as ischemic stroke, systemic embolism, myocardial infarction (MI), or bleeding (intracranial hemorrhage (ICH), other major
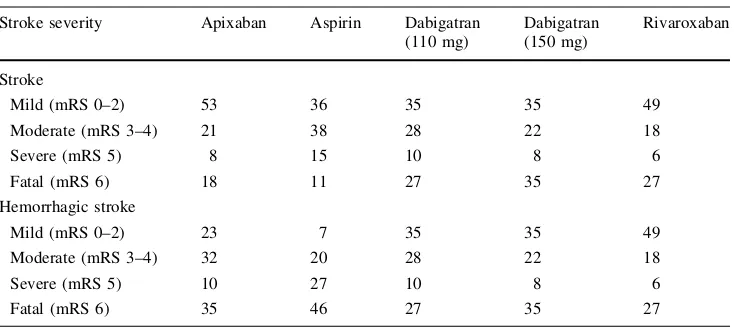
bleeds, clinically relevant nonmajor (CRNM) bleeds). Non-fatal strokes (ischemic as well as hemorrhagic) are classi-fied according to severity, based on the modiclassi-fied Rankin Scale (mRS) score into three categories, i.e., mild (mRS: 0–2), moderate (mRS: 3–4), and severe (mRS: 5). Patients in the model are subject to the risk of one recurrent stroke event, in which case they are transitioned to the most severe health state between primary and recurrent stroke and remain there until death. An additional health state in the model (‘‘NVAF with subsequent aspirin treatment’’) is incorporated to simulate the clinical pathway of patients that discontinue their first-line treatment and switch to aspirin. In this case, transition probabilities for subsequent events are those that correspond to the second-line therapy with aspirin.
Finally, death is an absorbing state in the model with patients simulated to die either due to a clinical event or general (‘‘background’’) mortality.
Fig. 1 Outline of the Markov model. Modified after Lip et al. [30].ACanticoagulant,ASAaspirin,CRNMclinically relevant nonmajor,HS
The model follows a lifetime horizon (running until all patients in the initial cohort are transferred to the ‘‘death’’ state) in 6-week cycle iterations.
2.2 Patient Characteristics
The baseline patient cohort of the model follows the characteristics of patients enrolled in the ARISTOTLE (Apixaban for Reduction in Stroke and Other Throm-boembolic Events in Atrial Fibrillation) clinical trial [20], i.e., patients in the analysis are VKA suitable, 64.7 % were male, with a mean age of 70 years and an average CHADS2
score of 2.1.
2.3 Efficacy Considerations for Comparators
The key efficacy measure for each comparator is the rate of occurrence of the clinical events included in the model. In the case of apixaban, clinical event rates were obtained from the ARISTOTLE clinical trial (apixaban 5 mg twice daily vs. warfarin, dose adjusted to maintain an interna-tional normalized ratio of 2.0–3.0) [20]. In the absence of head-to-head clinical trials between NOACs, comparative efficacy data for other NOACs are based on an indirect comparison as per the strategy and methodology proposed by Lip et al. [30]. Specifically, comparative efficacy data are produced by an indirect treatment comparison based on the data from the phase III clinical trials of each of the medication under evaluation, using warfarin as the com-mon comparator. Studies included in the indirect compar-ison were the ARISTOTLE [20] trial, the ROCKET–AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) [31] trial (rivaroxaban, 20 mg once daily vs warfarin, INR 2.0–3.0), and the RE-LY (Randomized Evaluation of Long-Term
Anticoagulation Therapy) trial (dabigatran, 110 mg twice daily vs dabigatran 150 mg twice daily vs. warfarin, INR 2.0–3.0) [32]. For each comparator, hazard ratios (HRs) were calculated that were subsequently applied to the clinical event rates observed in patients treated with apix-aban. Table1depicts clinical event rates for apixaban and the HRs calculated for comparator treatments. The indirect comparison strategy is described in detail in the publication by Lip et al. [30].
The rates of clinical events for patients in the ‘‘NVAF with subsequent aspirin treatment’’ health state (Table2) were based on a subgroup analysis of patients in the AVERROES (Apixaban Versus Acetylsalicylic Acid to Prevent Strokes) trial [21], who had discon-tinued VKA treatment and were subsequently treated with aspirin.
Because aging is an important risk factor for the occurrence of cardiovascular events, an adjustment should be made to avoid miscalculations during the projections in the model. Based on literature data, ischemic stroke, bleeding, and MI risks were adjusted by a factor of 1.46 [33], 1.97 [34], and 1.30 [35] per decade, respectively, to
Table 1 Clinical event rates for apixaban and comparators
Clinical event Apixaban (rate/100 PY) Hazard ratio vs. apixaban
Dabigatran (110 mg) Dabigatran (150 mg) Rivaroxaban
Stroke 0.981 1.198 0.823 0.980
Intracranial hemorrhage 0.330 0.733 1.020 1.731
Other major bleed 1.790 1.205 1.371 1.436
Clinically relevant nonmajor bleed 2.083 1.155 1.303 1.488
Myocardial infarction 0.530 1.474 1.456 0.935
Systemic embolism 0.090 1.000 1.000 1.000
Other CV hospitalization 10.460 1.000 1.000 1.000
Other death rate 3.0825 1.000 1.000 1.000
Other treatment discontinuation 13.177 1.452 1.505 1.184
Source: modified after Lip et al. [30]
CVcardiovascular,PYpatient-years
Table 2 Clinical event rates for patients who receive aspirin after
first-line treatment discontinuation
Clinical event Rate/100 PY
Stroke 3.453
Intracranial hemorrhage 0.322
Other major bleed 0.887
Clinically relevant nonmajor bleed 2.936
Myocardial infarction 1.110
Systemic embolism 0.400
Source: modified after Lip et al. [30]
incorporate the factor of aging. Risk of stroke recurrence was derived from a subgroup analysis of patients with AF from the South London Stroke Registry [36]. Severity distributions for stroke and hemorrhagic stroke, respec-tively, by treatment were used as previously published [30] (Table3).
Data regarding fatal events due to other ICH and other major bleeds were derived from pooling the events observed in the AVERROES and the ARISTOTLE trials, calculating a case fatality rate of 13 % for other ICH and 2 % for other major bleeds in the case of apixaban and aspirin. Results were applied across all treatment comparators.
2.4 Cost Calculations
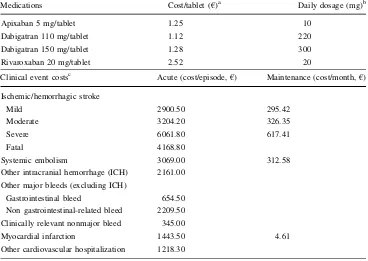
Cost calculations in the analysis follow a third-party payer perspective, include direct medical costs (only), and are reported in year 2013 values. Given the time horizon of the analysis, discounting was deemed necessary; therefore costs and outcomes were discounted at a 3 % rate per annum. Prices for medications were sourced from the most recent official price list available at the time of model adaptation [37]. Patients who experience an event in the model are assigned a cost per acute care episode as well as a per month long-term maintenance cost. Acute care epi-sode costs were sourced from the Greek Diagnosis-related group (DRG) price list and the study by Gioldasis et al. [38] (costs updated to year 2013 values). Maintenance costs were calculated based on the proportion of the acute to total per year costs, as reported previously [39], and the allocation of (the remaining) maintenance costs on a monthly basis. Resource use associated with patient mon-itoring was elicited via a panel of experts (cardiologists and internists). Unit costs for medications and events are reported in Table4.
2.5 Utilities
Utility inputs were derived from a UK-based utility catalog [40] and presented in Table5. The calculation method for utility estimations subsequent to events is based on assigning a baseline utility to all patients with AF and subtracting a disutility decrement, specific to the event that is modeled for a particular duration of time; utility decre-ment durations were used as previously published [30].
2.6 Sensitivity Analyses
To test the robustness of findings, probabilistic sensitivity analysis (PSA) was conducted by simultaneously drawing random values from a series of variables, based on a set of pre-specified types of distributions for each variable and calculating incremental cost- effectiveness ratios (ICERs). Beta distributions were used for utilities and for the prob-abilities of clinical events. Dirichlet distributions were fit-ted for the distributions of patients among a number of different occurrences, such as by stroke severity. The characteristics of the gamma distribution (non-negative results, skewness) rendered it suitable for the simulation of cost inputs [41].
In addition to the PSA, a series of one-way deterministic sensitivity analyses were performed by varying a number of variables by ?10/-10 % of their original (baseline) values to estimate their effect to the baseline ICER calculations.
3 Results
Based on simulation of a cohort of 1000 patients with AF in a lifetime horizon, apixaban was predicted to reduce the occurrence of major CV events (mainly ischemic and
Table 3 Distribution (%) of
stroke severity and case fatality rate (mRS 6) by treatment
Stroke severity Apixaban Aspirin Dabigatran
(110 mg)
Source: Modified after Lip et al. [30]
hemorrhagic strokes and cases of systemic embolism) as well as CV deaths, compared with rivaroxaban or dabiga-tran (regardless of dosage) (Table6).
Apixaban was accompanied by higher incremental costs of treatment as well as higher gains in quality-adjusted life
expectancy, compared with alternatives, thus leading to favorable ICERs across all comparisons. Compared with the least effective alternative (dabigatran 110 mg), dabi-gatran 150 mg (next best alternative) presented an ICER of €3464.1 per QALY gained whereas the ICERs for rivaroxaban vs. dabigatran 150 mg and apixaban vs. dabigatran 150 mg were €24,576.3 and €13,781.6 per QALY gained, respectively. In this light, apixaban and dabigatran 150 mg presented extended dominance [42] over rivaroxaban (Fig.2).
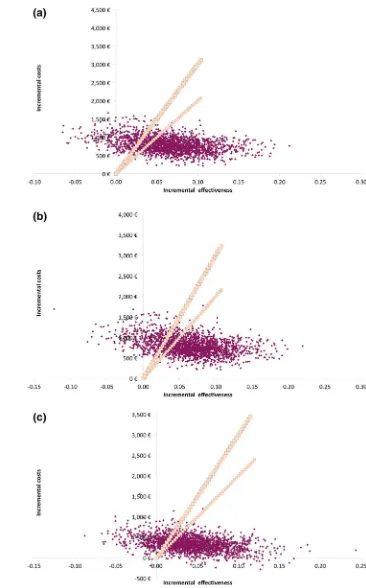
3.1 Probabilistic Sensitivity Analysis
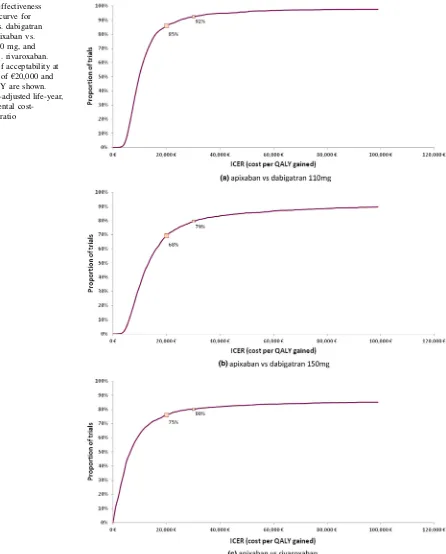
Figure3depicts the results of the PSA. Cost-effectiveness planes for apixaban vs. all other NOACs show a con-centration of points (i.e., results from PSA iterations) in the North-East quadrant and below the €30,000/QALY implicit threshold, suggesting a more effective and more costly outcome for apixaban compared with all treat-ments. Using a threshold of €30,000 cost per QALY gained, apixaban was a cost-effective treatment choice compared with dabigatran 110 mg, dabigatran 150 mg, and rivaroxaban in 92, 79 and 80 % of the iterations, respectively (Fig.4).
Table 5 Utility scores and decrements
Health state Utility score
Non-valvular atrial fibrillation (baseline) 0.7270
Stroke
Clinical events Utility decrement
Other intracranial hemorrhage (excluding hemorrhagic stroke)
0.1511
Other major bleed 0.1511
Clinically relevant nonmajor bleed 0.0582
Other cardiovascular hospitalization 0.1276
Source: modified after Lip et al. [30]
Table 4 Medication and
clinical event costs Medications Cost/tablet (
€)a Daily dosage (mg)b
Apixaban 5 mg/tablet 1.25 10
Dabigatran 110 mg/tablet 1.12 220
Dabigatran 150 mg/tablet 1.28 300
Rivaroxaban 20 mg/tablet 2.52 20
Clinical event costsc Acute (cost/episode,€) Maintenance (cost/month,€)
Ischemic/hemorrhagic stroke
Mild 2900.50 295.42
Moderate 3204.20 326.35
Severe 6061.80 617.41
Fatal 4168.80
Systemic embolism 3069.00 312.58
Other intracranial hemorrhage (ICH) 2161.00
Other major bleeds (excluding ICH)
Gastrointestinal bleed 654.50
Non gastrointestinal-related bleed 2209.50
Clinically relevant nonmajor bleed 345.00
Myocardial infarction 1443.50 4.61
Other cardiovascular hospitalization 1218.30
ICHintracranial hemorrage
a Cost of drugs was based on latest available price bulletin (February 2014) at the time of model adaptation
b The daily dosage was based on each approved summary of product characteristic for apixaban,
dabi-gatran, and rivaroxaban, respectively
3.2 One-Way Sensitivity Analysis
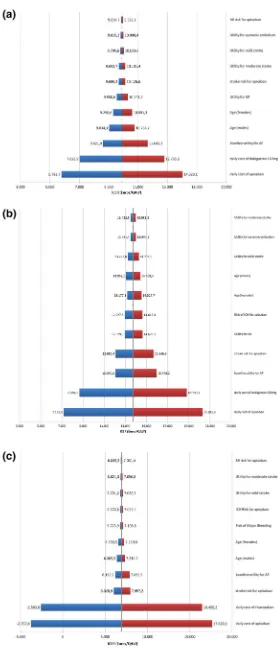
The results of the one-way sensitivity analyses of the ICERs for apixaban vs. comparators are presented in Fig.5. Variables with the highest impact on the baseline
ICERs in all three cases were the daily cost of apixaban as well as the daily cost of the comparator. Nevertheless, the results remained favorable for apixaban, on an implicit threshold of €30,000/QALY. Variables with significant impact, although at a quite lesser extent than daily costs, were the baseline utility for atrial fibrilation and MI, mean age, as well as the risks for major events (mainly stroke, myocardial infarction, and intracranial haemorrhage).
4 Discussion
The present analysis assessed the incremental costs and benefits of apixaban compared with other NOACs as a first-line treatment in the prevention of stroke for patients with AF suitable for treatment with VKAs in Greece. The analysis was performed from a third-party payer perspec-tive (social insurance fund).
According to CEA results, in a cohort of 1000 patients simulated over a lifetime, apixaban was found to be a cost-effective therapeutic option compared with other NOACs at an implicit threshold of €30,000/QALY with an ICER
Fig. 2 Cost-effectiveness plane: costs and QALYs per person for
each comparator. The bold line represents the efficiency frontier. Rivaroxaban is extendedly dominated by dabigatran 150 mg and apixaban.QALYsquality-adjusted life-years
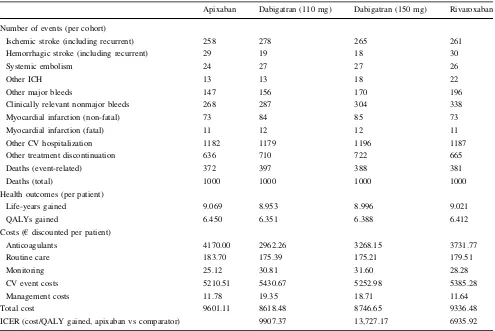
Table 6 Occurrence of cardiovascular events, life-years gained (quality adjusted and un-adjusted), and costs by treatment (lifetime horizon)
Apixaban Dabigatran (110 mg) Dabigatran (150 mg) Rivaroxaban
Number of events (per cohort)
Ischemic stroke (including recurrent) 258 278 265 261
Hemorrhagic stroke (including recurrent) 29 19 18 30
Systemic embolism 24 27 27 26
Other ICH 13 13 18 22
Other major bleeds 147 156 170 196
Clinically relevant nonmajor bleeds 268 287 304 338
Myocardial infarction (non-fatal) 73 84 85 73
Myocardial infarction (fatal) 11 12 12 11
Other CV hospitalization 1182 1179 1196 1187
Other treatment discontinuation 636 710 722 665
Deaths (event-related) 372 397 388 381
Deaths (total) 1000 1000 1000 1000
Health outcomes (per patient)
Life-years gained 9.069 8.953 8.996 9.021
QALYs gained 6.450 6.351 6.388 6.412
Costs (€discounted per patient)
Anticoagulants 4170.00 2962.26 3268.15 3731.77
Routine care 183.70 175.39 175.21 179.51
Monitoring 25.12 30.81 31.60 28.28
CV event costs 5210.51 5430.67 5252.98 5385.28
Management costs 11.78 19.35 18.71 11.64
Total cost 9601.11 8618.48 8746.65 9336.48
ICER (cost/QALY gained, apixaban vs comparator) 9907.37 13,727.17 6935.92
relative to dabigatran 110 mg, dabigatran 150 mg, and rivaroxaban over-projected lifetime of€9907,€13,727, and €6936 per QALY gained respectively. Overall, patients treated with apixaban were predicted to experience fewer major CV events as well as a lower number of CV-related
deaths and hospitalizations compared with those taking dabigatran and rivaroxaban. Although the treatment cost of apixaban compared with dabigatran 110 mg, dabigatran 150 mg, and rivaroxaban over projected lifetime was higher, it was off-set to a great extent by the reduction in
Fig. 3 Probabilistic sensitivity
analysis scatterplots for
aapixaban vs. dabigatran 110 mg,bapixaban vs. dabigatran 150 mg, and
capixaban vs. rivaroxaban.
Input linesrepresent the
the occurrence of clinical events, leading to modest incremental costs (€983,€854, and€265 respectively).
Apixaban as well as other NOACs have demonstrated acceptable ICERs when independently compared with warfarin and/or aspirin in various settings [43–45]. How-ever, cost-effectiveness comparisons between the three
NOACs are rather scarce in the literature, probably owing to the absence of head-to-head clinical trials and, thus, the need to use indirect comparisons for the production of relative efficacy data.
The strengths of the present analysis include the use of data from completed phase III randomized trials, the
Fig. 4 Cost-effectiveness
acceptability curve for
aapixaban vs. dabigatran 110 mg,bapixaban vs. dabigatran 150 mg, and
capixaban vs. rivaroxaban. Percentages of acceptability at the threshold of€20,000 and
€30,000/QALY are shown.
QALYquality-adjusted life-year,
Fig. 5 Tornado diagrams of the one-way sensitivity analyses of the ICERs foraapixaban vs. dabigatran 110 mg,bapixaban vs. dabigatran 150 mg, and
capixaban vs. rivaroxaban.
QALYquality-adjusted life-year,
combination of data on efficacy, major bleeding and tol-erability profile for the assessment of drugs in the analysis, detailed modeling of mortality for patients with AF, and the lifetime horizon of the analysis. However, this study has some limitations, as well, that should be acknowledged. First, the use of indirect treatment comparison of available NOACs in the absence of head-to-head trials, which con-stitutes a source of uncertainty; the analysis was based on a comparison of the pivotal trials ARISTOTLE, ROCKET-AF, and RE-LY using warfarin as the common comparator as per the strategy and methodology described in Lip et al. [30]. The reason for the use of this comparison vs. a more broad meta-analysis that could potentially include a larger sample of studies is based on the observation by Mitchell et al. [46] that there exist significant between-study dif-ferences (in the total population of studies available) that could introduce additional statistical heterogeneity into the network meta analysis (NMA) for a particular outcome in the case of a broad meta-analysis. Nevertheless, the results generated from this analysis are highly consistent with earlier indirect comparisons [47–49]; however, as more data become available, an analysis in a systematic manner could be the scope of a future manuscript. Another limi-tation is the third-party payer perspective from which the analysis is undertaken and does not include costs to society, mainly the productivity losses as a result of the disease and the costs of informal care. The latter constitute an impor-tant cost variable, especially for patients whose daily activities are severely impaired by the CV events associ-ated with AF. Indicatively, informal care costs can account for up to 21 or 25 % of the total cost for patients that have experienced a cerebrovascular or coronary heart disease episode, respectively [50, 51]. Inclusion of such costs typically favors the treatment that averts the most clinical events, compared with alternatives.
5 Conclusions
A significant advantage attributed to NOACs is that they do not require routine INR monitoring. This is especially important in the Greek healthcare setting, where antithrombotic therapy in eligible patients remains subop-timal [23,52,53]. Although all NOACs have successfully reduced the risk of thromboembolism, treatment with apixaban is predicted to lead to reduced morbidity and mortality, compared with other NOACs. Furthermore, the ICER of apixaban relative to other NOACs ranged between €5300 and €12,900 per QALY gained, a ratio well under commonly accepted thresholds. Apixaban therefore could be considered a cost-effective therapeutic option for the prevention of stroke in AF patients with one or more risk
factors and suitable for treatment with VKAs, from a third-party payer perspective.
Acknowledgments The authors would like to thank Elena
Armelidou and Periklis Giovas from Pfizer Hellas for their in-depth review of the draft manuscript, and Ms. Daphne Arzoumanidou for her assistance in the early stages of the study.
Author contributions KA and AB participated in the design and
coordination of the study and KA conducted the analyses and inter-preted the results. KA and EK wrote the manuscript. AB and DT revised the manuscript for important intellectual content. JK super-vised the study and made critical comments to the final draft of the paper. All authors read and approved the final manuscript.
Compliance with Ethical Standards
The study was sponsored by Pfizer and Bristol Myers Squibb. KA, EK, and JK are employees of the National School of Public Health who were paid consultants to Pfizer in connection with conducting this analysis and the development of this manuscript. AB is full-time employee at Pfizer Hellas. At the time of manuscript submission, DT was a full-time employee at Pfizer Hellas. Nothing contained in this paper is intended to guarantee the appropriateness of any medical treatment or to be used for therapeutic purposes or as a substitute for a health professional’s advice.
References
1. Kirchhof P, Bax J, Blomstrom-Lundquist C, et al. Early and comprehensive management of atrial fibrillation: executive summary of the proceedings from the 2nd AFNET-EHRA con-sensus conference ‘research perspectives in AF’. Eur Heart J. 2009;30:2969–80.
2. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: the anticoagulation and risk
factors in atrial fibrillation (atria) study. JAMA.
2001;285:2370–5.
3. Stewart S, Hart CL, Hole DJ, et al. Population prevalence, inci-dence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart. 2001;86:516–21.
4. Heeringa J, van der Kuip DAM, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27(8):949–53.
5. Stewart S, MacIntyre K, MacLeod MMC, et al. Trends in hospital activity, morbidity and case fatality related to atrial fibrillation in Scotland, 1986–1996. Eur Heart J. 2001;22:693–701.
6. Sanoski CA. Clinical, economic, and quality of life impact of atrial fibrillation. J Manag Care Pharm. 2009;15:S4–9.
7. Savelieva I, Camm J. Update on atrial fibrillation: part I. Clin Cardiol. 2008;31:55–62.
8. Stewart S, Murphy N, Walker A, et al. Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart. 2004;90:286–92.
9. Coyne KS, Paramore C, Grandy S, et al. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health. 2006;9:348–56.
11. Bru¨ggenju¨rgen B, Rossnagel K, Roll S, et al. The impact of atrial fibrillation on the cost of stroke: the Berlin Acute Stroke study. Value Health. 2007;10:137–43.
12. Potpara T, Polovina M, Licina M, et al. Novel oral anticoagulants for stroke prevention in atrial fibrillation: focus on apixaban. Adv Ther. 2012;29:491–507.
13. Fuster V, Ryde´n LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to revise the 2001 guidelines for the man-agement of patients with atrial fibrillation): developed in col-laboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–354. 14. Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the
man-agement of atrial fibrillation: the Task Force for the Manman-agement of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–429.
15. Go AS. Efficacy of anticoagulation for stroke prevention and risk stratification in atrial fibrillation: translating trials into clinical practice. Am J Manag Care. 2004;10:S58–65.
16. Maan A, Padmanabhan R, Shaikh AY, et al. Newer anticoagu-lants in cardiovascular disease: a systematic review of the liter-ature. Cardiol Rev. 2012;20:209–21.
17. Bushnell CD, Matchar DB. Pharmacoeconomics of atrial fibril-lation and stroke prevention. Am J Manag Care. 2004;10:S66–71. 18. Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrilla-tion. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–47.
19. European Medicines Agency. Summary of opinion (post autho-risation): Eliquis (apixaban). EMA. 2012. Available from:http:// www.ema.europa.eu/docs/en_GB/document_library/Summary_ of_opinion/human/002148/WC500132869.pdf. Accessed 6 Sep 2013.
20. Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92.
21. Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–17. 22. Vemmos KN, Bots ML, Tsibouris PK, et al. Stroke incidence and
case fatality in southern Greece: the Arcadia stroke registry. Stroke. 1999;30:363–70.
23. Vemmos KN, Takis CE, Georgilis K, et al. The Athens stroke registry: results of a five-year hospital-based study. Cerebrovasc Dis. 2000;10:133–41.
24. Korantzopoulos P, Andrikopoulos G, Vemmos K, et al. Atrial fibrillation and thromboembolic risk in Greece. Hellenic J Car-diol. 2012;53:48–54.
25. OECD. Pharmaceutical pricing policies in a global market. OECD Health Policy Studies. Paris: OECD Publishing; 2008. 26. Singh SM, Wijeysundera HC. Cost-Effectiveness of novel oral
anticoagulants for stroke prevention in non-valvular atrial fibril-lation. Curr Cardiol Rep. 2015;17:618.
27. Ferreira J, Mirco A. Systematic review of cost-effectiveness analyses of novel oral anticoagulants for stroke prevention in atrial fibrillation. Rev Port Cardiol. 2015;34:179–91.
28. Xu Y, Holbrook AM, Simpson CS, et al. Prescribing patterns of novel oral anticoagulants following regulatory approval for atrial fibrillation in Ontario, Canada: a population-based descriptive analysis. CMAJ Open. 2013;1:E115–9.
29. Desai NR, Krumme AA, Schneeweiss S, et al. Patterns of initi-ation of oral anticoagulants in patients with atrial fibrilliniti-ation: quality and cost implications. Am J Med. 2014;127:1075–82.
30. Lip GYH, Kongnakorn T, Phatak H, et al. Cost-effectiveness of apixaban versus other new oral anticoagulants for stroke pre-vention in atrial fibrillation. Clin Ther. 2014;36(192–210):e20. 31. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus
warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91.
32. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51.
33. [No authors listed]. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation: analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–57.
34. Ariesen MJ, Claus SP, Rinkel GJ, et al. Risk factors for intrac-erebral hemorrhage in the general population: a systematic review. Stroke. 2003;34:2060–5.
35. Expert Panel on Detection. Evaluation, and treatment of high blood cholesterol in adults. executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285:2486–97.
36. Mohan KM, Crichton SL, Grieve AP, et al. Frequency and pre-dictors for the risk of stroke recurrence up to 10 years after stroke: the South London Stroke Register. J Neurol Neurosurg Psychiatry. 2009;80:1012–8.
37. Ministry of Health. February 2014 price bulletin. Ministry of Health. 2014. Available from: http://www.moh.gov.gr/articles/ times-farmakwn/deltia-timwn. Accessed 4 Jul 2014.
38. Gioldasis G, Talelli P, Chroni E, et al. In-hospital direct cost of acute ischemic and hemorrhagic stroke in Greece. Acta Neurol Scand. 2008;118:268–74.
39. Geitona M, Papadimitriou A, Kyriopoulos J. Economic evalua-tion of prevenevalua-tion of cerebral strokes via the administraevalua-tion of dipyridamol and aspirin twice daily. Neurologia. 1999;8:250–7. 40. Sullivan PW, Slejko JF, Sculpher MJ, et al. Catalogue of EQ-5D
scores for the United Kingdom. Med Decis Making.
2011;31:800–4.
41. Briggs AH, Ades AE, Price MJ. Probabilistic sensitivity analysis for decision trees with multiple branches: use of the Dirichlet distribution in a Bayesian framework. Med Decis Making. 2003;23:341–50.
42. Cantor SB. Cost-effectiveness analysis, extended dominance, and
ethics: a quantitative assessment. Med Dec Making.
1994;14:259–65.
43. Limone BL, Baker WL, Kluger J, et al. Novel anticoagulants for stroke prevention in atrial fibrillation: a systematic review of cost-effectiveness models. PLoS One. 2013;8:e62183.
44. Kansal AR, Zheng Y, Pokora T, et al. Cost-effectiveness of new oral anticoagulants in the prevention of stroke in patients with atrial fibrillation. Best Pract Res Clin Haematol. 2013;26:225–37. 45. Kongnakorn T, Lanitis T, Annemans L, et al. Cost effectiveness of apixaban versus aspirin for stroke prevention in patients with non-valvular atrial fibrillation in Belgium. Clin Drug Investig. 2014;34:709–21.
46. Mitchell SA, Simon TA, Raza S, et al. The efficacy and safety of oral anticoagulants in warfarin-suitable patients with nonvalvular atrial fibrillation: systematic review and meta-analysis. Clin Appl Thromb Hemost. 2013;19(6):619–31.
47. Baker WL, Phung OJ. Systematic review and adjusted indirect comparison meta-analysis of oral anticoagulants in atrial fibril-lation. Circ Cardiovasc Qual Outcomes. 2012;5:711–9. 48. Lip GY, LarsenTB Skjoth F, et al. Indirect comparisons of new
49. Mantha S, Ansell J. An indirect comparison of dabigatran, rivaroxaban and apixaban for atrial fibrillation. Thromb Haemost. 2012;108:476–84.
50. Luengo-Fernandez R, Leal J, Gray A, et al. Cost of cardiovas-cular diseases in the United Kingdom. Heart. 2006;92:1384–9. 51. Liu JL, Maniadakis N, Gray A, et al. The economic burden of
coronary heart disease in the UK. Heart. 2002;88:597–603.
52. Andrikopoulos G. Comments on the 2012 update of the ESC guidelines for the management of atrial fibrillation: what is new and what is important for the clinician? Hellenic J Cardiol. 2012;53:407–11.
53. Goudevenos J, Pipilis A, Vardas P. Novel oral anticoagulants in atrial fibrillation: will the benefit outweigh the cost? Hellenic J Cardiol. 2012;53:137–41.
![Fig. 1 Outline of the Markov model. Modified after Lip et al. [hemorrhagic stroke,30]. AC anticoagulant, ASA aspirin, CRNM clinically relevant nonmajor, HS ICH intracranial hemorrhage, IS ischemic stroke, NVAF non-valvular atrial fibrillation](https://thumb-ap.123doks.com/thumbv2/123dok/3964059.1907479/4.595.56.543.293.684/modied-hemorrhagic-anticoagulant-clinically-relevant-intracranial-hemorrhage-brillation.webp)