A computational model of membrane lipid electronic
properties in relation to neural signaling
Harry L. Price
a,*, Ron Wallace
b,1aDepartment of Chemistry,Uni
6ersity of Central Florida,Orlando,FL32816-2366,USA bDepartment of Sociology and Anthropology,Uni
6ersity of Central Florida,Orlando,FL32816-1360,USA Accepted 24 September 2000
Abstract
We present a computational model of a transiently-organized neural membrane molecular system with possible information-processing capacity. The model examines field-induced dipole and quadrupole moments and polarizabil-ity in monomeric, dimeric, and trimeric ethenes. Polarization of the ethenes is strongly indicated. This result is interpreted as a significant electronic feature of a molecular computing system based on organization of membrane lipids into a transient (10−4s) crystalline state due to lipid-protein hydrophobic mismatch at the
membrane-ion-channel interface. Predictive implications of the model’s electronic features are briefly discussed. © 2001 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Hydrophobic mismatch; Neural membrane; Lipids; Computational modeling; Molecular computing; Spectroscopy www.elsevier.com/locate/biosystems
1. Introduction
Polyunsaturated fatty acids (PUFAs) consist of a phosphorus head group joined to two hydrocar-bon chains containing at least one ethene hydrocar-bond (CC). These molecules are present in all biologi-cal membranes including that of the neuron. The degree of unsaturation varies markedly but the most common ratios are 18:1, 18:2, 18:3 and 20:4, where the first number designates the chain length and the second indicates the number of ethenic
bonds (Gennis, 1989). Since nearly all the ethenes are cis, there is a kink in the molecule, which prevents orderly packing of bilayer diacyls. This feature, in combination with the lack of a func-tional explanation for lipid hetereogeneity (\100
species in any membrane) has suggested that biomembrane molecular structure is stochastic. The Singer – Nicolson ‘fluid-mosaic model’ of the membrane (Singer and Nicolson, 1972) construes the structure as a homogeneous 2-D liquid in which the constituent molecules have considerable lateral mobility. However, more recent studies suggest that biomembranes are liquid crystals in which protein-associated lipid microdomains of 108– 1010 molecules with lateral lengths of 10 –
300 A, and typical associative lifetimes of 10−4 s
* Corresponding author. Fax: +407-823-2252.
E-mail addresses: [email protected] (H.L. Price), [email protected] (R. Wallace).
1Fax: +407-823-3026.
play important regulatory roles in membrane per-meability to Na+ and other ionic species (Alfsen,
1989; Mouritsen and Jørgensen, 1992; Jørgensen and Mouritsen, 1995; Marsh, 1995; Simons and Ikonen, 1997). This unordered-to-ordered phase transition may also affect formation of exocytotic vesicles as well as voltage- and ligand-gated chan-nel activity, all of which are important in nerve cell communication. Evidence from myocyte and neural membranes suggests that these microdo-main functions may be modulated by PUFAs in a dose-dependent fashion (Vreugdenhil et al., 1996). Increased membrane omega-3 PUFA concentra-tion in guinea pig cardiac myocytes reduces acconcentra-tion potential duration while prolonging its duration in rats. PUFA-concentration-dependent shifts to more hyperpolarized potentials have been demon-strated in vitro for rat hippocampal CA1 neurons. These molecular data appear consistent with neurobiological evidence for axonal and dendritic conduction block documented in a wide variety of species (leech, crayfish, rabbit, rat). In this well-known but poorly understood phenomenon, a neural impulse, once initiated, is not inevitably propagated in a cable-like manner to the presy-naptic terminal (Krnjevic´ and Miledi, 1959; Par-nas, 1972; Swadlow and Waxman, 1976). The evidence suggests that subneural (molecular) sys-tems may ‘decide’ at several stages of impulse propagation, whether or not the impulse should be ‘allowed’ to continue. Put somewhat differ-ently, the data suggest that the neuron is not a single switch, as postulated originally in the Hodgkin – Huxley model and more recently in the McCulloch – Pitts (MP) heuristic utilized in artifi-cial intelligence (McCulloch and Pitts, 1943; Hodgkin and Huxley, 1952) but may in fact be ‘a series of switches’ (Scott, 1995). Based on these findings, a number of possible mechanisms by which membrane electronic properties could mod-ulate microdomain functions, and ultimately regu-late neuron electrical activity, have been examined through simulation studies and analyses of artifi-cial membranes (Kinnunen and Virtanen, 1986; Wallace et al., 1998; Wallace and Price, 1999).
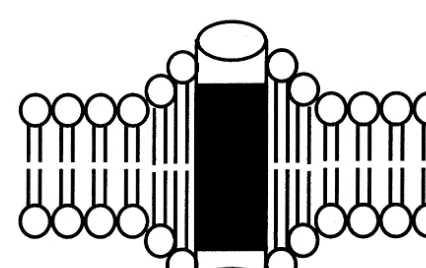
In this article, we present a computational model of molecular computing in the neural mem-brane. The most significant feature of the model is
a two-stage potential energy search of protein-as-sociated membrane phospholipids. The first stage of the search is initiated by ligand or voltage gating of the ion-channel protein, a perturbation which generates protein conformational change from a-helix to random coil, in turn producing lipid-protein hydrophobic mismatch (Mouritsen and Bloom, 1993; Sperotto and Mouritsen, 1993; Mouritsen and Jørgensen, 1994; Lehtonen et al., 1996; Lehtonen and Kinnunen, 1997; Mouritsen and Jørgensen, 1997; Mouritsen, 1998; Sabra and Mouritsen, 1998; Killian, 1998). As a consequence of the mismatch, membrane lipids self-assemble such that lipids with hydrophobic lengths most closely approximating that of the protein become more abundant in the protein’s vicinity (Fig. 1). The effect of the self-assembly (in combination with the lipid-condensing effect of cholesterol) is the alignment of lipid ethenes in the plane of the membrane bilayer (Hyslop et al., 1990; Raffy and Teissie´, 1999). Subsequent permeant ion move-ment through anion and cation specific channels generates a field orthogonal to ion movement, which polarizes the aligned ethenes. Ethene reor-ganization due to dipole – dipole interactions con-stitutes the second stage of the potential energy search. When the difference Ec−Em=dMIN
(where, Em is the potential energy of the lipid
microdomain, Ec is the potential energy of the
ion-channel protein in the random coil conforma-tion, and dMIN is the minimal energy difference
required to close the channel pore) the system is at the threshold value. Following cessation of ion movement and concomitant neutralization of membrane ethenes, the channel protein relaxes to the a-helix conformation which restores hydro-phobic matching (Leuchtag, 1994). In this man-ner, microdomain dynamics regulate the duration of channel opening, a model consistent with in vitro experimental manipulation of ion channel activity by variations in concentration of unsatu-rated membrane lipids (Vreugdenhil et al., 1996). We describe the above process in terms of biomolecular computing (Wallace and Price, 1999), i.e. as a mechanism by which the frequency coding of classical neural networks is regulated by molecular minimum potential energy searches (Conrad, 1992). Finally, we propose experiments involving Raman and fluorescence resonance en-ergy transfer (FRET) spectroscopy as a means of investigating the above features in a liposomal system.
2. Computational modeling of membrane ethene system stability and polarization
2.1. Rationale
Our primary objective was to construct a com-putational model of ethene polarization. We in-vestigated dipole and quadrupole moments, and polarizability in the adjacent double bonds of a monomer, dimer, and trimer. The electrical per-manent moments (dipole, quadrupole) represent derivatives of the energy E with respect to the applied electric field vector Ea. Specifically, dipole moment is given as −(dE/dEa) and quadrupole
moment is given as 1 2(d
2E/dEa). Similarly,
polariz-ability is given as−(d2
E/dEa2
) and varies with the oscillation of an applied electric field (Dykstra, 1997). This latter feature is consistent with the complex, interacting fields (with time-dependent variation in strength) that are encountered in actual membranes. The use of a polymer model stabilized by methylene linkages was justified by computational pragmatics. When ab initio meth-ods are applied to disconnected model compo-nents, radical changes in system geometry
frequently result. We further wished to demon-strate that systematically increasing the number of aligned ethenes would produce a dramatic in-crease in the observed polarization.
2.2. Method
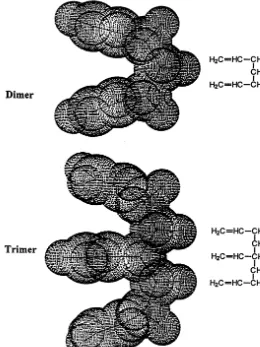
Calculations were performed on an ethene monomer, dimer, and trimer using Hyperchem v. 4.5 (Hypercube Inc., 1995) and Gaussian 95 W (Frisch et al., 1995). The latter two components were stabilized by methylene linkages (Fig. 2). The 4-31G* basis set was used to optimize all structures. Dipole and quadrupole moments and polarizability values were then calculated on the optimized system using the same basis set.
2.3. Results
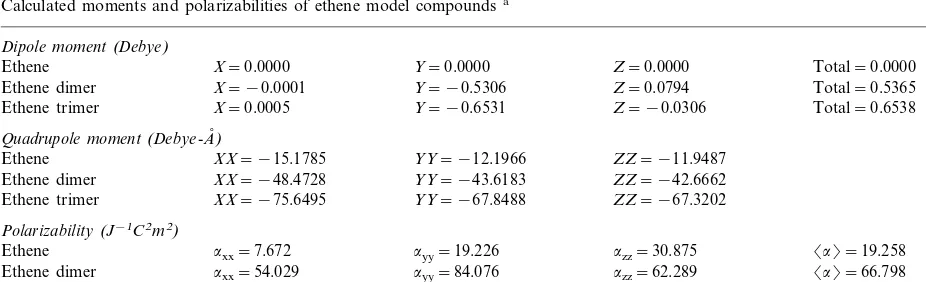
Sensitivity of the aligned ethenes to an applied electric field was indicated by our analysis of dipole and quadrupole moments, and polarizabil-ity (Table 1). Proceeding from the ethene monomer to the trimer, total dipole moment in-creases from 0.0000 to 0.6538 Debye. Examina-tion of the quadrupole moments reveals a consistent 5-fold increase in the axial moments (i.e. xx, yy, zz) from monomer to trimer. These cumulative increases suggest a progressive mobi-lization of p electrons. Pronounced fluctuation of the p electronic structure in the applied field is indicated by component increases in polarizability from monomer to trimer (axx from 7.672 to 93.644, ayy from 19.226 to 125.284, azz from 30.875 to 91.345 J−1 C2 m2). These increases in
turn produced an increase in mean polarizability a from 19.258 to 103.424 [where a=(axx+ ayy+azz/3)]. Together these results indicate an overall increase in the stability of the aligned ethenes, as well as a pronounced increase in the mobilized electron polarizability.
3. Ethene polarization and neuromolecular computing
Fig. 2. Structures of ethene model compounds used in ab initio calculations of dipole and quadrupole moments, and polarizability.
of a biomolecular computing system (Conrad, 1984, 1989, 1992; Wallace, 1996; Wallace and Price, 1999). Although these systems are believed to operate on quantum-mechanical (QM) princi-ples, it may actually be somewhat misleading (al-though technically accurate) to refer to them as ‘quantum computers’. The latter term, coined by Feynman (1982) originally referred to a device, which utilized quantum mechanics to simulate a QM system. More exactly, Feynman argued that the feature of linear superposition, in which the values of an observable (e.g. spin, position, en-ergy) exist simultaneously in many dimensional (Hilbert) space, could be the basis for massively parallel computations. Such parallelism, he
ar-gued, would make it possible to solve the ordinar-ily intractable (exponential) problem (i.e. of the form HD(N)=XN where HD is computational
steps,Nis problem size andXis a real number) of describing a large QM system within a realistic (polynomial) time frame (i.e. in the form HD(N)=NX) (Garey and Johnson, 1979). Based
experi-mental manipulation of two-valued QM systems (e.g. the spin states of an electron , ¡ which are construed as a superposition of 1 and 0, respectively). Unlike these devices, however, the biomolecular computers that may exist in living systems, following 3.5 billion years of cellular
evolution, utilize superposition of many-valued QM observables (e.g. position) interacting in an ensemble search for a minimum local energy value. The difference is important because the molecular search process is under thermodynamic constraints, which permit open-system mapping from classical input to QM state changes to threshold value to classical output (Conrad, 1989; Wallace et al., 1998). In this regard, biomolecular computers based on ensemble molecular searches are conceptually distinctive even from recent ar-tificial approaches that superficially resemble them (such as nuclear magnetic manipulation of molecular nuclei in a liquid (Gershenfeld and Chuang, 1997). The distinction raises the ques-tion; which features of the neural membrane would exemplify open-system mapping? We sug-gest that the following model identifies the key architectural elements.
We begin with a picture of a membrane region in which the constituent molecules are in the liquid phase. Contrary to the earlier models (Singer and Nicolson, 1972; Churchland and Se-jnowski, 1992) this phase is not entirely ‘pattern-less’. Lateral movements of molecules within the
region do indeed occur (Alfsen, 1989; Zachowski, 1993) but are thermodynamically constrained by lipid-protein hydrophobic matching (Sperotto and Mouritsen, 1993; Lehtonen et al., 1996; Lehtonen and Kinnunen, 1997; Killian, 1998). Moreover, the cholesterol molecule induces close interaction between membrane phospholipids, and especially between the hydrocarbon ethenes (Hyslop et al., 1990; Raffy and Teissie´, 1999). This dynamic equilibrium is disturbed by external inputs. Two physical forms of inputs are neurotransmitter binding with an ion-channel receptor, and an electromagnetic field applied (at or above a threshold level) to an ion-channel-associated polypeptide voltage sensor (Catterall, 1988; Jan and Jan, 1989; Stuhmer et al., 1989; Hall, 1992). Response to the external input is instantiated as protein conformational change from thea-helix to random coil (open channel) followed by a mini-mum local potential energy search of lipid and protein molecules comprising the microdomain (lipid selectivity) (Mouritsen and Bloom, 1993; Horvath et al., 1995; Mouritsen, 1998; Sabra and Mouritsen, 1998). Given the probabilistic struc-ture of the lipid and protein molecular hypersur-faces, successive instances of the ensemble search will utilize different pathways and will lose or recruit lipids from neighboring microdomains. Thus changes in interactions and elements exist within the system. The search process term-inates at threshold value; i.e. when the difference
Table 1
Calculated moments and polarizabilities of ethene model compoundsa
Dipole moment(Debye)
Ethene X=0.0000 Y=0.0000 Z=0.0000 Total=0.0000
Ethene dimer X= −0.0001 Y= −0.5306 Z=0.0794 Total=0.5365 X=0.0005 Y= −0.6531
Ethene trimer Z= −0.0306 Total=0.6538
Quadrupole moment(Debye-A,)
XX= −15.1785 YY= −12.1966
Ethene ZZ= −11.9487
Ethene dimer XX= −48.4728 YY= −43.6183 ZZ= −42.6662 Ethene trimer XX= −75.6495 YY= −67.8488 ZZ= −67.3202
Polarizability(J−1C2m2)
Ethene axx=7.672 ayy=19.226 azz=30.875 a=19.258
axx=54.029 ayy=84.076 azz=62.289 a=66.798 Ethene dimer
axx=93.644 ayy=125.284 azz=91.345 a=103.424 Ethene trimer
aGeometries of model compounds were optimized using the 4-31G* basis set. Polarizabilities were obtained by performing
Ec−Em=dMIN(see Section 1). A candidate
phys-ical mechanism for dMIN is the specific potential energy difference between the membrane mi-crodomain and the ion-channel protein required to thermodynamically constrain the protein to the lower-potential-energy a-helix conformation (closed state). In this manner, the duration of the channel open state, and thus, neuron spike fre-quency (macroscopic output) are directly regu-lated by the molecular-ensemble search. Clearly, the search could be prolonged or varied by spa-tial-temporal variation in ion-channel gatings fol-lowing the initial perturbation (input). The implications of the latter property will be dis-cussed in Section 4.
4. Potential experiments
Can quantum computing in a model membrane be experimentally investigated? Clearly, we believe that the answer is yes, but our optimism is tem-pered by an awareness of technical challenges. In our laboratory, we are designing a set of artificial-membrane spectroscopy experiments based on our computational evidence for ethylenic stacking and polarization. It is our intention to investigate membrane-molecular regulation of a ligand-gated ion channel embedded in a model liposome sys-tem. In the first stage of the experiments, we will obtain open-channel and closed-channel spectro-scopic read-outs of the liposome and the embed-ded receptor through the use of ‘caged’ L-glutamate. Difference spectra (open-channel – closed-channel) will provide information regard-ing changes in protein-lipid interactions as well as alterations in microdomain lipid organization. We predict that this protocol will reveal an inverse correlation between duration of channel opening and PUFA clustering in the lipid bilayer. (See the anesthetic-epileptic opposition discussion in Vreugdenhil et al., 1996). These experiments will also permit determination of PUFA ethylenic bond polarizability during permeant ion move-ment. Closely related to this set of experiments is a subsequent set of measurements involving fluorescence resonance energy transfer (FRET), a
technique widely utilized to investigate lipid struc-ture and dynamics in membranes (Edidin, 1989). Using FRET, we will monitor the distance be-tween a fluorescein donor and a tetramethylrho-damine acceptor as a function of ion-channel activity. We predict that acceptor-donor distances will be reduced in direct relation to PUFA con-centration, a prediction consistent with the hy-pothesis of hydrophobic mismatch and lipid selectivity.
A second stage of experimentation would in-volve evaluating the adaptation of the system to increasing input complexity (Garey and Johnson, 1979; Wallace, 1989, 1993, 1995; Wallace and Price, 1999). Through the use of Raman and FRET techniques, the computational limit of membrane molecular response (minimum local potential energy search) to gatings (mediated via caged glutamate) of an increasing number of re-ceptors could be instrumentally identified as a plateau response in the spectroscopic read-out (Wallace and Price, 1999).
5. Conclusion
nat-ural membranes will be challenging but possible. We are designing experiments to further investi-gate the role of lipid selectivity and ethene polar-ization as regulators of neural activity.
References
Alfsen, A., 1989. Membrane dynamics and molecular traffic and sorting in mammalian cells. Prog. Biophys. Mol. Biol. 54, 145 – 157.
Bennett, C., 1995. Quantum information and computation. Phys. Today 48, 24 – 30.
Catterall, W., 1988. Structure and function of voltage-sensitive ion channels. Science 242, 50 – 61.
Churchland, P., Sejnowski, T., 1992. The Computational Brain. The MIT Press, Cambridge.
Conrad, M., 1984. Microscopic-macroscopic interface in bio-logical information processing. BioSystems 16, 345 – 363. Conrad, M., 1989. Physics and biology: toward a unified
model. Appl. Math. Comput. 32, 75 – 102.
Conrad, M., 1992. Quantum molecular computing: the self-as-sembly model. Int. J. Quantum Chem. Quantum Biol. Symp. 19, 125 – 143.
Deutsch, D., 1985. Quantum theory, the Church-Turing Prin-ciple, and the universal quantum computer. Proc. R. Soc. London A 400, 97 – 117.
Deutsch, D., Jozsa, R., 1992. Rapid solution of problems by quantum computation. Proc. R. Soc. London A 439, 553 – 558.
Dykstra, C., 1997. Physical Chemistry: A Modern Introduc-tion. Prentice Hall, Upper Saddle River.
Edidin, M., 1989. Fluorescent labeling of cell surfaces. In: Wang, Y.-L., Taylor, D. (Eds.), Fluorescent Microscopy of Living Cells in Culture: Part A. Academic Press, New York, pp. 87 – 102A.
Feynman, R., 1982. Simulating physics with computers. Int. J. Theor. Phys. 21, 467 – 488.
Frisch, M., Trucks, G., Schlegel, H., Gill, P., Johnson, B., Robb, M., Cheeseman, J., Keith, T., Petersson, G., Mont-gomery, J., Raghavachari, K., Al-Laham, M., Zakrzewski, V., Ortiz, J., Foresman, J., Cioslowski, J., Stefanov, B., Nanayakkara, A., Challacombe, M., Peng, C., Ayala, P., Chen, W., Wong, M., Andres, J., Replogle, E., Gomperts, R., Martin, R., Fox, D., Brinkley, J., Defrees, D., Baker, J., Stewart, J., Head-Gordon, M., Gonzalez, C., Pople, J., 1995. Gaussian 95, Gaussian Inc., Pittsburgh.
Garey, M., Johnson, D., 1979. Computers and Intractability: A Guide to the Theory of NP-Completeness. Freeman, San Francisco.
Gennis, R., 1989. Biomembranes: Molecular Structure and Function. Springer, New York.
Gershenfeld, N., Chuang, I., 1997. Bulk spin-resonance quan-tum computation. Science 275, 350 – 356.
Hall, Z., 1992. Ion channels. In: Hall, Z. (Ed.), An Introduc-tion to Molecular Neurobiology. Sinauer, Sunderland.
Hodgkin, A., Huxley, A., 1952. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 116, 500 – 544.
Horvath, L., Heimburg, T., Kovachev, P., Findlay, J., Hideg, K., Marsh, D., 1995. Integration of a K+ channel-associ-ated peptide in a lipid bilayer: conformation, lipid-protein interactions, and rotational diffusion. Biochemistry 34, 3893 – 3898.
Hyperchem 4.5 1995. Hypercube Inc., 1115 N.W. 4th Street, Gainesville, FL 32601.
Hyslop, P., Morel, B., Sauerheber, R., 1990. Organization and interaction of cholesterol and phosphatidylcholine in model bilayer membranes. Biochemistry 29, 1025 – 1038. Jan, L., Jan, Y., 1989. Voltage-sensitive ion channels. Cell 56,
13 – 25.
Jørgensen, K., Mouritsen, O., 1995. Phase separation dynam-ics and lateral organization of two-component lipid mem-branes. Biophys. J. 95, 942 – 954.
Killian, J., 1998. Hydrophobic mismatch between proteins and lipids in membranes. Biochim. Biophys. Acta 1376, 401 – 415.
Kinnunen, P., Virtanen, J., 1986. A qualitative, molecular model of the nerve impulse: conductive properties of unsat-urated, lyotropic liquid crystals. In: Gutman, F., Keyzer, H. (Eds.), Modern Bioelectrochemistry. Plenum Press, New York.
Krnjevic´, K., Miledi, R., 1959. Presynaptic failure of neuro-muscular propagation in rats. J. Physiol. 149, 1 – 22. Lehtonen, J., Kinnunen, P., 1997. Evidence for phospholipid
microdomain formation in liquid crystalline liposomes re-constituted with Escherichia coli lactose permease. Bio-phys. J. 72, 1247 – 1257.
Lehtonen, J., Holopainen, J., Kinnunen, P., 1996. Evidence for the formation of microdomains in liquid crystalline large unilamellar vesicles caused by hydrophobic mismatch of the constitutent phospholipids. Biophys. J. 70, 1753 – 1760.
Leuchtag, H., 1994. Long-range interactions, voltage sensitiv-ity, and ion conduction in S4 voltage segments of excitable channels. Biophys. J. 66, 217 – 224.
Lloyd, S., 1993. A potentially realizable quantum computer. Science 61, 1569 – 1571.
Marsh, D., 1995. Lipid-protein interactions and hetereoge-neous lipid distribution in membranes. Mol. Membrane Biol. 12, 59 – 64.
McCulloch, W., Pitts, W., 1943. A logical calculus of the ideas immanent in nervous activity. Bull. Math. Biophys. 5, 115 – 133.
Mouritsen, O., 1998. Self-assembly and organization of lipid-protein membranes. Curr. Opin. Coll. Interf. Sci. 3, 78 – 87. Mouritsen, O., Bloom, M., 1993. Models of lipid-protein interactions in membranes. In: Engleman, D., Cantor, C., Pollard, T., (eds) Annual Review of Biophysics and Biomolecular Structure vol. 22. Annual Reviews, Palo Alto, pp 145 – 171.
Mouritsen, O., Jørgensen, K., 1994. Dynamical order and disorder in lipid bilayers. Chem. Phys. Lipids 73, 3. Mouritsen, O., Jørgensen, K., 1997. Small-scale
lipid-mem-brane structure: simulation versus experiment. Curr. Opin. Struct. Biol. 7, 518 – 527.
Parnas, I., 1972. Differential block at high frequencies of branches of a single axon innvervating two muscles. J. Neurophys. 35, 903 – 914.
Raffy, S., Teissie´, J., 1999. Control of lipid membrane stability by cholesterol content. Biophys. J. 76, 2072 – 2080. Sabra, M., Mouritsen, O., 1998. Steady-state
compartmental-ization of lipid membranes by active proteins. Biophys. J. 74, 745 – 752.
Scott, A., 1995. Stairway to the Mind: The Controversial New Science of Consciousness. Copernicus, New York. Singer, S., Nicolson, G., 1972. The fluid mosaic structure of
the structure of cell membranes. Science 173, 720 – 731. Sperotto, M., Mouritsen, O., 1993. Lipid enrichment and
selectivity of integral membrane proteins in two-compo-nent lipid bilayers. Eur. Biophys. J. 22, 323 – 328. Simons, K., Ikonen, E., 1997. Functional rafts in cell
mem-branes. Nature 387, 569 – 572.
Stuhmer, W., Conti, F., Suzuki, H., Wang, X., Noda, M., Yahagi, N., Kubo, H., Numa, S., 1989. Structural parts involved in activation and inactivation of the sodium chan-nel. Nature 339, 597 – 603.
Swadlow, H., Waxman, S., 1976. Variations in conduction
velocity and excitability following single and multiple im-pulses of visual callosal axons in the rabbit. Exp. Neurol. 53, 128 – 150.
Vreugdenhil, M., Bruehl, C., Voskuyl, R., Kang, J., Leaf, A., Wadman, W., 1996. Polyunsaturated fatty acids modulate sodium and calcium currents in CA1 neurons. Proc. Natl. Acad. Sci. USA 93, 12559 – 12563.
Wallace, R., 1989. Cognitive mapping and the origin of lan-guage and mind. Curr. Anthropol. 30, 518 – 526. Wallace, R., 1993. Cognitive mapping and algorithmic
com-plexity: is there a role for quantum. Processes in the evolution of human consciousness? Behav. Brain Sci. 16, 614 – 615.
Wallace, R., 1995. Microscopic computation in human brain evolution. Behav. Sci. 40, 133 – 158.
Wallace, R., 1996. Microcomputational evolution of the neu-ral membrane. Nanobiology 4, 25 – 37.
Wallace, R., Price, H., 1999. Neuromolecular computing: a new approach to human brain evolution. Biol. Cyber. 81, 189 – 197.
Wallace, R., Price, H., Breitbeil, F., 1998. Toward a charge-transfer model of neuromolecular computing. Int. J. Quan-tum Chem. 69, 3 – 10.
Zachowski, A., 1993. Phospholipids in animal eukaryotic membranes: transverse asymmetry and movement. Biochem. J. 294, 1 – 14.