www.elsevier.com / locate / bres
Research report
The effects of
L
-NAME on vestibular compensation and NOS activity
in the vestibular nucleus, cerebellum and cortex of the guinea pig
a a a ,
*
bSylvia Paterson , Yiwen Zheng , Paul F. Smith
, Cynthia L. Darlington
a
Vestibular Research Group, Department of Pharmacology, School of Medical Sciences, University of Otago Medical School, Dunedin, New Zealand
b
Vestibular Research Group, Department of Psychology and the Neuroscience Research Centre, University of Otago, Dunedin, New Zealand Accepted 25 July 2000
Abstract
Nitric oxide (NO) has been implicated in the processes by which animals recover from peripheral vestibular damage (‘vestibular compensation’). However, few data exist on the dose–response effects of systemic administration of the nitric oxide synthase (NOS)
G
inhibitor, N -nitro-L-arginine methyl ester (L-NAME), on the vestibular compensation process. The aim of this study was to investigate the effects on compensation of 5, 10, 50 or 100 mML-NAME administered by s.c osmotic minipump for 50 h following unilateral vestibular deafferentation (UVD) in guinea pig, either commencing the drug treatment at 4 h pre-UVD or at the time of the UVD (i.e., post-UVD). Post-UVD treatment withL-NAME, at any of the four concentrations used, had no effect on the compensation of spontaneous nystagmus (SN), yaw head tilt (YHT) or roll head tilt (RHT). By contrast, pre-UVD treatment with 100 mML-NAME resulted in a significant decrease in SN frequency (P,0.05) and a change in the rate of its compensation (P,0.0005). Pre-UVDL-NAME resulted in a significant increase in the overall magnitude of YHT (P,0.005); however, post-hoc comparisons revealed no significant differences between any specific L-NAME and vehicle groups. Pre-UVDL-NAME had no effect on RHT at any concentration. Analysis of NOS activity in the pre-UVD L-NAME treatment groups at 50 h post-UVD showed that only 100 mML-NAME resulted in a significant decrease in NOS activity in the contralateral medial vestibular nucleus (MVN) / prepositus hypoglossi (PH) (P,0.05) and that NOS activity in the ipsilateral MVN / PH was not significantly affected. However, NOS activity was significantly inhibited in the bilateral cerebellum and cortices for several concentrations ofL-NAME. These results suggest that pre-UVD systemic administration ofL-NAME can significantly increase the rate of SN compensation in guinea pig and that this effect is correlated with inhibition of NOS activity in several regions of the CNS. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Nitric oxide; Nitric oxide synthase; Vestibular compensation; Unilateral vestibular deafferentation; Unilateral labyrinthectomy
1. Introduction symptoms (i.e., ‘static symptoms’, such as spontaneous
ocular nystagmus (SN), yaw head tilt (YHT) and roll head Damage to the peripheral vestibular system results in a tilt (RHT)) related to the imbalance in resting activity syndrome of ocular motor and postural disorders due to the between the bilateral VNCs, gradually subside within a disruption of central vestibulo-ocular (VOR) and vestibulo- few days to a week, whereas those symptoms related to the spinal (VSR) reflex pathways. In the case of unilateral loss of the dynamic sensitivity of VNC neurons to head vestibular deafferentation (UVD), the symptoms are par- movement (i.e., ‘dynamic symptoms’, such as abnormal ticularly dramatic, as a result of the severe imbalance in gain and phase of the VORs and VSRs), compensate much neuronal activity between the ipsilateral and contralateral less completely, more variably and over a longer period of vestibular nucleus complexes (VNCs). However, over time. To the extent that vestibular compensation does time, many but not all of these symptoms subside in a occur, it appears to be relatively independent of any process of behavioural recovery known as ‘vestibular recovery in the deafferented vestibular nerve, and therefore compensation’. Vestibular compensation is a heterogeneous is attributed to plasticity in the central nervous system process in which many of the ocular motor and postural (CNS). Many neurophysiological studies, including studies in alert animals, have shown that the static compensation process is approximately correlated with a partial recovery *Corresponding author. Tel.:164-3-479-5747; fax:164-3-479-5747.
E-mail address: [email protected] (P.F. Smith). of resting activity in the ipsilateral VNC (see Refs. 0006-8993 / 00 / $ – see front matter 2000 Elsevier Science B.V. All rights reserved.
[3,4,6,7,11,20] for reviews). However, the biochemical administration on NOS activity in the VNC, and compared basis of the compensation process is still poorly under- these effects with those in the cerebellum and cortex. stood (e.g. [9,15]).
The fact that excitatory amino acid receptors such as the
N-methyl-D-aspartate (NMDA) receptor and associated 2. Materials and methods second messengers and protein kinases have been
impli-cated in vestibular compensation suggests that other 2.1. Subjects NMDA receptor-related neuromodulators, such as nitric
oxide (NO), are also likely to be involved (see [19] for Data were obtained from a total of 60 male and female review). NO is a free radical gas which is synthesised from pigmented guinea pigs (300–600 g). Five of these animals arginine by the enzyme, nitric oxide synthase (NOS), and were used to examine NOS activity in the absence of UVD which is released from neurons by simple diffusion rather (‘non-UVD controls’); these animals were cervically dislo-than exocytosis, acting on intracellular targets in other cated and decapitated without anaesthetic (see below). The neurons via the activation of guanylate cyclase to produce remaining animals were allocated to one of the following cyclic guanosine monophosphate (cGMP) (see [21] for 10 groups, depending upon whether the drug administra-review). tion began before or after the UVD: (1) pre-UVD saline Flohr and colleagues [8] were the first to investigate NO control (n55); (2) pre-UVD L-NAME 5 mM (n54); (3) in the context of vestibular compensation and reported that pre-UVDL-NAME 10 mM (n55); (4) pre-UVDL-NAME
G
the selective NOS inhibitor, N -nitro-L-arginine methyl 50 mM (n56); (5) pre-UVDL-NAME 100 mM (n55); (6) ester (L-NAME), delivered post-UVD into the dorsal post-UVD saline control (n59); (7) post-UVDL-NAME 5 lymph sac of the frog, delayed vestibular compensation. mM (n55); (8) post-UVD L-NAME 10 mM (n56); (9) Kitahara et al. [10], using rats, showed that changes in post-UVD L-NAME 50 mM (n55); (10) post-UVD L -NOS expression occur in the cerebellar flocculus following NAME 100 mM (n55).
UVD and that post-UVD injection of L-NAME into the
flocculus also inhibited the compensation process. Kitahara 2.2. Drug administration et al. [12] have further reported that NOS expression in the
flocculus modulates the expression of the immediate early In order to maintain constant levels of NOS inhibition in gene protein, c-Fos, in the medial vestibular nucleus the CNS for up to 50 h post-UVD,L-NAME was adminis-(MVN) and prepositus hypoglossi (PH) following UVD. tered using continuous infusion by osmotic minipump In our previous studies we found that NOS activity (Alzet 1003D) implanted s.c between the shoulder blades. decreases in the ipsilateral MVN and bilateral PH follow- The minipump was incubated in 0.9% saline at 378C ing UVD and that NOS activity levels remain low up to 50 overnight so that it was ready to pump at 0.98ml / h from h post-UVD [1]. Taken together, these studies suggest that the time of implantation. In all cases L-NAME was the uncompensated stage following UVD is associated dissolved in 0.9% saline. Because of the limited amount of with low levels of NOS activity in the ipsilateral MVN and data available on the effects of L-NAME on vestibular PH and that inhibition of NOS results in a retardation of compensation, we decided to use a wide range of con-the vestibular compensation process. centrations (from 5 to 100 mM) in order to provide a There are many possible sites of action in the CNS dose–response analysis. The decision to use this con-where L-NAME could influence the development and centration range was based on our preliminary studies maintenance of vestibular compensation. Although Kita- which showed that 10 and 50 mM L-NAME treatment hara et al. [10] investigated the effects of local injection of using an s.c osmotic minipump resulted in a decrease in L-NAME into the cerebellar flocculus in rat, to date the NOS activity of 15 and 61% in the MVN / PH, respectively only study to examine the effects of systemic administra- [Paterson et al., unpublished observations]. We decided to tion ofL-NAME on vestibular compensation has used frog use 100 mM L-NAME in addition in order to achieve [8]. Because of the known species differences between greater than 61% inhibition of NOS activity in the MVN / frogs and mammalian species in the compensation process PH. For the pre-UVD conditions, the animal was anaes-(see Refs. [3,4,6,7,11,20] for reviews), we reasoned that it thetised with ether and the minipump implanted 4 h prior was important to conduct a systematic dose–response to the UVD. For the post-UVD conditions, the minipump analysis of the effects of systemic administration of L- was implanted at the time of the UVD under fentazin NAME on vestibular compensation in a mammalian anaesthesia (see below).
species. Because of the different effects of NMDA receptor
antagonists depending upon whether they are administered 2.3. Surgery pre- or post-UVD ([16,17]; see [19] for review), we also
Elec-trocardiograph (ECG) electrodes were inserted into the to avoid contamination of SN by VOR nystagmus induced forelimb muscles and heart rate was monitored throughout by head movement. Animals were allowed to choose a the surgery using a Beckman amplifier and an au- natural posture and were not restrained since stress induced diomonitor. Procaine hydrochloride was injected (s.c.) into by restraint has been shown to increase SN frequency. We all pressure points and wound margins. The temporal bone estimate that the measurement error involved in using this was exposed using blunt dissection and the skin retracted method of SN frequency analysis is of the order of 61 using hemostats, and then the bony vestibular labyrinth beat / 15 s [17].
was exposed using a high speed dental drill with a fine YHT was defined as the angle of deviation (in degrees) burr, with the aid of an otolaryngological operating between a line through the midscapular point and sacrum microscope (Zeiss OPM). The sensory components of the and a line through the midscapular point and the centre of vestibular apparatus (the horizontal and anterior semicircu- the animal’s head in the horizontal plane; RHT was lar canal ampullae, and the maculae of the utricle and defined as the angle of deviation (in degrees) between saccule) were destroyed by drilling and aspiration. Al- gravitational vertical and the centre of the animal’s head in though the posterior canal ampulla was not visualized, it the vertical plane. Postural symptoms were measured by was also destroyed by drilling and aspiration. At the end of using the freeze-frame facility on the video recorder and the surgery, antibiotic cream (2% mupirocin) was inserted fitting a large protractor over the screen of the monitor. We into the labyrinthine cavity and the temporal bone sealed estimate that the measurement error involved in measuring with dental cement. Histological examination has shown YHT and RHT in this way is of the order of6108. All that this UVD procedure results in complete destruction of measurements began at 10 h post-UVD when animals had the labyrinthine receptors [5]. Animals were allowed to recovered sufficiently from the anaesthetic to display recover under a warm lamp with food and water available typical UVD symptoms. Mean SN, YHT and RHT were ad libitum. calculated for each measurement time within each group of
animals [17]. 2.4. Behavioural measurements
2.5. NOS assays All behavioural measurements were made double-blind:
all drug solutions were coded so that the person making The specific purpose of the NOS assay was to determine the measurements did not know which solution was being the extent to which the pre-UVD L-NAME treatment, administered. Three static symptoms of UVD were quan- which resulted in effects on vestibular compensation, had tified: spontaneous ocular nystagmus (SN), yaw head tilt been effective in reducing NOS activity in the VNC, (YHT) and roll head tilt (RHT). Measurements were made cerebellum and cortex. For this reason and because our at 10, 20, 30, 40 and 50 h post-UVD. These times were interest was not in studying NOS activity in individual chosen because in our previous studies they have been subnuclei, the entire right MVN / PH and left MVN / PH shown to provide an accurate characterisation of the complexes were removed for analysis. For comparison, in vestibular compensation process. Symptoms were vid- the same animals, the entire ipsilateral and contralateral eotaped at each measurement time using two video cerebella and cortices were also removed.
cameras (Panasonic NV-M50), each with a zoom lens, In all cases, at 50 h post-UVD, animals were killed by connected to a video recorder (Mitsubishi HS-641V) and a cervical dislocation without anaesthesia. The brains were colour monitor (Sony Trinitron). One video camera was rapidly removed and immediately chilled in iced-water for positioned in front of the animal to videotape SN and 45 s. The ipsilateral and contralateral MVN / PHs and RHT, the other was positioned directly above the animal in ipsilateral and contralateral cerebella and cortices were order to videotape YHT. Animals were housed in in- rapidly dissected from the unfrozen brains following the dividual boxes with perspex windows at the front so that methods of Anderson et al. [1]. The tissues were immedi-video recording could take place without moving the ately frozen on dry ice and stored at2848C until the time animal, since changes in vestibular, proprioceptive and of the assay. We employed a radioenzymatic assay tech-visual information produced by lifting the animal can nique which was modified from the one developed by cause decompensation [17]. Bredt and Snyder [2], in which NOS enzyme activity is
3
SN was defined as a rapid, large amplitude, mainly measured by monitoring the conversion of [ H]arginine to 3
reaction was started by adding 25 ml of the sample supernatant to an assay tube containing 75 ml of 1 mM
3
NADPH, 0.75 mM CaCl2 and 150 nM [ H]-L-arginine. Following incubation in a water bath for 1 min at 378C, the reaction was stopped by adding 1 ml of 1:1 (v / v) Dowex /
1
H O-50W (200–400, 8% cross-linked, Na2 form) and 2 ml of 20 mM HEPES buffer (containing 2 mM EDTA, pH 5.5) into the tube and transferring the tube onto the ice. After 10 min, 750ml of the supernatant were removed and
3 added to 4.25 ml of scintillation fluid in a vial. [ H]-citrulline was quantified by liquid scintillation spectros-copy, using a Beckman scintillation counter. The counts-per-minute (cpm) for duplicate samples were averaged and corrected with respect to the blank control and background radioactivity. NOS activity was expressed as pmol / min / mg protein. Protein concentrations in the supernatant were measured based on the Bradford method [18] using a Bio-Rad protein assay dye reagent concentrate and spec-tramax microplate reader. Ten microlitres of supernatant were removed from the same tissue sample used for the NOS assay and added to 390ml of distilled water in a 1.5 ml Eppendorf tube and vortexed. Ten microlitres of standard (0–500mg / ml protein) or sample was then added to 190 ml of reagent that consisted of 40 ml of BioRad reagent and 150 ml of water in a 96 well tray. Protein formation was then assessed using spectrophotometry at a wavelength of 595 nm. The counts for duplicate samples were averaged.
2.6. Statistical analysis
For SN, YHT and RHT, a single two-factor analysis of variance (ANOVA) with repeated measures on time was performed [22]. Factor A represented the drug effect on SN frequency, YHT or RHT; factor B, the repeated measure, represented time; and the interaction (AB) repre-sented the change in the rate of compensation as a result of treatment. As factor B, the repeated measure, was always significant (i.e., since vestibular compensation occurred in all cases), it will not be discussed further. Pairwise comparisons were conducted, where appropriate, using the
´
Scheffe F-test; linear and exponential regression analyses were also used on the SN data [22]. The NOS activity data were analysed using one-way ANOVAs and post-hoc student Newman–Keuls multiple comparison tests [22]. The significance level was set at 0.05 for all comparisons.
3. Results
3.1. Behavioural results
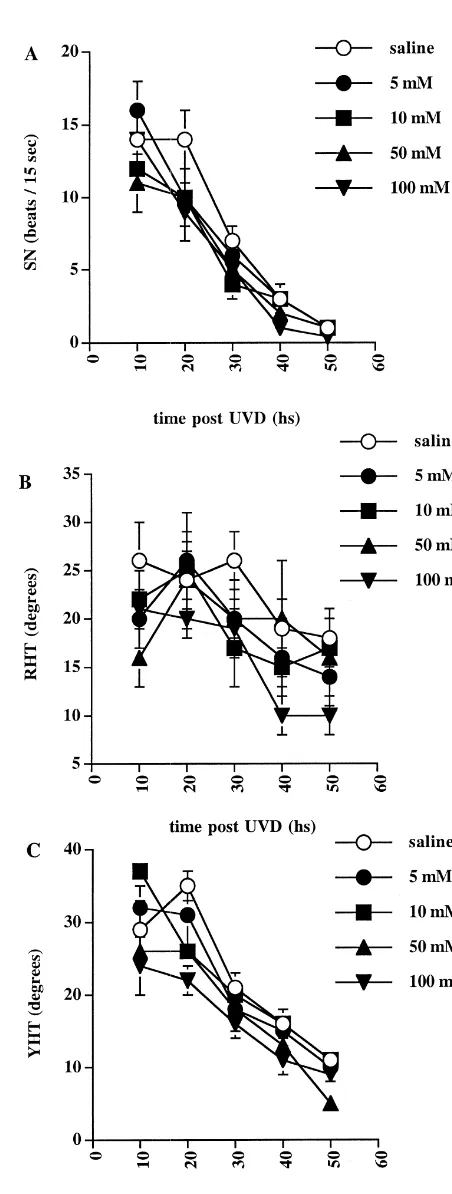
Post-UVD treatment with L-NAME, at any of the four
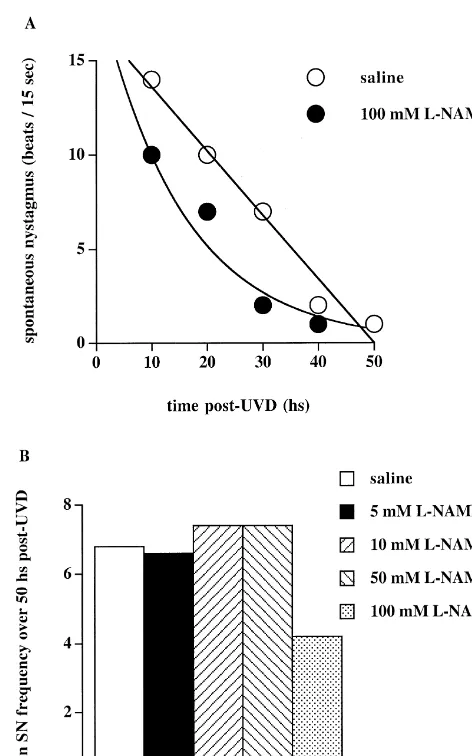
frequency (P,0.05) and a change in the rate of its compensation (P,0.0005) (Fig. 2A). Post-hoc analysis showed that these significant effects were due to a large decrease in SN frequency in the 100 mM group at 10 h post-UVD compared to the other drug groups (P,0.05) and to a change in the pattern of the decrease in SN over time compared to the vehicle group. Regression analysis revealed that the 100 mM L-NAME group showed a distinct exponential pattern of SN decrease over time compared to the vehicle controls (P,0.001) (Fig. 3). Pre-UVD L-NAME treatment also resulted in a significant increase in the overall magnitude of YHT using ANOVA (P,0.005) (Fig. 2C); however, post-hoc analysis failed to detect significant differences between L-NAME and ve-hicle groups at any particular time point. Pre-UVD L -NAME had no significant effect on RHT at any con-centration (Fig. 2B).
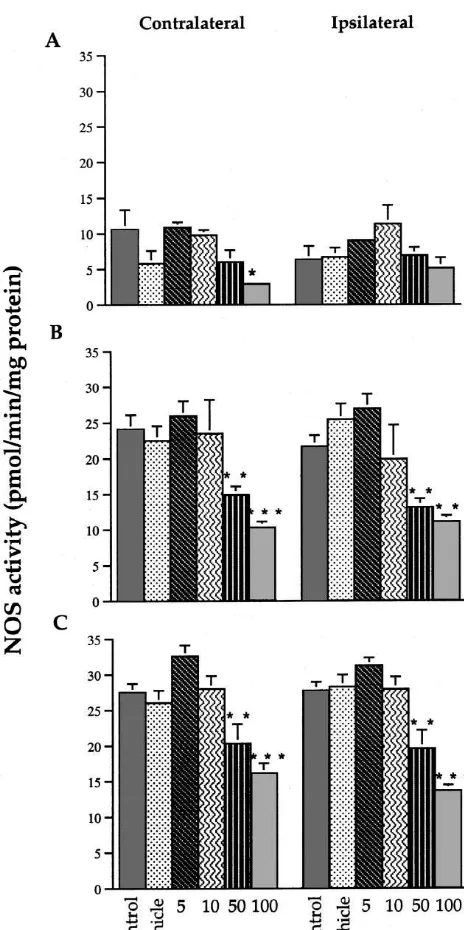
3.2. NOS assay results and NOS activity in the ipsilateral MVN / PH was not significantly affected (Fig. 4A). By contrast, in both the Fig. 4 shows that levels of NOS activity were generally contralateral and ipsilateral cerebellum and cortex, 50 and much lower in the MVN / PH than in the cerebellum and 100 mM L-NAME resulted in a significant decrease in cortex, and this raised the possibility that even high NOS activity (P,0.01 and P,0.001, respectively; Fig. 4B, concentrations ofL-NAME might not result in a significant C).
inhibition of NOS activity. In fact, amongst the pre-UVD L-NAME treatment groups, only 100 mM L-NAME
re-sulted in a significant (72%) decrease in NOS activity in 4. Discussion
the contralateral MVN / PH compared to controls (P,0.05)
The results of the present study demonstrate that, in guinea pig, continuous systemic infusion of high con-centrations (ie. 100 mM) ofL-NAME from 4 h pre-UVD until 50 h post-UVD result in a marked increase in the rate of compensation of SN. By contrast, similar post-UVD administration does not result in a change in either the frequency of SN at any particular time point nor the rate of SN compensation. Pre-UVD administration of L-NAME caused a small increase in the overall magnitude of YHT, although post-hoc analysis failed to detect significant differences between the drug and vehicle conditions at any particular time point. There were no significant effects of L-NAME on YHT in the post-UVD conditions or on RHT in either the pre- or post-UVD conditions.
Although NOS activity levels were lower in the MVN / PH than in the cerebellum and cortex, our results support the hypothesis that pre-UVD administration of the highest concentration of L-NAME (i.e., 100 mM) resulted in a significant inhibition of NOS activity in at least the contralateral MVN / PH, as well as in the bilateral cere-bellum and cortex. These results, which pertain specifically to NOS activity levels in these various regions at 50 h post-UVD when the tissue samples were extracted, suggest that the highest concentrations of L-NAME that we used (50–100 mM) did effectively inhibit NOS activity, al-though they do not prove that the effects of pre-UVD administration of L-NAME on SN and YHT were due to this inhibition, nor do they indicate which site of action might be responsible. Although the analyses of NOS activity indicate that a dose of 50 mML-NAME effectively inhibited NOS activity in many parts of the brain, the behavioural study indicated that this caused no significant effects on vestibular compensation. We have no explana-tion for this finding at present and can only conclude that this degree of inhibition of NOS activity must have been insufficient to affect vestibular compensation. We believe that it is unlikely that the higher doses ofL-NAME used in the current study produced toxic effects which in some way reduced the expression of vestibular symptoms since: (i) only SN frequency decreased in the 100 mM pre-UVD condition whereas YHT increased and RHT showed no Fig. 4. NOS activity in the contralateral and ipsilateral medial vestibular change; (ii) these changes occurred only in the pre-UVD nucleus / prepositus hypoglossi (top), cerebellum (middle) and cortices 100 mM condition and not in the post-UVD 100 mM (bottom) at 50 h post-op. for animals receiving no treatment (‘control’),
treatment group; (iii) animals receiving L-NAME did not
vehicle, or 5, 10, 50 or 100 mML-NAME pre-UVD. Asterisks represent
show any evidence of ill-health (e.g., piloerection). significant differences relative to the control group. Columns represent
different from those that have been reported previously, extensive literature suggesting that even lesions to many although direct comparison between studies is limited by areas of the cerebellum and cortex do not significantly the different species and drug administration protocols affect at least the static aspects of vestibular compensation used. Flugel et al. [8] reported that post-UVD administra- (see [6] for review).
tion of L-NAME into the dorsal lymph sac of the frog One important aspect of the present study is the com-resulted in a delayed compensation of RHT. However, we parison of pre- and post-UVD administration ofL-NAME. found no effects of L-NAME on RHT irrespective of Many previous studies have shown that pre- and post-UVD whether it was administered pre- or post-UVD at any of a administration of NMDA receptor / channel and voltage-range of concentrations. It is difficult to compare our study dependent calcium channel antagonists results in quite with that of Flugel et al. [8] because: (i) they used daily different effects on static compensation, with pre-UVD bolus injections ofL-NAME whereas we used continuous treatment often causing a decrease in the severity of the infusion by osmotic minipump; (ii) we have no infor- symptoms [19]. It is therefore interesting that pre-UVD mation on the biochemical effects of theL-NAME on NOS administration of 100 mM L-NAME in this study should in the study by Flugel et al. [8]; and (iii) it is well accelerate the compensation of SN. Why this is the case, established that vestibular compensation differs in many we do not know at present. We have previously suggested important respects between frogs and mammalian species that UVD might result in an increased release of glutamate (e.g., frogs do not exhibit SN or YHT). It is conceivable into the ipsilateral VNC, causing increased activation of that discrete, bolus injections of a NOS inhibitor, because NMDA receptors [19]; this might then lead to an increased the levels of NOS inhibition would not be constant, would production of NO. However, our previous study of changes have quite different effects on vestibular compensation in NOS activity did not support this prediction: NOS compared to continuous infusion of the same drug with activity was significantly lower rather than higher in the constant levels of NOS inhibition. However, in the absence ipsilateral MVN and bilateral PH following UVD [1]. The of data on the biochemical effects ofL-NAME in the frog, results of the NOS assays from the current experiment it is impossible to resolve this issue. cannot be compared directly with those of Anderson et al. Kitahara et al. [10] also reported that L-NAME delayed [1] because in that study MVN and PH were analysed compensation of SN in rats when it was administered just separately, whereas in the current study the entire MVN / prior to the UVD; however, these investigators injected the PH complex on each side of the brainstem was analysed drug directly into the cerebellar flocculus by cannula. together. However, in our previous study [1] the initial Because NOS activity was inhibited in the cerebellum in decrease in NOS activity in the ipsilateral MVN and PH the present study, it is therefore surprising that we did not following UVD was partially resolved by 50 h post-op. observe a retardation of SN compensation in guinea pig. It With respect to the current study, it must be remembered is possible that concurrent inhibition of NOS activity in that the MVN / PH samples were removed at 50 h post-other areas of the CNS altered the effects of inhibition of UVD and therefore the lack of difference in NOS activity cerebellar NOS activity in the current study, since in the between the vehicle and control conditions is reasonably study by Kitahara et al. NOS would have been inhibited in consistent with our previous study. It is interesting that at the cerebellar flocculus only. Another possibility is that 50 h post-UVD in the 100 mM L-NAME condition, SN there is a species difference in the effects ofL-NAME on compensation was accelerated despite an imbalance in vestibular compensation in rat and guinea pig. Although NOS activity between the ipsilateral and contralateral behavioural compensation following UVD is known to be MVN / PH (Fig. 4A). Moreno-Lopez et al. [14] reported very similar in the two species, there is increasing evidence that symmetrical levels of NO production in the bilateral for at least some differences in the neurophysiological and PH were necessary for normal horizontal eye movement. neurochemical changes underlying the behavioural re- Therefore, the imbalance in NOS activity between the covery (see [6] and [20] for reviews). However, we feel bilateral MVN / PH in the present study may be due largely that an adequate understanding of the effects ofL-NAME, to the MVN rather than PH, or alternatively, acceleration like any other drug, on vestibular compensation, requires a of SN compensation may be possible even in the presence systematic dose–response analysis rather than an arbitrary of this kind of asymmetry in NOS activity.
in: S. Moncada, M. Feelisch, R. Busse, E.A. Higgs (Eds.), The concentrations of L-NAME on these isoforms. Our
pre-Biology of Nitric Oxide: Physiological and Clinical Aspects, liminary Western blotting data, obtained using
electro-Portland Press, London, 1994, pp. 381–387.
blotting with enhanced chemiluminescence (ECL), suggest [9] D.R. Kerr, A.J. Sansom, P.F. Smith, C.L. Darlington, Comparison of that nNOS does exist in the MVN / PH and that it is likely protein kinase activity and protein phosphorylation in prepositus to be partly responsible for the action ofL-NAME on NOS hypoglossi and medial vestibular nuclei of labyrinthine-intact and labyrinthectomized guinea pigs, J. Vest. Res. 10 (2000) 107–117. activity in the current study [Paterson, Zheng, Appleton,
[10] T. Kitahara, N. Takeda, P.C. Emson, T. Kubo, H. Kiyama, Changes Smith and Darlington, unpublished observations].
in nitric oxide synthase-like immunoreactivities in unipolar brush cells in the rat cerebellar flocculus after unilateral labyrinthectomy, Brain Res. 765 (1997) 1–6.
Acknowledgements [11] T. Kitahara, N. Takeda, H. Kiyama, T. Kubo, Molecular
mecha-nisms of vestibular compensation in the central vestibular system — review, Acta Otolaryngol. (Stockh.) 539 (Suppl.) (1998) 19–27. This research was supported by Project Grants from the
[12] T. Kitahara, N. Takeda, T. Kubo, H. Kiyama, Nitric oxide in the Health Research Council of New Zealand (to CD and PS) flocculus works the inhibitory circuits after unilateral labyrinthec-and the New Zeallabyrinthec-and Neurological Foundation (to PS labyrinthec-and tomy, Brain Res. 815 (1999) 405–409.
CD). We thank Dr D.R. Kerr, Murray Craig and the [13] P.K. Moore, R.L. Handy, Selective inhibitors of neuronal nitric oxide synthase — is no NOS really good NOS for the nervous technical staff of the Department of Pharmacology for their
system?, Trends Pharmacol. Sci. 18 (1997) 204–211. advice and excellent technical assistance.
[14] B. Moreno-Lopez, M. Escudero, J.M. Delgado-Garcia, C. Estrada, Nitric oxide production by brainstem neurons is required for normal performance of eye movements in alert animals, Neuron 17 (1996)
References 739–745.
[15] A.J. Sansom, V.A. Brent, P.E. Jarvie, C.L. Darlington, P.F. Smith, R. Laverty, J.A.P. Rostas, In vitro phosphorylation of medial vestibular [1] T. Anderson, A. Moulton, A.J. Sansom, D. Kerr, R. Laverty, C.L.
nucleus and prepositus hypoglossi proteins during behavioural Darlington, P.F. Smith, Evidence for reduced nitric oxide synthase
recovery from unilateral vestibular deafferentation in guinea pig, activity in the ipsilateral medial vestibular nucleus and bilateral
Brain Res. 778 (1997) 166–177. prepositus hypoglossi following unilateral vestibular deafferentation
[16] A.J. Sansom, C.L. Darlington, P.F. Smith, Pre-treatment with MK-in guMK-inea pig, BraMK-in Res. 787 (1998) 311–314.
801 reduces spontaneous nystagmus following unilateral labyrin-[2] D.S. Bredt, S.H. Snyder, Isolation of nitric oxide synthetase, a
thectomy, Eur. J. Pharmacol. 220 (1992) 123–127. calmodulin-requiring enzyme, Proc. Natl. Acad. Sci. USA 87 (1990)
[17] A.J. Sansom, P.F. Smith, C.L. Darlington, R. Laverty, Vestibular 682–685.
nucleus NMDA receptors contribute to spontaneous nystagmus [3] I.S. Curthoys, G.M. Halmagyi, Vestibular compensation: a review of
generation following unilateral labyrinthectomy in guinea pig, the oculomotor, neural and clinical consequences of unilateral
Neurosci. Lett. 283 (2000) 117–120. vestibular loss, J. Vest. Res. 5 (1995) 67–107.
[18] C.V. Sapan, R.L. Lundblad, N.C. Price, Review — Colorimetric [4] I.S. Curthoys, G.M. Halmagyi, Vestibular compensation. Vestibular
protein assay techniques, Biotechnol. Appl. Biochem. 29 (1999) Dysfunction and its Therapy, Vol. 2, 1999, pp. 82–110.
99–108. [5] I.S. Curthoys, P.F. Smith, C.L. Darlington, Postural compensation in
[19] P.F. Smith, C.L. Darlington, The contribution of NMDA receptors to the guinea pig following unilateral labyrinthectomy, Prog. Brain
lesion-induced plasticity in the vestibular nucleus, Prog. Neurobiol. Res. 76 (1988) 375–384.
53 (1997) 517–531. [6] C.L. Darlington, P.F. Smith, Molecular mechanisms of recovery
[20] P.P. Vidal, C. De Waele, N. Vibert, M. Muhlethaler, Vestibular from vestibular damage in mammals: recent advances, Prog.
Neuro-compensation revisited, Otolaryngol. Head / Neck Surg. 119 (1998) biol. 62 (2000) 313–325.
34–42. [7] N. Dieringer, ‘Vestibular compensation’: neural plasticity and its
[21] J. Zhang, S.H. Snyder, Nitric oxide in the nervous system, A. Rev. relations to functional recovery after labyrinthine lesions in frogs
Pharmacol. Toxicol. 35 (1995) 213–233. and other vertebrates, Prog. Neurobiol. 46 (1995) 97–129.
[22] J.F. Zolman, Biostatistics: Experimental Design and Statistical [8] G. Flugel, S. Holm, H. Flohr, Chronic inhibition of nitric oxide