Essential role for the (hepatic) LDL receptor in macrophage
apolipoprotein E-induced reduction in serum cholesterol levels and
atherosclerosis
Miranda Van Eck
a,*, Ko Willems Van Dijk
b, Nicole Herijgers
a, Marten H. Hofker
b,
Pieter H.E. Groot
c, Theo J.C. Van Berkel
aaDi6ision of Biopharmaceutics,Leiden/Amsterdam Center for Drug Research,Syl6ius Laboratories,Leiden Uni6ersity,P.O.Box9503, 2300RA Leiden,The Netherlands
bDepartment of Human Genetics,Syl6ius Laboratories,Leiden Uni6ersity,Leiden,The Netherlands cSmithKline Beecham Pharmaceuticals,Department of Vascular Biology,Harlow,Essex,United Kingdom
Received 12 October 1999; received in revised form 2 March 2000; accepted 10 March 2000
Abstract
Apolipoprotein E (apoE) is a high affinity ligand for several receptor systems in the liver, including the low-density lipoprotein (LDL) receptor, and non-LDL receptor sites, like the LDL receptor-related protein (LRP), the putative remnant receptor and/or proteoglycans. Although the liver is the major source of apoE synthesis, apoE is also produced by a wide variety of other cell types, including macrophages. In the present study, the role of the LDL receptor in the removal of lipoprotein remnants, enriched with macrophage-derived apoE from the circulation, was determined using the technique of bone marrow transplantation (BMT). Reconstitution of macrophage apoE production in apoE-deficient mice resulted in a serum apoE concentration of only 2% of the concentration in wild-type C57Bl/6 mice. This low level of apoE nevertheless reduced VLDL and LDL cholesterol 12-fold (PB0.001) and fourfold (PB0.001), respectively, thereby reducing serum cholesterol levels and the susceptibility to atherosclero-sis. In contrast, reconstitution of macrophage apoE synthesis in mice lacking both apoE and the LDL receptor induced only a twofold (PB0.001) reduction in VLDL cholesterol and had no significant effect on atherosclerotic lesion development, although serum apoE levels were 93% of the concentration in normal C57Bl/6 mice. In conclusion, a functional (hepatic) LDL receptor is essential for the efficient removal of macrophage apoE-enriched lipoprotein remnants from the circulation and thus for normalization of serum cholesterol levels and protection against atherosclerotic lesion development in apoE-deficient mice. © 2001 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Apolipoprotein E; Bone marrow transplantation; mouse apoE ELISA
www.elsevier.com/locate/atherosclerosis
1. Introduction
Chylomicrons and very-low-density lipoproteins (VLDL) are processed by lipoprotein lipase after enter-ing the blood circulation, resultenter-ing in the formation of cholesterol and apolipoprotein E (apoE) enriched lipo-protein remnants [1]. ApoE is a 34 kDa arginine-rich lipoprotein, that serves as a high-affinity ligand for several receptor systems in the liver, including the low-density lipoprotein (LDL) receptor, LDL
receptor-re-lated protein (LRP), proteoglycans, and/or the putative remnant receptor [2 – 7]. Targeted inactivation of the apoE gene in mice is associated with a prominent increase in VLDL and LDL cholesterol levels, indicat-ing that apoE plays an important role in the removal of these lipoproteins from the circulation [8 – 11]. Choi et al. found that an anti-LDL receptor antibody reduced the liver uptake of lipoprotein remnants by 50%, indi-cating that the LDL receptor plays a major role in the clearance of remnant lipoproteins [12]. However, in humans, rabbits, and mice, lacking a functional LDL receptor, mainly LDL accumulates and not remnant lipoproteins [13 – 15]. These data indicate that in vivo back-up receptor systems are present that can
compen-* Corresponding author. Tel.: +31-71-5276238; fax: + 31-71-5276032.
E-mail address:[email protected] (M. Van Eck).
sate for lipoprotein remnant clearance when the LDL receptor is absent. A likely other candidate for lipo-protein remnant clearance is the LRP. LRP is a multi-functional receptor, that binds a broad range of ligands, including apoE-enriched lipoproteins, lipo-protein lipase, and a2-macroglobulin-proteinase
com-plexes [3,4]. A 39 k-Da receptor associated protein (RAP) was also identified that binds with high affinity to the multiple binding sites of LRP, thereby blocking the receptor-ligand interaction [16]. Recently, Willnow et al. demonstrated that transient inactivation of LRP in vivo in wild-type mice by injection of an adenoviral vector containing RAP did not induce an increase in lipoprotein remnants, whereas overexpression of RAP in the liver of LDL receptor-deficient mice resulted in a massive accumulation of VLDL and LDL cholesterol [17]. Furthermore, liver-specific inactivation of LRP in LDL receptor-deficient mice by conditional gene target-ing induced accumulation of mainly LDL cholesterol and to a lesser extent VLDL cholesterol, whereas in wild-type animals this caused a compensatory up regu-lation of the LDL receptor in the liver [18]. Herz et al. further elucidated that the initial hepatic removal of remnant lipoproteins is largely independent of either the LDL receptor or LRP [19]. Endocytosis of surface bound remnants in wild-type mice is predominantly mediated by the LDL receptor and in LDL receptor-deficient animals by slow endocytosis via LRP. The current model for liver uptake of lipoproteins involves two successive stages, including sequestration of lipo-protein remnants by proteoglycans on parenchymal liver cells and subsequent internalization by either the LDL receptor or LRP. For uptake via LRP further enrichment with exogenous apoE during the phase of sequestration is suggested to be required [20,21].
Although the liver is the major source of apoE synthesis, apoE is also produced by a wide variety of other cell types, including macrophages [22 – 24]. Re-cently, by transplantation of wild-type bone marrow into apoE-deficient mice we [25] and others [26 – 28] found that macrophage-derived apoE can reduce hyper-cholesterolemia in apoE-deficient mice due to increased recognition and uptake of lipoprotein remnants by parenchymal liver cells, leading to a decreased suscepti-bility to atherosclerosis.
The aim of the present study was to investigate the role of the (hepatic) LDL receptor in macrophage apoE induced reduction in serum cholesterol levels and atherosclerosis. To address this question, mice solely deficient for apoE and mice deficient for both apoE and LDL receptors are reconstituted with apoE positive
bone marrow. Our results demonstrate that
macrophage-derived apoE is unable to normalize hy-percholesterolemia and decrease susceptibility to atherosclerosis in apoE-deficient mice, lacking the LDL receptor, despite high levels of apoE in the circulation.
Therefore, it is concluded that for efficient clearance of remnant lipoproteins that are enriched with extrahep-atic apoE, a functional (hepextrahep-atic) LDL receptor is essential.
2. Materials and methods
2.1. Animals
ApoE knock-out (apoE− / −.LDLr+ / +), LDL receptor knock-out (apoE+ / +.LDLr− / −), apo-E.LDL receptor double knock-out (apoE− / −
.LDLr− / −), and wild-type C57Bl/6NCrlBR (apoE+ / +.LDLr+ / +) mice were used. ApoE− / −. LDLr+ / + mice were created as previously described [11]. ApoE+ / +.LDLr− / − mice were obtained from the Jacksons Laboratory (Bar Harbour, ME, USA) as mating pairs and were bred in the Gaubius Laboratory, Leiden, The Netherlands. ApoE− / −.LDLr− / −
mice were obtained by cross-breeding of apoE− / −
.LDLr+ / + and apoE+ / +.LDLr− / − mice. All knock-out mice were hybrids between C57Bl/6 and 129 Sv strains (at least three backcrosses to C57Bl/6). C57Bl/6NCrlBR (apoE+ / +.LDLr+ / +) mice were purchased from Broekman Institute B.V., Someren, The Netherlands.
All mice used for bone marrow transplantation ex-periments were housed in sterilized filter-top cages and given free access to food and water. Animals were maintained on sterilized regular chow (SRM-A), con-taining 5.7% (w/
w) fat (Hope Farms, Woerden, The
Netherlands) and drinking water was supplied with antibiotics (83 mg/l ciprofloxacin and 67 mg/l polymyxin B sulfate) and 6.5 g/l sugar.
Animal procedures were performed at the Sylvius Laboratories of the Leiden/Amsterdam Center for Drug Research in accordance with the national laws. All experimental protocols were approved by the Ethics
Committee for Animal Experiments of Leiden
University.
2.2. Irradiation and bone marrow transplantation
Female ApoE− / −.LDLr+ / + and ApoE− / −
prepared by passing the cells through a 30-mm nylon gauze. Irradiated recipients received 1.0×107 bone
marrow cells by intravenous injection into the tail vein.
2.3. Serum cholesterol and triglyceride analysis
After an overnight fasting-period, approximately 100
ml blood was drawn from each individual mouse by tail bleeding. The concentrations of total cholesterol in serum were determined using enzymatic procedures (Boehringer Mannheim, Germany). Precipath (stan-dardized serum; Boehringer Mannheim, Germany) was used as an internal standard.
The distribution of cholesterol over the different lipoproteins in serum was determined by loading of 30
ml serum of each mouse onto a Superose six column (3.2×30 mm, Smart-system, Pharmacia, Uppsala, Swe-den). Serum was fractionated at a constant flow rate of 50 ml/min, using phosphate-buffered saline. Total cholesterol content in the effluent was determined enzymatically.
2.4. Quantification of apoE
ApoE was measured using a sandwich ELISA spe-cific for mouse apoE, as described earlier [25]. Briefly, for determination of apoE, a rabbit-anti-mouse apoE polyclonal antibody (SB Rabbit 67-AH, SmithKline Beecham, Harlow, UK) was used as primary antibody, biotinylated rabbit-mouse apoE polyclonal anti-body was used as secondary antianti-body (SB Rabbit 67-AH-biotin, SmithKline Beecham, Harlow, UK), and finally biotinylated HRP conjugated streptavidin was applied. HRP was detected by incubation with 3,3%,5,5%
-tetramethylbenzidin (TMB; Pierce, USA) for 30 min at room temperature. The reaction was stopped with 2 mol/l H2SO4 and the absorbance was read at 450 nm.
Pooled serum from C57Bl/6 mice, with known apoE level, was used as standard.
2.5. (b)VLDL isolation and characterization
(b)VLDL was isolated from pooled serum of three mice from each transplantation group at 4 weeks after BMT by discontinuous KBr gradient ultracentrifuga-tion at 250 000×g for 18 h, as described by Redgrave et al. [30]. The fraction ofdB1.006 g/ml was isolated, dialyzed against PBS/1 mmol/l EDTA, and subse-quently characterized with respect to the free choles-terol, cholesteryl ester, phospholipid, and triglyceride content, using enzymatic procedures (Boehringer Mannheim, Germany). The protein content was deter-mined according to Lowry et al. [31].
2.6. Histological analysis of hearts and aortas for atherosclerosis
To analyze the development of atherosclerosis, mice were sacrificed at 4 months after BMT. Hearts and aortas were perfused in situ with oxygenated Krebs buffer (37°C, 100 mm Hg) for 20 – 30 min via a cannula in the left ventricle, followed by a post-perfusion fixa-tion with 3.7% neutral-buffered formalin (Formal-fixx, Shandon Scientific, England) and subsequent storage in formalin. To evaluate the development of atheroscle-rotic lesions, the aortas were separated from the hearts. Hearts were bisected at the level of the atria and the base of the heart plus aortic root were taken for analysis. Cryostat 10 mm cross sections of the aortic root were made and stained with oil red O (BDH, England). The atherosclerotic lesion area in the sections was quantified using a light microscope connected with a 24-bits full color video camera and Optimas 6.1 image analysis software (BioScan, Edmonds, WA). Mean le-sion area was calculated (in mm2) from 10 sections,
starting at the appearance of the tricuspid valves as described previously [32].
2.7. Statistical analysis
Statistically significant differences among the means of the different populations were tested by ANOVA. To compare pairs of groups, the Student-Newman-Keuls multiple comparison test was performed after ANOVA.
3. Results
3.1. Effect of reconstitution of macrophage apoE
synthesis in apoE− / −. LDLr+/+ and
apoE− / −.LDLr− / − mice on serum cholesterol le6els
To examine the role of the (hepatic) LDL receptor in the macrophage apoE-induced reduction in serum cholesterol levels and atherosclerosis, apoE knockout (apoE− / −.LDLr+ / +) and apoE.LDLr double knockout (apoE− / −.LDLr− / −) mice were trans-planted with wild-type (apoE+ / +.LDLr+ / +) and LDLr knockout (apoE+ / +.LDLr− / −) bone mar-row, respectively. By using apoE− / −.LDLr+ / +
majority of the LDL receptor activity is found in the liver we will refer to the absence or presence of the (hepatic) LDL receptor for functional simplicity.
The effect on serum cholesterol levels and atheroscle-rosis were determined in time after bone marrow trans-plantation. The effect of reconstitution of apoE− / −.LDLr+ / + and apoE− / −.LDLr− / −
mice with apoE+ / +.LDLr+ / + and apoE+ / +
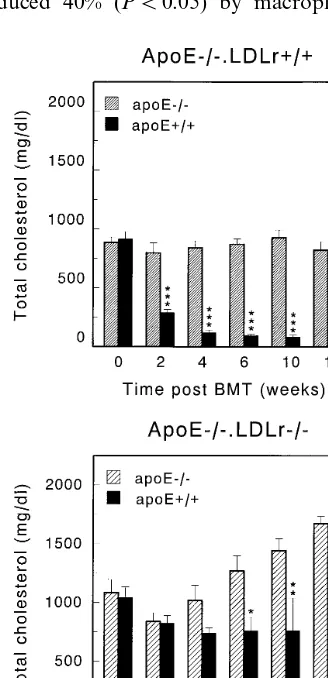
.LDLr− / − bone marrow on serum cholesterol levels is depicted in Fig. 1. As previously reported [25], pro-duction of apoE by macrophages induced a 86% (PB
0.001) reduction in serum cholesterol levels at both 4 and 16 weeks after BMT, indicating that macrophage-derived apoE can promote the clearance of cholesterol-rich lipoproteins in the presence of the LDL receptor (Fig. 1A). Interestingly, in the absence of a functional hepatic LDL receptor (apoE+ / +.LDLr− / −
apoE− / −.LDLr− / −), serum cholesterol levels were only reduced 40% (PB0.05) by macrophage-derived
apoE at 6 weeks after BMT and 31% (PB0.01) at 16 weeks after BMT as compared to control transplanted mice (apoE− / −.LDLr− / −apoE− / −.LDLr− / −; Fig. 1B). Strikingly, both apoE− / −.LDLr− / −
mice, transplanted with apoE− / −.LDLr− / − bone marrow, and apoE− / −.LDLr− / − mice, trans-planted with apoE+ / +.LDLr− / − bone marrow, show a gradual increase in serum cholesterol levels after bone marrow transplantation. Although the exact mechanism for this increase in both groups is unknown, a possible explanation might be down regulation of an alternative uptake route in the absence of the LDL receptor.
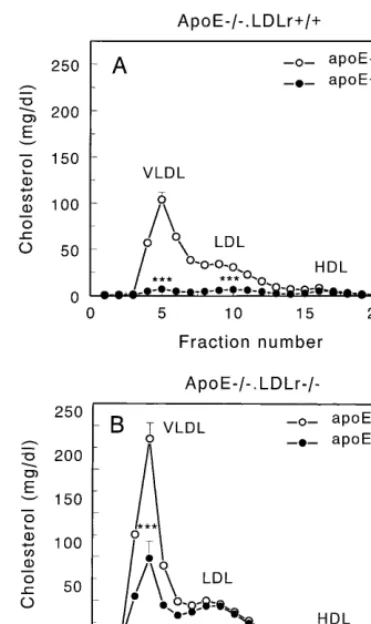
At 16 weeks after BMT, the distribution of choles-terol over the different lipoprotein fractions was deter-mined by liquid chromatography of serum of each individual animal. In control transplanted apoE− / −
.LDLr+ / +apoE− / −.LDLr+ / + mice the ma-jority of cholesterol is transported by VLDL and LDL. Introduction of apoE-producing macrophages in apoE− / −.LDLr+ / + animals induced a large de-crease in VLDL (12-fold; PB0.001) and LDL (four-fold;PB0.001) cholesterol levels, thereby reducing the total serum cholesterol levels dramatically (Fig. 2A). In contrast, introduction of apoE producing macrophages in apoE− / −.LDLr− / − mice resulted only in a two-fold (PB0.001) decrease in serum VLDL cholesterol, whereas no effect could be demonstrated on LDL cholesterol levels (Fig. 2B). Serum VLDL cholesterol levels in control transplanted apoE− / −.LDLr− / − apoE− / −.LDLr− / − mice were approximately twofold higher as compared to apoE− / −.LDLr+ / + apoE− / −.LDLr+ / + mice. In Table 1, a sum-mary is given of the data on the effect of macrophage apoE synthesis in the absence or presence of the (hep-atic) LDL receptor on cholesterol levels.
The effect of BMT on the composition and mean diameter of VLDL, the major cholesterol transporting lipoprotein in apoE− / −.LDLr+ / + and apoE− / −
.LDLr− / − mice was analyzed at 4 weeks after BMT. VLDL from both apoE− / −.LDLr+ / + and apoE− / −.LDLr− / −mice is enriched in cholesteryl esters as compared to triglycerides, due to the impaired clear-ance of these lipoproteins by the liver and the thereby increased circulation time and exposure to lipoprotein lipase. As indicated in Table 2, introduction of apoE-producing monocytes/macrophages in apoE− / −
.LDLr+ / + mice induced a decrease in cholesteryl esters in the VLDL core, while the relative proportion of core triglycerides was increased. Replacement of cholesteryl esters in the VLDL core was found to be associated with an increase in the mean diameter of these particles. These compositional changes of the VLDL core from cholesteryl ester-rich to triglyceririch indicate that the circulation time is largely de-creased. In the absence of a functional LDL receptor,
Fig. 1. Effect of reconstitution of macrophage apoE-synthesis in apoE− / −.LDLr+ / + and apoE− / −.LDLr− / −mice on serum cholesterol levels. After transplantation of either apoE− / −
Fig. 2. Distribution of serum cholesterol over the different lipoprotein fractions in apoE− / −.LDLr+ / + (panel A) and apoE− / −
.LDLr− / − (panel B) mice, transplanted with apoE− / − or apoE+ / + bone marrow at 16 weeks after BMT. Blood samples were drawn by tail bleeding after an overnight fasting period. A 30ml aliquot of serum of each individual mouse was loaded onto a Superose six column and fractions were collected. Fractions 3 – 7 represent VLDL and chylomicrons, fractions 8 – 14 LDL, and frac-tions 15 – 19 HDL. Open circles () represent transplantation with apoE− / −bone marrow and closed circles ( ) transplantation with apoE+ / + bone marrow. Values are means9SEM of 4 – 7 mice. Statistically significant difference ***PB0.001 versus control trans-planted mice.
Table 2
Percentual lipid composition ofdB1.006 lipoproteins, isolated at 4 weeks after BMTa
ApoE−/−.LDLr+/+ ApoE−/−.LDLr−/−
Recipient
ApoE−/− ApoE+/+ ApoE−/− ApoE+/+
Donor
%of total composition
Free 9.2 5.2 7.9 9.3
cholesterol
5.2 25.6
32.4 23.3
Cholesterol esters
68.6 32.4
Triglycerides 38.1 44.1
Phospholipids 16.4 16.0 17.3 15.9
9.7 5.2 11.0
Proteins 7.2
mean particle diameter(nm) 47910.5 74.7920.1
Diameter 49.0910.6 57.7912.2
aAt 4 weeks after BMT, the effect of reconstitution of macrophage apoE synthesis in apoE−/−.LDLr+/+and apoE−/−.LDLr−/−
mice on the composition and mean diameter was determined of (b)VLDL isolated from pooled serum of six mice.
however, no change in either the ratio of triglycerides and cholesteryl esters or mean particle diameter was observed.
3.2. Effect of reconstitution of macrophage apoE
synthesis in apoE− / −. LDLr+/+ and
apoE− / −.LDLr− / − mice on serum apoE le6els
Since the availability of macrophage-derived apoE to lipoproteins in the circulation may be of critical impor-tance for uptake via several receptor systems in the liver, serum apoE levels were measured at 4 weeks after transplantation in the different transplantation groups using an ELISA, specific for mouse apoE. In apoE+ / +.LDLr+ / +apoE− / −.LDLr+ / + mice serum apoE levels reached a steady state concentration of 0.1390.028 mg/dl, which is :2% of the concentration
Table 1
Summary of the effects of macrophage versus hepatic LDL receptor expression or apoE synthesis on cholesterol levels
ApoE−/−.LDLr+/+ ApoE+/+.LDLr+/+ ApoE−/−.LDLr−/− apoE+/+.LDLr−/− apoE+/+.LDLr−/−[29] Donor
ApoE−/−.LDLr+/+ ApoE−/−.LDLr+/+ ApoE−/−.LDLr−/− apoE−/−.LDLr−/−
Recipient apoE+/+.LDLr−/−
+ −
+ +
−
Macrophage apoE
−
− +
−
Hepatic apoE −
+ +
Macrophage LDLr − − −
+ +
Hepatic LDLr − − −
926966 85917
Total cholesterol (mg/dl) 14449100 7639277 365916
VLDL (%) 74.8 37.9 85.0 70.0 3.9
24.8 45.1
LDL (%) 22.4 35.7 13.4
41.4 5.2
1.5 26.4
in wild-type C57Bl/6 mice. However, in the absence of a functional (hepatic) LDL receptor in apoE+ / +
.LDLr− / −apoE− / −.LDLr− / − mice, serum apoE levels increased to 6.6790.47 mg/dl, which is
:93% of the concentration in wild-type C57Bl/6 mice. No further increase in serum apoE concentrations was observed at later time points after transplantation, indi-cating that no further accumulation of lipoproteins is occurring, which correlates with the steady cholesterol levels in the circulation.
3.3. Effect of reconstitution of macrophage apoE
synthesis in apoE− / −. LDLr+/+ and
apoE− / −.LDLr− / − mice on atherosclerosis
Macrophage-derived apoE may not only function as a high affinity ligand for uptake by several receptor systems in the liver; local enrichment of lipoproteins with extrahepatic apoE in the vessel wall may also influence the uptake by macrophages, thereby influenc-ing the atherosclerotic process [34,35]. To investigate
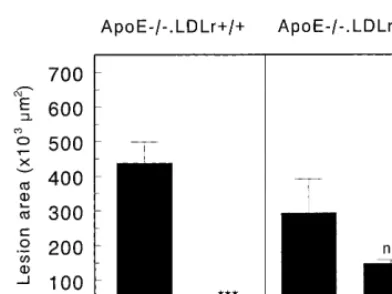
Fig. 4. Effect of reconstitution of macrophage apoE-synthesis in apoE− / −.LDLr+ / + and apoE− / −.LDLr− / − mice on sus-ceptibility to atherosclerosis. The mean atherosclerotic lesion area was calculated from oil red O-stained cross sections of the aortic root at the level of the tricuspid valves. Values indicate the mean atherosclerotic lesion area of 10 cross sections of the aortic root in each mouse. Statistically significant difference ***PB0.001 versus control transplanted mice.
ing that hepatic production of apoE and subsequent local enrichment of lipoproteins with apoE is crucial for the LRP-mediated uptake of lipoprotein remnants [36]. The present study demonstrates that macrophage-derived apoE is unable to normalize hypercholes-terolemia and significantly decrease the susceptibility to atherosclerosis in apoE-deficient mice, lacking the LDL receptor, despite high levels of apoE in the circulation. Therefore, it is concluded that for efficient clearance of remnant lipoproteins, associated with extrahepatically produced apoE, expression of functional LDL recep-tors in the liver is crucial.
The role of the (hepatic) LDL receptor in the macrophage apoE-induced reduction in serum choles-terol levels and atherosclerosis was investigated by com-parison of the effect of reconstitution of macrophage apoE production in apoE− / −.LDLr+ / + and apoE− / −.LDLr− / − mice by bone marrow trans-plantation. Bone marrow transplantation is a powerful tool to investigate the role of apoE production in hematopoietic stem cell-derived cells. ApoE is produced by monocytes and macrophages, but not by other hematopoietic cells [23,24]. Therefore, using bone mar-row transplantation, apoE production can be specifi-cally modified in monocytes and macrophages. Although several recent reports have proposed that endothelial cells [37,38], muscle cells [39], and hepatic oval cells [40] can also be bone marrow-derived, these processes do not play a role in our studies, as induction of differentiation of bone marrow to these cell types requires acute, severe injury to the tissues involved.
As previously reported [25], reconstitution of macrophage apoE production in apoE− / −.LDLr+ / + mice resulted in normalization of hypercholes-terolemia and protection against atherosclerosis, at serum apoE levels of only 2% of the concentration in wild-type mice. Recent data of Thorngate and Williams demonstrated that this ability is not restricted to macrophage-derived apoE, as apoE production by the adrenal gland was also able to normalize serum choles-terol levels in apoE-deficient mice [41]. However, in the absence of functional (hepatic) LDL receptors, macrophage-derived apoE was unable to normalize hy-percholesterolemia and prevent atherosclerosis develop-ment in these mice, although serum apoE levels were increased to 93% of the level in C57Bl/6 mice. From these results it can be concluded that expression of LDL receptors in the liver is crucial for the efficient clearance of remnant lipoproteins, associated with ex-trahepatically produced apoE. In these apoE+ / +
.LDLr− / −apoE− / −.LDLr− / − mice a small decrease in serum cholesterol levels due to a reduction in VLDL cholesterol was observed, indicating that in addition to the LDL receptor, although less important, a non-LDL receptor system also can recognize lipo-protein remnants loaded with macrophage-derived apoE.
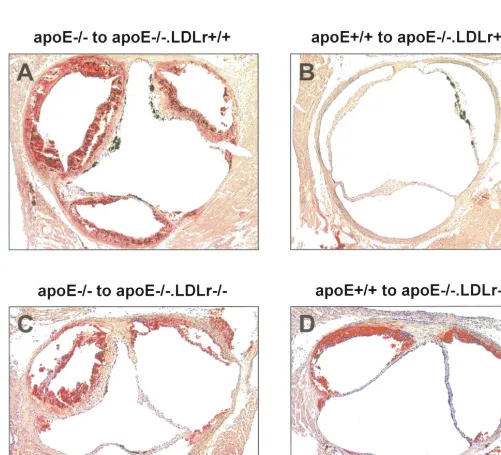
the effect of macrophage apoE production on atherosclerotic lesion development in the absence or presence of the LDL receptor, the mean atherosclerotic lesion area was determined in the transplanted animals. The hearts and aortas were perfused, fixed, and exam-ined histologically at 4 months after BMT. Representa-tive photomicrographs of lesions in cross sections of the different transplantation groups stained for lipid with oil red O are shown in Fig. 3. Quantification of the mean atherosclerotic lesion area in the aortic root revealed that macrophage-derived apoE induced a 17-fold reduction in the mean atherosclerotic lesion area from 437961×103 mm2 (n=11; mean9SEM) to
25916×103 mm2 (n=4; mean9SEM, PB0.001) in
apoE− / −.LDLr+ / + mice (Fig. 4). In the absence of functional LDL receptors, however, no significant reduction in the mean atherosclerotic lesion area was observed in apoE+ / +.LDLr− / −apoE− / −
7; mean9SEM) animals. Although there was a tendency to slightly smaller lesions, which correlates with the observed small decrease in serum cholesterol levels, the effect is not comparable to the 17-fold reduc-tion in the presence of funcreduc-tional (hepatic) LDL recep-tors.
4. Discussion
accumulat-In a recent report of Linton et al. [42], it was demonstrated that reconstitution of macrophage apoE-synthesis in apoE− / −.LDLr− / − mice had no ef-fect on serum cholesterol levels, although plasma apoE levels increased to levels of 4 – 16 times higher than the level in wild-type C57Bl/6 mice. The increasing serum apoE levels and the continuous high levels of choles-terol indicate that in their study remnant lipoproteins are not cleared from the circulation at all. In contrast, in our study we did observe a small reduction in serum cholesterol levels, while serum apoE concentra-tions were comparable to the level in wild-type C57Bl/
6 mice and did not increase in time, indicating that no further accumulation of remnant lipoproteins occurred in our study. At the moment it is unclear what causes this difference in response upon reconstitution of macrophage apoE production in apoE− / −.LDLr− / − mice. Two possible mechanisms can be distin-guished: 1) Linton et al. observed levels of apoE of 4 – 16 times the triglyceride level in wild-type mice, which might inhibit lipolysis and thus VLDL clearance [43,44] and 2) Variation in background genes between the mice of both institutes [45,46].
In apoE+ / +.LDLr− / − mice, apoE-containing lipoproteins can still bind to LRP, proteoglycans, and the putative remnant receptor, and hence no dramatic accumulation of lipoprotein remnants occurs [15]. In contrast, in apoE− / −.LDLr+ / + mice, apoE, a high affinity ligand for several binding sites in the liver, is absent, resulting in a profound accumulation of lipoprotein remnants [8 – 11]. Reconstitution of macrophage apoE production rescued these apoE− / −.LDLr+ / + mice from hypercholesterolemia and development of premature atherosclerosis [25 – 28]. Re-constitution of macrophage apoE production in apoE− / −.LDLr− / − mice, however, induced only a small decrease in serum VLDL cholesterol levels, despite normal serum apoE concentrations. It can be postulated that the decreased ability of macrophage-derived apoE to reduce serum cholesterol levels in the absence of the LDL receptor is either due to insuffi-cient levels of apoE on lipoprotein remnant particles or by the necessity of production of apoE by par-enchymal liver cells to trigger uptake by non-LDL receptor pathways, including LRP. In Table 1, in which a summary is given of the data obtained in this study, data of a previous study by our group, per-formed under the same conditions [29] are also in-cluded, thereby clearly demonstrating the effects of macrophage versus hepatic LDL receptor expression and apoE synthesis on serum cholesterol levels. Comparison of our recent data with the previously published data [29] reveals that in apoE+ / +
.LDLr− / −apoE+ / +.LDLr− / − transplanted mice with apoE production by liver, macrophages, and other extrahepatic tissues, serum cholesterol levels are
highly decreased as compared
to apoE+ / +.LDLr− / −apoE− / −.LDLr− / −
mice due to a complete normalization of VLDL cholesterol levels. These data indicate that, at least for VLDL uptake by the liver, hepatic apoE production plays an important role in the absence of the hepatic LDL receptor. In contrast, Ishibashi et al. previously demonstrated that feeding LDL receptor deficient mice a high cholesterol diet induces a profound increase in lipoprotein remnants, despite abundant levels of apoE in the circulation and production of apoE by par-enchymal liver cells [47].
Recently, Rensen et al. demonstrated that particle size is an important determinant for the affinity of lipoprotein remnant-like particles for the LDL recep-tor in vivo [48]. ApoE-containing emulsions with a mean diameter of :150 nm were taken up by the liver via a non-LDL receptor-dependent pathway, whereas small apoE-containing emulsions with a mean diameter of 50 nm were solely taken up via an LDL receptor-dependent mechanism. Windler et al. demon-strated that the rate of removal of small chylomicron remnants by isolated perfused rat livers followed closely the activity of the LDL receptor, whereas the uptake of large chylomicrons was unaffected by changes of LDL receptor activity [49]. Determination of the mean diameter of VLDL-sized lipoproteins, isolated from serum of apoE− / −.LDLr− / −
apoE− / −.LDLr− / − mice and apoE+ / +
.LDLr− / −apoE− / −.LDLr− / − mice, revealed that the circulating lipoprotein remnants were small with a mean diameter of :50 nm. Thus, uptake of these lipoprotein remnants by the liver is expected to be mainly LDL receptor-dependent. This is in agree-ment with our observation that expression of the hep-atic LDL receptor is crucial for macrophage apoE-mediated normalization of hypercholesterolemia and protection against atherosclerosis in apoE-defi-cient mice.
In conclusion, a functional (hepatic) LDL receptor is essential for the efficient removal of small lipo-protein remnants that are enriched with extrahepatic apoE from the circulation and thus for the normaliza-tion of serum cholesterol levels and protecnormaliza-tion against atherosclerotic lesion development in apoE-deficient mice.
Acknowledgements
References
[1] Windler E, Chao Y-S, Havel RJ. Determinants of hepatic uptake of triglyceride-rich lipoproteins and their remnants in the rat. J Biol Chem 1980;255:5475 – 80.
[2] Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 1988;240:622 – 30. [3] Moestrup SK. The a2-macroglobulin receptor and epithelial
glycoprotein-330: two giant receptors mediating endocytosis of multiple ligands. Biochim Biophys Acta 1994;1197:197 – 213. [4] Krieger M, Herz J. Structures and functions of multiligand
receptors: macrophage scavenger receptors and LDL receptor related protein (LRP). Annu Rev Biochem 1994;62:601 – 37. [5] Van Dijk MCM, Ziere GJ, Boers W, Linthorst C, Bijsterbosch
MK, Van Berkel ThJC. Recognition of chylomicron remnants and b-migrating very-low-density lipoproteins by the remnant receptor of parenchymal liver cells is distinct from the liver a2-macroglobulin-recognition site. Biochem J 1991;279:863 – 70. [6] Ziere GJ, Van der Kaaden ME, Vogelezang CJM, Boers W,
Bihain BE, Kuiper J, Kruijt JK, Van Berkel ThJC. Blockade of the a2-macroglobulin receptor/ low-density-lipoprotein-receptor-related protein on rat liver parenchymal cells by the 39-kDa receptor-associated protein leaves the interaction ofb-migrating very-low-density lipoprotein with the lipoprotein remnant recep-tor unaffected. Eur J Biochem 1996;242:703 – 11.
[7] De Faria E, Fong LG, Komaromy M, Cooper AD. Relative roles of the LDL receptor, the LDL receptor-like protein, and hepatic lipase in chylomicron remnant removal by the liver. J Lipid Res 1996;37:197 – 209.
[8] Ghiselli G, Schaefer EJ, Gascon P, Brewer HB, jr. Type III hyperlipoproteinemia associated with apolipoprotein E defi-ciency. Science 1981;214:1239 – 41.
[9] Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 1992;258:468 – 71.
[10] Plump AS, Smith JD, Hayek T, et al. Severe hypercholes-terolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 1992;71:343 – 53.
[11] Van Ree JH, Van den Broek WJAA, Dahlmans VEH, et al. Diet-induced hypercholesterolemia and atherosclerosis in het-erozygous apolipoprotein E-deficient mice. Atherosclerosis 1994;111:25 – 37.
[12] Choi SY, Fong LG, Kirven MJ, Cooper AD. Use of an anti-low density lipoprotein receptor antibody to quantify the role of the LDL receptor in the removal of chylomicron remnants in the mouse in vivo. J Clin Invest 1991;88:1173 – 81.
[13] Rubinsztein DC, Cohen JC, Berger GM, Van der Westhuyzen DR, Coetzee GA, Gevers W. Chylomicron remnant clearance from the plasma is normal in familial hypercholesterolemic homozygotes with defined receptor defects. J Clin Invest 1990;86:1306 – 12.
[14] Kita T, Goldstein JL, Brown MS, Watanabe Y, Hornick CA, Havel RJ. Hepatic uptake of chylomicron remnants in WHHL rabbits: a mechanism genetically distinct from the low density lipoprotein receptor. Proc Natl Acad Sci USA 1982;79:3623 – 7. [15] Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest 1993;92:883 – 93.
[16] Herz J, Goldstein JL, Strickland DK, Ho YK, Brown MS. 39-kDa protein modulates binding of ligands to low density lipoprotein receptor-related protein/a2-macroglobulin receptor. J Biol Chem 1991;266:21232 – 8.
[17] Willnow TE, Sheng Z, Ishibashi S, Herz J. Inhibition of hepatic chylomicron remnant uptake by gene transfer of a receptor antagonist. Science 1994;264:1471 – 4.
[18] Rohlmann A, Gotthardt M, Hammer RE, Herz J. Inducible inactivation of the hepatic LRP gene by cre-mediated recombi-nation confirms role of LRP in clearance of chylomicron rem-nants. J Clin Invest 1998;101:689 – 95.
[19] Herz J, Qiu S-Q, Oesterle A, DeSilva HV, Shafi S, Havel RJ. Initial hepatic removal of chylomicron remnants is unaffected but endocytosis is delayed in mice lacking the low density lipoprotein receptor. Proc Natl Acad Sci USA 1995;92:4611 – 5. [20] Kowal RC, Herz J, Goldstein JL, Esser V, Brown MS. Low density lipoprotein receptor-related protein mediates uptake of cholesteryl esters derived from apoprotein E-enriched lipo-proteins. Proc Natl Acad Sci USA 1989;86:5810 – 4.
[21] Ji Z-S, Fazio S, Lee Y-L, Mahley RW. Secretion-capture role for apolipoprotein E in remnant lipoprotein metabolism involving cell surface heparan sulfate proteoglycans. J Biol Chem 1994;269:2764 – 72.
[22] Elshourbagy NA, Liao WS, Mahley RW, Taylor JM. Apolipo-protein mRNA is abundant in the brain and adrenals, as well as in the liver, and is present in other peripheral tissues of rats and marmosets. Proc Natl Acad Sci USA 1985;82:203 – 7.
[23] Lin C-T, Xu JJ, Chan L. Immunoreactive apolipoprotein E is a widely distributed cellular protein. Immunohistochemical locali-sation of apolipoprotein E in baboon tissues. J Clin Invest 1986;78:947 – 58.
[24] Basu SK, Brown MS, Ho YK, Havel RJ, Goldstein JL. Mouse macrophages synthesize and secrete a protein resembling apolipoprotein E. Proc Natl Acad Sci USA 1981;78:7545 – 9. [25] Van Eck M, Herijgers N, Yates J, Pearce NJ, Hoogerbrugge
PM, Groot PHE, Van Berkel ThJC. Bone marrow transplanta-tion in apolipoprotein E-deficient mice. Effect of apoE gene dosage on serum lipid concentrations, (b)VLDL catabolism, and atherosclerosis. Arterioscler Thromb Vasc Biol 1997;17:3117 – 26. [26] Linton MF, Atkinson JB, Fazio S. Prevention of atherosclerosis in apolipoprotein E-deficient mice by bone marrow transplanta-tion. Science 1995;267:1034 – 7.
[27] Fazio S, Linton MF. Murine bone marrow transplantation as a novel approach to studying the role of macrophages in lipo-protein metabolism and atherogenesis. Trends Cardiovasc Med 1996;6:58 – 65.
[28] Boisvert WA, Spangenberg J, Curtiss LK. Treatment of severe hypercholesterolemia in apolipoprotein E-deficient mice by bone marrow transplantation. J Clin Invest 1995;96:1118 – 24. [29] Herijgers N, Van Eck M, Groot PHE, Hoogerbrugge PM, Van
Berkel ThJC. Effect of bone marrow transplantation on lipo-protein metabolism and atherosclerosis in LDL receptor-knock-out mice. Arterioscler Thromb Vasc Biol 1997;17:1995 – 2003. [30] Redgrave TG, Roberts DCK, West CE. Separation of plasma
lipoproteins by density-gradient ultracentrifugation. Anal Biochem 1975;65:42 – 9.
[31] Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265 – 75.
[32] Groot PHE, Van Vlijmen BJM, Benson GM, Hofker MH, Schiffelers R, Vidgeon-Hart M, Havekes LM. Quantitative as-sessment of aortic atherosclerosis in apoE*3 Leiden transgenic mice and its relation to serum cholesterol exposure. Arterioscler Thromb Vasc Biol 1996;16:926 – 33.
[33] Brown MS, Goldstein JL. Lipoprotein receptors in the liver. Control signals for plasma cholesterol traffic. J Clin Invest 1983;72:743 – 7.
[34] Shimano H, Ohsuga J, Shimada M, et al. Inhibition of diet-in-duced atheroma formation in transgenic mice expressing apolipoprotein E in the arterial wall. J Clin Invest 1995;95:469 – 76.
[36] Brown MS, Herz J, Kowal RC, Goldstein JL. The low density lipoprotein receptor-related protein: double agent or decoy? Curr Opin Lipidol 1991;2:65 – 72.
[37] Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964 – 7.
[38] Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial pro-genitor cells for neovascularization. Nat Med 1999;5:434 – 8. [39] Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E,
Stor-naiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science 1998;279:1528 – 30.
[40] Petersen BE, Bowen WC, Patrene KD, et al. Bone marrow as a potential source of hepatic oval cells. Science 1999;284:1168 – 70. [41] Thorngate FE, Williams DL. The minimum concentration of apolipoprotein E required to maintain cholesterol homeostasis in apolipoprotein E knockout mice. Circulation 1998;17:1 – 305. [42] Linton MF, Hasty AH, Babaev VR, Fazio S. Hepatic apoE
expression is required for remnant lipoprotein clearance in the absence of the low density lipoprotein receptor. J Clin Invest 1998;101:1726 – 36.
[43] Rensen PCN, Van Berkel ThJC. Apolipoprotein E effectively inhibits lipoprotein lipase-mediated lipolysis of chylomicron-like triglyceride-rich emulsions in vitro and in vivo. J Biol Chem 1996;271:14791 – 9.
[44] Huang Y, Liu XQ, Rall SC, jr, Mahley RW. Apolipoprotein E2 reduces the low density lipoprotein level in transgenic mice by impairing lipoprotein lipase-mediated lipolysis of triglyceride-rich lipoproteins. J Biol Chem 1998;273:17483 – 90.
[45] Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development 1995;121:1845 – 54.
[46] Kirikae T, Kodama T, Kirikae F, Suzuki H, Nakano M. The role of scavenger receptors in LPS-induced macrophage activa-tion. Prog Clin Biol Res 1998;397:97 – 105.
[47] Ishibashi S, Herz J, Maeda N, Goldstein JL, Brown MS. The two-receptor model of lipoprotein clearance: tests of the hypoth-esis in ‘knockout’ mice lacking the low density lipoprotein receptor, apolipoprotein E, or both lipoproteins. Proc Natl Acad Sci USA 1994;91:4431 – 5.
[48] Rensen PCN, Herijgers N, Netscher MH, Meskers SCJ, Van Eck M, Van Berkel ThJC. Particle size determines the specificity of apolipoprotein E-containing triglyceride-rich emulsions for the LDL receptor versus hepatic remnant receptor in vivo. J Lipid Res 1997;38:1070 – 84.
[49] Windler E, Greeve J, Robenek H, Rinninger F, Greten H, Ja¨ckle S. Differences in the mechanisms of uptake and endocytosis of small and large chylomicron remnants by rat liver. Hepatology 1996;24:344 – 51.