High serum concentrations of soluble E-selectin in patients with
impaired glucose tolerance with hyperinsulinemia
Kazunari Matsumoto *, Seibei Miyake, Mayumi Yano, Yukitaka Ueki,
Yuko Tominaga
Department of Internal Medicine,Sasebo Chuo Hospital,15Yamato-cho,Sasebo,Nagasaki857-1195,Japan
Received 28 June 1999; received in revised form 27 October 1999; accepted 6 December 1999
Abstract
High serum concentrations of soluble adhesion molecules are present in diabetics, but whether similar levels are present in patients with impaired glucose tolerance (IGT) is unclear. We measured serum concentrations of soluble intercellular adhesion molecule-1 (sICAM-1), vascular cell adhesion molecule-1 (sVCAM-1), and sE-selectin in 128 nondiabetic Japanese subjects. The concentrations of sICAM-1, sVCAM-1, and sE-selectin in IGT patients (n=47) were not different from those in subjects with normal glucose tolerance (NGT;n=81). IGT patients were subdivided into two groups by the results of 75 g OGTT, those with low- (hypoinsulinemia;n=23) or high-insulin (hyperinsulinemia;n=24). The levels of sICAM-1 and sVCAM-1 were not different among NGT and IGT with high-insulin or with low-insulin. However, sE-selectin concentrations were significantly higher in IGT patients with high-insulin than in NGT and IGT with low-insulin (61.193.4, 47.191.8 and 43.793.9 ng/ml, respectively,
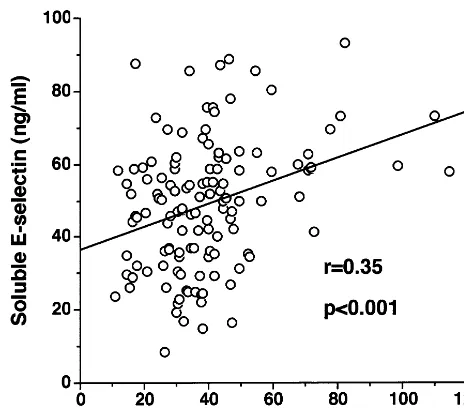
PB0.001). Adjustment for age and gender did not influence the results. Serum sE-selectin concentrations correlated significantly with the area under the curve of insulin (AUCinsulin), AUCglucose, diastolic blood pressure, and triglyceride levels (r=0.35, 0.26,
0.18 and 0.21, respectively), and negatively with HDL-cholesterol levels (r= −0.20). Multiple regression analysis showed that AUCinsulin was the only independent factor that correlated with sE-selectin levels (PB0.001). Our results indicate that
hyperinsulinemia/insulin resistance may be responsible for the elevation of sE-selectin levels. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Adhesion molecule; E-selectin; Impaired glucose tolerance; Hyperinsulinemia; Insulin resistance
www.elsevier.com/locate/atherosclerosis
1. Introduction
Adhesion of leucocytes to arterial endothelial cells and subsequent transendothelial infiltration is thought to be an important step in the development of atherosclerosis [1 – 3]. This process depends on a group of receptors and binding proteins, i.e. adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin [1,2]. Recent studies have reported the presence of high serum concentrations of soluble adhesion molecules (sICAM-1, sVCAM-1, and sE-se-lectin) in patients with type 2 diabetes [4 – 7]. Moreover, the levels of sE-selectin correlate positively with the degree of hyperglycemia [8 – 10]. Despite the association
between increased soluble adhesion molecules and clini-cally overt diabetes mellitus, it is not clear whether increased concentrations of soluble adhesion molecules are present in patients with impaired glucose tolerance (IGT).
IGT is often complicated with dyslipidemia, hyper-tension, and obesity, and is characterized by insulin resistance [11]. Insulin resistance and compensatory hyperinsulinemia may play a central role in the multi-faceted clustering syndrome, and accelerate the devel-opment of atherosclerosis [11 – 13]. Such insulin resistance is common in patients with IGT among Pima Indians and Hispanics [14,15]. However, some Japanese patients with IGT do not necessarily show hyperinsu-linemia/insulin resistance [16]. Taniguchi et al. [17] re-ported two subtypes of Japanese patients with IGT; those who are insulin sensitive and others who are insulin resistant. The relationship between concentra-* Corresponding author. Tel.: +81-956-337151; fax: +
81-956-343241.
tions of soluble adhesion molecules and hyperinsuline-mia is also not clear at present.
Therefore, we measured sICAM-1, sVCAM-1, and sE-selectin in Japanese patients with IGT with respect to hyperinsulinemia.
2. Research design and methods
A total of 150 Japanese subjects gave their informed consent to participate in this cross-sectional study. All subjects visited the Health Care Center of the Sasebo Chuo Hospital for health checks of lifestyle related diseases (e.g. diabetes, hypertension and hyperlipi-demia). Excluded from the study were patients who had been already diagnosed with diabetes mellitus, those with chronic heart or renal failure, ischemic heart dis-ease, stroke, peripheral obstructive artery disdis-ease, en-docrine disease or intercurrent infections. Each patient completed a medical questionnaire, and physical check up, routine collection of biochemical data, and a 75 g oral glucose tolerance test (OGTT) were performed. Blood pressure was measured with a standard mercury sphygmomanometer after at least 10 min of rest.
A 75 g OGTT was performed after an overnight fast. Subjects ingested carbohydrate equivalent to 75 g of glucose (Tolelan-G, Shimizu, Japan), and blood sam-ples were withdrawn at 0, 30, 60, 90 and 120 min.
Based on the results of OGTT, 22 subjects were found to have diabetes and accordingly were excluded from the study. The remaining 128 subjects were classified into two groups according to their glucose tolerance status as those with normal glucose tolerance (NGT; n=81) or impaired glucose tolerance (IGT; n=47). The definition of glucose tolerance was based on the report of the Expert Committee of American Diabetes Association [18]. In this study, patients with impaired fasting glucose were included among those with IGT. IGT patients were subdivided into two classes; IGT with high-insulin and IGT with low-in-sulin. Area under the curve of insulin during OGTT (AUCinsulin) over median level (39.48 nmol min per l) in
IGT patients was defined as high-insulin (hyperinsuline-mia; n=24), and AUCinsulin below median level was
defined as low-insulin (hypoinsulinemia; n=23). AUCglucoseand AUCinsulinwere calculated by the
trape-zoidal methods.
Plasma glucose was measured in duplicate with an automatic analyzer (Kyoto-Daiichi-Kagaku, Kyoto, Japan) by the glucose oxidase method. Intra- and in-terassay coefficients of variations (CVs) were 1.5%. Immunoreactive insulin was measured by a commercial radioimmunoassay (RIA) kit (Shionogi, Osaka, Japan). The intra- and interassay CVs were less than 5%. Total cholesterol and triglyceride were measured by enzy-matic methods (Kokusai Shiyaku, Kobe, Japan).
HDL-cholesterol was determined after isolation by the precipitation method (Kyowa, Tokyo, Japan). CVs for intra- and interassay in total cholesterol, triglyceride, and HDL-cholesterol were less than 3%.
Serum concentrations of sICAM-1, sVCAM-1, and sE-selectin were measured by a commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Abington, UK). The intra- and interas-say CVs were less than 6%.
3. Statistical analysis
Unpaired data were analyzed by the x2
test or Stu-dent’s t-test. Multiple comparisons were conducted by one-way analysis of variance (ANOVA) with post hoc Bonferroni test. Analysis of covariance (ANCOVA) and contrasts were used to compare data after adjust-ment for other variables. The relationship between sE-selectin concentrations and other variables was estimated by Pearson’s correlation coefficients. To de-termine the independent factor(s) related to sE-selectin concentrations, we performed the multiple regression analysis. Data were presented as means9SE. Differ-ences were considered statistically significant at PB 0.05. Statistical analysis was performed using Statview 4.5 or Super ANOVA (Abacus, Berkeley, CA) software package.
4. Results
4.1. Clinical characteristics
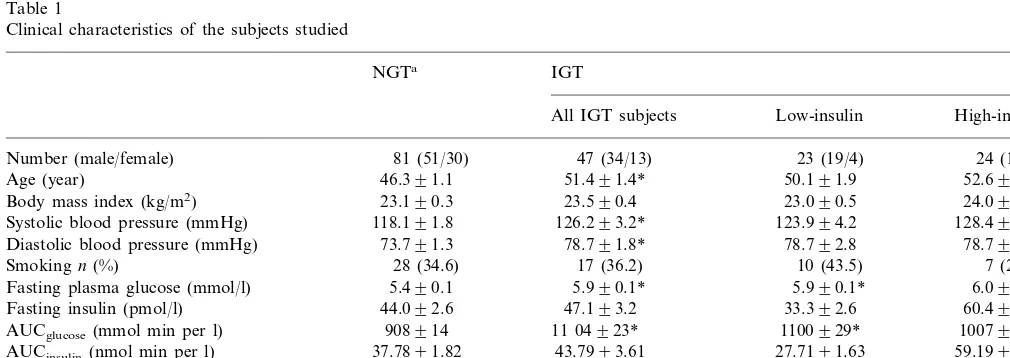
Table 1 shows the clinical characteristics of patients with NGT and IGT. Patients with IGT were signifi-cantly older than NGT subjects and had a higher blood pressure, fasting plasma glucose, and serum triglyceride concentrations. However, body mass index, the percent-age of smokers among each group, fasting insulin con-centrations, and total- and HDL-cholesterol concentrations were comparable between IGT and NGT. When patients with IGT were subdivided into two groups based on the levels of insulin, patients with high-insulin showed significant fasting and post glucose challenge hyperinsulinemia. This result suggested the presence of insulin resistance in patients with high-insulin.
4.2. Serum concentration of soluble adhesion molecules
statis-tical significance. We also investigated the effects of hyperinsulinemia/insulin resistance on the serum con-centrations of soluble adhesion molecules. Soluble ICAM-1 levels in NGT, IGT with low-insulin, and IGT with high-insulin were comparable by ANOVA (P= 0.308, Table 2). Soluble VCAM-1 levels in IGT with high-insulin tended to be higher than in NGT and IGT with low-insulin albeit insignificantly (P=0.174, Table 2). On the other hand, sE-selectin levels in IGT with high-insulin were significantly higher than in NGT and IGT with low-insulin (PB0.001, Table 2). Adjustments for age and gender by ANCOVA did not influence these results.
4.3. Relationship between sE-selectin and other 6ariables
In the simple correlation coefficients, age, body mass index, AUCglucose, AUCinsulin, systolic blood pressure,
diastolic blood pressure, total cholesterol, triglyceride, and HDL-cholesterol were entered as independent
vari-ables. Serum concentrations of sE-selectin significantly correlated with AUCglucose (r=0.26, P=0.003),
AUCinsulin(r=0.35,PB0.001), diastolic blood pressure
(r=0.18, P=0.042), and triglyceride (r=0.21, P= 0.018). The levels of sE-selectin also significantly but negatively correlated with HDL-cholesterol (r= − 0.20, P=0.021). The correlation between AUCinsulin
and sE-selectin levels is depicted in Fig. 1. To determine the independent factor(s) related to sE-selectin levels, we performed multiple regression analysis by entering AUCglucose, AUCinsulin, diastolic blood pressure,
triglyc-eride, and HDL-cholesterol as independent variables. Multiple regression analysis identified AUCinsulinas the
only independent factor that determined sE-selectin levels (b=0.353, F=17.98;PB0.001).
5. Discussion
In the present study, we demonstrated the presence of high serum concentrations of sE-selectin in Japanese
Table 1
Clinical characteristics of the subjects studied
NGTa IGT
High-insulin Low-insulin
All IGT subjects
81 (51/30) 47 (34/13) 23 (19/4) 24 (15/9) Number (male/female)
46.391.1 51.491.4*
Age (year) 50.191.9 52.691.9*
23.090.5
23.190.3 23.590.4 24.090.5
Body mass index (kg/m2)
126.293.2* 123.994.2 128.494.9* Systolic blood pressure (mmHg) 118.191.8
78.791.8* 78.792.8 78.792.5
Diastolic blood pressure (mmHg) 73.791.3
7 (29.1) 10 (43.5)
17 (36.2)
Smokingn(%) 28 (34.6)
5.990.1*
5.490.1 5.990.1* 6.090.1*
Fasting plasma glucose (mmol/l)
44.092.6 47.193.2
Fasting insulin (pmol/l) 33.392.6 60.494.3†,*
AUCglucose(mmol min per l) 908914 11 04923* 1100929* 1007935* 27.7191.63
43.7993.61 59.1995.25†,*
37.7891.82 AUCinsulin(nmol min per l)
Total cholesterol (mmol/l) 5.1490.10 5.4390.14 5.2890.21 5.5890.18
Triglyceride (mmol/l) 1.1590.06 1.5690.11* 1.4990.16 1.6290.17*
1.4590.05 1.3990.05 1.4290.07 1.3690.05
HDL-cholesterol (mmol/l)
aNGT, normal glucose tolerance; IGT, impaired glucose tolerance. AUC, area under the curve during OGTT. Data aren, means9SE, orn (%).
*PB0.05 versus NGT.
†PB0.05 versus IGT-hyperinsulinemia (−).
Table 2
Serum concentrations of soluble ICAM-1, VCAM-1, and E-selectin in subjects with NGT and patients with IGTa
NGT IGT
High-insulin Low-insulin
All IGT subjects
204.099.3
slCAM-1 (ng/ml) 220.0910.5 232.9914.4 207.6915.2
711.0917.4
sVCAM-1 (ng/ml) 752.4936.0 709.7956.1 793.3945.1
61.193.4†,*
sE-selectin (ng/ml) 47.191.8 52.692.8 43.793.9
aNGT, normal glucose tolerance; IGT, impaired glucose tolerance. Data are means9SE. *PB0.05 versus NGT.
Fig. 1. Relationships between sE-selectin levels and AUCinsulin mea-sured during OGTT.
sents a prediabetic stage. Similar high concentrations of sE-selectin have been reported in other patients with prediabetic conditions such as former gestational diabetes mellitus or first degree relatives of type 2 diabetes mellitus [24,25].
Several studies have investigated the relationship between adhesion molecules and hypertension or dys-lipidemia [26 – 28]. In our present study, sE-selectin levels correlated with elevated AUCglucose, elevated
AUCinsulin, high blood pressure, elevated triglyceride
concentrations, and low HDL-cholesterol. These con-ditions are strongly related to insulin resistance [11 – 13,29]. Our study showed that only hyperinsulinemia is an independent factor related to elevated sE-se-lectin. These results suggest that hyperinsulinemia/ in-sulin resistance may be a basic factor related to endothelial activation. Another possible cause of ele-vated sE-selectin levels is endothelium damaged by insulin resistant state [4]. In the present study, we have not measured markers of endothelial cell dam-age, like von Willebrand factor [1,21], so we are un-able to confirm the suggestion that elevated sE-selectin levels reflect activation or injury of the endothelium.
How does insulin resistance increase the expression of E-selectin on the endothelium? While the exact mechanisms are unknown at present, recent studies demonstrated a blunted endothelium-dependent va-sodilation in the insulin resistant state [30]. This phe-nomenon was also associated with low nitric oxide (NO) release from the endothelium [30,31]. Further-more, NO limits endothelial activation and reduces the expression of E-selectin in vitro [32]. Therefore, high serum concentrations of sE-selectin in insulin re-sistance may be, at least in part, related to low NO levels.
Agents that improve insulin resistance are reported to decrease serum levels of sE-selectin [28,33]. For example, Cominacini et al. [33] reported that the treatment with troglitazone resulted in decrease of sE-selectin levels in patients with type 2 diabetes. Ferri et al. [28] reported that the treatment with enalapril, an angiotensin converting enzyme inhibitor, decreased sE-selectin concentrations in patients with essential hypertension. Another study has also shown that in-tensive insulin treatment reduced sE-selectin levels in patients with type 2 diabetes [34]. These results sug-gest that improvement in insulin resistance may be associated with a concomitant decrease in sE-selectin levels.
In conclusion, we demonstrated in the present study the presence of high concentrations of sE-se-lectin in Japanese patients with IGT with hyperinsu-linemia (suggesting an insulin resistant state). This may be associated with a premature atherogenesis in insulin resistance syndrome.
patients with IGT with hyperinsulinemia. Our results also showed that sE-selectin levels were not elevated in IGT patients with hypoinsulinemia. Further analy-sis showed that serum sE-selectin concentrations cor-related with AUCinsulin, AUCglucose, blood pressure,
triglyceride, and negative with HDL-cholesterol. Recent studies have demonstrated the presence of significantly high concentrations of sICAM-1, sV-CAM-1, and sE-selectin in patients with overt dia-betes [5 – 7]. In the present study, sICAM-1 and sVCAM-1 levels in Japanese IGT patients were not elevated, and the rise in sE-selectin levels was depen-dent on the presence or absence of hyperinsulinemia. Therefore, it seems that elevation of sICAM-1 and sVCAM-1 may appear only after the full manifesta-tion of diabetes [19,20].
Other recent studies have shown that high levels of soluble adhesion molecules, especially sICAM-1 and sVCAM-1, were strongly associated with established atherosclerosis such as coronary artery, carotid artery, and peripheral artery [6,21 – 23]. The subjects in our present study lacked clinical evidence of ischemic heart disease, stroke, and peripheral obstructive artery disease. Thus, it is likely that the normal levels of sICAM-1 and sVCAM-1 noticed in the present study in patients with IGT were due to the inclusion crite-ria used in the present study.
repre-Acknowledgements
The authors wish to thank the laboratory staff of the Sasebo Chuo Hospital for performing the biochemical assays. We also thank Dr F.G. Issa (Word-Medex) for careful reading and editing of the manuscript.
References
[1] Carter AM, Grant PJ. Vascular homeostasis, adhesion molecules, and macrovascular disease in non-insulin-dependent diabetes mellitus. Diabet Med 1997;14:423 – 32.
[2] Richardson M, Hadcock SJ, DeReske M, Cybulsky MI. In-creased expression in vivo of VCAM-1 and E-selectin by the aortic endothelium of normolipemic and hyperlipemic diabetic rabbits. Arterioscler Thromb 1994;14:760 – 9.
[3] Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993;362:801 – 9.
[4] Gearing AJH, Newman W. Circulating adhesion molecules in disease. Immunol Today 1993;14:506 – 12.
[5] Ceriello A, Falleti E, Bortolotti N, Motz E, Cavarape A, Russo A, Gonano F, Bartoli E. Increased circulating intercel-lular adhesion molecule-1 levels in type II diabetic patients: the possible role of metabolic control and oxidative stress. Metabolism 1996;45:498 – 501.
[6] Otsuki M, Hashimoto K, Morimoto Y, Kishimoto T, Kasayama D. Circulating vascular cell adhesion molecule-1 (VCAM-1) in atherosclerotic NIDDM patients. Diabetes 1997;46:2096 – 101.
[7] Olson JA, Whitelaw CM, McHardy KC, Pearson DWM, For-rester JV. Soluble leucocyte adhesion molecules in diabetic retinopathy stimulate retinal capillary endothelial cell migra-tion. Diabetologia 1997;40:1166 – 71.
[8] Cominacini L, Pasini AF, Garbin U, Davoli A, De Santis A, Campagnola M, Rigoni A, Zenti MG, Moghetti P, Lo Cascio V. Elevated levels of soluble E-selectin in patients with IDDM and NIDDM: relation to metabolic control. Diabetologia 1995;38:1122 – 4.
[9] Fasching P, Waldha¨usl W, Wagner OF. Elevated circulating adhesion molecules in NIDDM-potential mediators in diabetic macroangiopathy. Diabetologia 1996;39:1242 – 4.
[10] Cominacini L, Pasini AF, Garbin U, Campagnola M, Davoli A, Rigoni A, Zenti MG, Pastorino AM, Lo Cascio V. E-se-lectin plasma concentration is influenced by glycaemic control in NIDDM patients: possible role of oxidative stress. Dia-betologia 1997;40:584 – 9.
[11] Reaven GM. Role of insulin resistance in human disease. Dia-betes 1988;37:1595 – 607.
[12] Kaplan NM. The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch In-tern Med 1989;149:1514 – 20.
[13] DeFronzo RA, Ferrannini E. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dys-lipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991;14:173 – 94.
[14] Lillioja S, Mott DM, Howard BV, Bennett PH, Yki-Jarvinen H, Freymond D, Nyomba BL, Zurlo F, Swinburn B, Bogar-dus C. Impaired glucose tolerance as a disorder of insulin action. Longitudinal and cross-sectional studies in Pima Indi-ans. New Engl J Med 1988;318:1217 – 25.
[15] DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM: a balanced overview. Diabetes Care 1992;15:318 – 68. [16] Matsumoto K, Miyake S, Yano M, Ueki Y, Yamaguchi Y,
Akazawa S, Tominaga Y. Glucose tolerance, insulin secretion,
and insulin sensitivity in nonobese and obese Japanese sub-jects. Diabetes Care 1997;20:1562 – 8.
[17] Taniguchi A, Nakai Y, Doi K, Fukushima M, Nagata I, Kawamura H, Imura H, Suzuki M, Tokuyama K. Glucose effectiveness in two subtypes within impaired glucose tolerance. A minimal model analysis. Diabetes 1994;43:1211 – 7.
[18] The expert committee on the diagnosis and classification of diabetes mellitus: report of the Expert committee on the diag-nosis and classification of diabetes mellitus, Diabetes Care 1997;20:1183 – 1197.
[19] Steiner M, Reinhardt KM, Krammer B, Ernst B, Blann AD. Increased levels of soluble adhesion molecules in type 2 (non-insulin-dependent) diabetes mellitus are independent of gly-caemic control. Thromb Haemost 1994;72:979 – 84.
[20] Baumgartner-Parzer SM, Wagner L, Pettermann M, Gessl A, Waldha¨usl W. Modulation by high glucose of adhesion molecule expression in cultured endothelial cells. Diabetologia 1995;38:1367 – 70.
[21] Blann AD, McCollum CN. Circulating endotherial cell/ leuko-cyte adhesion molecules in atherosclerosis. Thromb Haemost 1994;72:151 – 4.
[22] Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM, Boerwinkle E. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases. Circulation 1997;96:4219 – 25.
[23] Peter K, Nawroth P, Conradt C, Nordt T, Weiss T, Boehme M, Wunsch A, Allenberg J, Kubler W, Bode C. Circulating vascular cell adhesion molecule-1 correlates with the extent of human atherosclerosis in contrast to circulating intercellular adhesion molecule-1, E-selectin, P-selectin and thrombomod-ulin. Arterioscler Thromb Vasc Biol 1997;17:505 – 12.
[24] Kautzky-Willer A, Fasching P, Jilma B, Waldha¨usl W, Wag-ner OF. Persistent elevation and metabolic dependence of cir-culating E-selectin after delivery in women with gestational diabetes mellitus. J Clin Endocrinol Metab 1997;82:4117 – 21. [25] Bannan S, Mansfield MW, Grant PJ. Soluble vascular cell
adhesion molecule-1 and E-selectin levels in relation to vascu-lar risk factors and to E-selectin genotype in the first degree relatives of NIDDM patients and in NIDDM patients. Dia-betologia 1998;41:460 – 6.
[26] Blann AD, Tse W, Maxwell SJR, Waite MA. Increased levels of the soluble adhesion molecule E-selectin in essential hyper-tension. J Hypertens 1994;12:925 – 8.
[27] Allen S, Khan S, Al-Mohanna F, Batten P, Yacoub M. Na-tive low density lipoprotein-induced calcium transients trigger VCAM-1 and E-selectin expression in cultured human vascular endothelial cells. J Clin Invest 1998;101:1064 – 75.
[28] Ferri C, Desideri G, Baldoncini R, Bellini C, De Angelis C, Mazzocchi C, Santucci A. Early activation of vascular en-dothelium in nonobese, nondiabetic essential hypertensive pa-tients with multiple metabolic abnormalities. Diabetes 1998;47:660 – 7.
[29] Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Tar-gher G, Alberiche M, Bonadonna RC, Muggeo M. Prevalence of insulin resistance in metabolic disorders. Diabetes 1998;47:1643 – 9.
[30] Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with en-dothelial dysfunction. J Clin Invest 1996;97:2601 – 10.
[31] Zeng G, Quon MJ. Insulin-stimulated production of nitric ox-ide is inhibited by wortmannin. J Clin Invest 1996;98:894 – 8. [32] De caterina R, Libby P, Peng HB, Thannickal VJ,
[33] Cominacini L, Garbin U, Pasini AF, Campagnola M, Davoli A, Foot E, Sighieri G, Sironi AM, Lo Cascio V, Ferrannini E. Troglitazone reduces LDL oxidation and lowers plasma E-selectin concentration in NIDDM patients. Diabetes 1998;47:130 – 3.
[34] Albertini JP, Valensi P, Lormeau B, Aurousseau MH, Ferriere F, Attali JR, Gattegno L. Elevated concentrations of soluble E-se-lectin and vascular cell adhesion molecule-1 in NIDDM. Diabetes Care 1998;21:1008 – 13.