S
terols are part of the vast family of isoprenoids. The con-version of farnesyl diphosphate into squalene marks the channelling of the isoprenoid pathway into the branch that produces sterols. These compounds are known to be essential for all eukaryotes, either synthesized de novo or taken up from the environment. Since the discovery of cholesterol, they have con-tinued to be the focus of the research activities of many chemists, biochemists and clinicians, as attested by the 13 Nobel prizes awarded between 1910 and 1985 that have been associated with work on sterols. The sterols show a fascinating chemical com-plexity (over 200 natural 3b-monohydroxysterols so far indexed) and a remarkable diversity of function in living organisms.Sterols are membrane components and as such regulate mem-brane fluidity and permeability. This structural role is often described as the ‘bulk’ function, because it is played by signifi-cant amounts of sterols and can be fulfilled by virtually any of the compounds. However, sterols can also participate in the control of membrane-associated metabolic processes, a function for which only a few specific sterol molecules are required; their involve-ment in signal transduction events has also been reported in mam-malian cells. Moreover, sterols are the precursors of a vast array of compounds involved in important cellular and developmental processes in animals (e.g. steroid hormones and bile acids), fungi (e.g. ecdysteroids, antheridiol and oogoniol) and higher plants
(e.g. brassinosteroids1
). Finally, in plants, sterols are substrates for the synthesis of a wide range of secondary metabolites – such as cardenolides, glycoalkaloids, pregnane derivatives and saponins – to which precise physiological functions have not been assigned. Saponins have been proposed to play a role in resistance to fungal attack2
. Whereas mammalian and fungal cells generally contain one major sterol, cholesterol and ergosterol, respectively, plants have a characteristically complex sterol mixture. Thus, as many as 61 sterols and pentacyclic triterpenes have been identified in maize seedlings3
, although some of these compounds are biosyn-thetic precursors that occur in very low amounts.
Several questions urgently need to be addressed to understand the function of plant sterols:
• Why do plants require a mixture of sterols instead of one unique sterol?
• Does such a mixture bestow significant advantages to plants compared with mammals and fungi?
• Does each individual sterol play a specific role in plant cell metabolism?
Here, the structural roles of sterols in higher plant cells are exam-ined in an attempt to assess how they relate to functional aspects of membrane behaviour.
Structure, biosynthesis and cellular localization of sterols Structural patterns
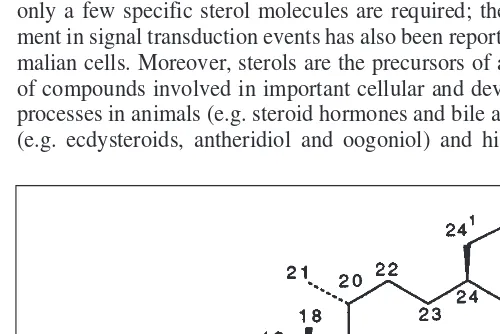
Sterol was the name originally proposed to describe a 3b -mono-hydroxy compound based upon the perhydro-1,2-cyclopentano phenanthrene ring system, with methyl substitution at C10 and C13 and a side chain with 8–10 carbon atoms (Fig. 1). The no-menclature of sterols is largely based on the 1989 IUPAC-IUB recommendations4
, although many phytochemists continue to use the old numbering system5
(Fig. 1). In plants, sterols are always present as a mixture. Structural variations arise from different substitutions in the side chain and number and position of double bonds in the tetracyclic skeleton6–8
. There have also been many reports on sterol composition of algae9
. Detailed and accurate sterol analyses of such complex mixtures have been made possible by the availability of powerful methods of separation (e.g. gas chromatography and high-performance liquid chromatography) and identification (high resolution 1
H- and 13
C-NMR spectroscopy and gas chromatography/mass spectrometry)7,8
. D5
-sterols with a 24-ethyl group at C24, such as sitosterol and stigmasterol (Fig. 2), are by far the most abundant compounds, but sterols with the D7 nucleus are frequently encountered in members of some plant families (e.g. Caryophyllaceae, Theaceae and Cucurbitaceae)6
. The introduction of an alkyl group at C24 renders this position chiral and thus two epimers are possible. Concerning the stereochem-istry of the sterol side chain, two sets of rules are also in use: the a/b and R/S nomenclatures5
. The a and bnotations are independent of
Plant sterols and the membrane
environment
Marie-Andrée Hartmann
Sterols are essential for all eukaryotes. In contrast to animal and fungal cells, which contain only one major sterol, plant cells synthesize a complex array of sterol mixtures in which sitosterol, stigmasterol and methylcholesterol often predominate. Sitosterol and 24-methylcholesterol are able to regulate membrane fluidity and permeability in a similar manner to cholesterol in mammalian cell membranes. Plant sterols can also modulate the activity of membrane-bound enzymes. In contrast, stigmasterol might be specifically required for cell proliferation.
substituents at neighbouring atoms, with a
in front of the plane and bbehind the plane (Fig. 1). According to the R/S nomencla-ture, the configuration depends on the sub-stituent pattern associated with C24. Thus, a 24a-alkyl group can be R or S depending on the absence or presence of a double bond at C22 (Ref. 5). Whereas 24-ethylsterols mainly have a side chain with only one type of configuration, usually 24a, 24-methyl-sterols consist of a mixture of epimers. Thus, 24-methylcholesterol is a mixture of campesterol (24a) and 22-dihydrobrassica-sterol (24b). Cholesterol, the major sterol in mammals, is also found in plants. It gen-erally accounts for a few percent of the sterol mixture of most plants, but some families (e.g. Solanaceae) contain higher amounts. In the early stages of apical devel-opment of Brassica campestris10
or in the epicuticular waxes of rape leaves11
, this sterol represents around 70% of total ste-rols. Plants also contain a great variety of minor 4,4-dimethyl- and 4a-methylsterols, which are biosynthetic precursors of the main sterols.
In most higher plants, sterols with a free 3b-hydroxyl group, also called free sterols, are the major end products. However, ste-rols also occur as conjugates in which the 3-hydroxyl group is either esterified (by a long-chain fatty acid to give steryl esters) or
b-linked (to the 1-position of a monosac-charide, usually glucose) to form steryl glu-cosides or, when the 6-position of the sugar is esterified by a fatty acyl chain, acylated steryl glucosides12
.
Biosynthesis
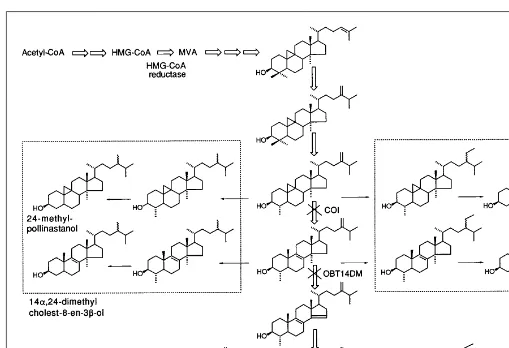
The occurrence of phylogenetic differ-ences in the sterol biosynthesis routes op-erating in various organisms is now well established (Fig. 2). In plants, the sterol pathway represents a sequence of more than 30 enzyme-catalyzed reactions, all as-sociated with membranes6,13
. It is charac-terized by steps restricted to plants, such as the cyclization of squalene oxide into cycloartenol; the opening of the 9b ,19-cyclopropane ring of cycloeucalenol, cata-lyzed by the cycloeucalenol-obtusifoliol isomerase; and the second stage of the side-chain alkylation at C24. In higher plants,
squalene oxide can also be converted into a wide range of penta-cyclic triterpenes such a- and b-amyrins. Cholesterol is synthe-sized via cycloartenol, but its biosynthetic pathway is not entirely known. The post-squalene biosynthetic pathway is clearly charac-terized by critical rate-limiting steps (e.g. the methylation of cycloartenol)14
. All plant tissues are able to form their own sterols. Most of the enzymes involved in sterol biosynthesis from squa-lene formation are associated with the membranes of endoplasmic reticulum (ER)15
, but the participation of the plasma membrane at final steps of the pathway (e.g. in stigmasterol synthesis) cannot be excluded.
Sterols appear to be very stable, as attested by the large increase in the sterol-to-phospholipid ratio observed in senescent mem-branes16
. So far, there are no available data about the mean half-life of plant sterol molecules or their catabolism.
Cellular distribution
As in animal and fungal cells, free sterols reside predominantly in the plasma membranes of plant cells15
. Compared with other cell membrane systems, the plasma membrane contains both the great-est sterol content in proportion to the amount of protein present and the highest sterol-to-phospholipid molar ratio. Sterols are
present in low amounts in ER (Ref. 15), tonoplast17
and mitochon-drial membranes18
. Also, a relatively small proportion of sterol molecules is present in the envelope of chloroplasts, but they are absent from thylakoid membranes15
(see note added in proof). It appears that there is no specific association of an individual sterol molecule with a given membrane compartment, and that all the membranes contain the same sterols in similar proportions15
. Cholesterol has been found in all the membranes. Cellular concen-trations of free sterols and their intracellular distribution are prob-ably tightly regulated, but factors that maintain sterols in the plasma membrane are unclear. Their partitioning between the two leaflets of the plasma membrane and their organization in the lateral plane of the membrane have not been investigated. The synthesis of ste-rols in the ER and accumulation in the plasma membrane implies transport between these two membrane compartments. Unfortu-nately, evidence for sterol domains in the plasma membrane is still lacking, although this might help to clarify whether they play a role in exocytosis, endocytosis, or during signal transduction in plants.
Experimental approaches for investigating sterol functions Biosynthesis inhibitors
The ergosterol biosynthetic pathway is the target of many antifun-gal drugs developed against animal and plant pathogens. Thus, a wide range of molecules is now available to inhibit this pathway at different levels19
. These compounds have also been shown to inter-fere with the phytosterol biosynthetic pathway13,20–22
. Two main groups of sterol biosynthesis inhibitors can be considered depend-ing on the enzyme target. If the target is an enzyme of the general isoprenoid pathway, such as the 3-hydroxymethyl-3-glutaryl co-enzyme A (HMG-CoA) reductase or an co-enzyme at the beginning of the sterol pathway (i.e. the squalene synthase or squalene epoxidase), the effect of the inhibitor is a net decrease in the total amount of membrane free sterols. In contrast, an inhibitor acting downstream of cycloartenol (the first sterol with a tetracyclic skeleton) triggers the replacement of the naturally occurring D5
-sterols by biosynthetic intermediates as well as by unusual -sterols such as 9b,19-cyclopropylsterols or 14a-methyl D8
-sterols13,20–22
(Fig. 3). Sterol biosynthesis inhibitors are therefore useful tools for manipulating plant sterol synthesis in vivo and thus for obtaining whole plants or plant cell suspensions with a wide variety of sterol profiles23
.Such plants are very convenient for studying the conse-quences of unusual sterols on the chemical composition, physical properties and functions of membranes. More-detailed studies have been performed with plants treated by two major classes of compounds, N-alkylmorpholines (e.g. fenpropimorph) and azole derivatives, which are the most commonly used in agriculture19
and inhibit the cycloeucalenol-obtusifoliol isomerase and the obtusifoliol 14-demethylase, respectively22
(Fig. 3). In the case of maize roots treated with fenpropimorph24
, it has been shown that: • The growth rate of seedlings is little affected, even after
replacement of 95% of the typical D5
-sterols by 9b ,19-cyclo-propylsterols.
• These unusual sterols are readily incorporated into membranes. • The modification of the sterol profile is accompanied by a dra-matic modification in the cellular distribution of sterols, the ER becoming the richest membrane in terms of free sterols instead of the plasma membrane.
• No change occurs in the partitioning of the main phospholipid classes, but an increase in the degree of unsaturation of ER phospholipid acyl chains is observed.
There are limitations to the use of inhibitors. First, there is the need to apply the inhibitor during the whole experiment to observe its effects. These compounds are also generally lipophilic molecules that can bind to membrane lipids and therefore interfere
with the parameter under study. Finally, the best modification of the sterol profile accounts for 95% of total sterols, the remaining 5% D5
-sterols corresponding to compounds already present in the seed. This means that some catalytic roles played by minute quan-tities of sterols might be not detected.
Membrane model systems
To gain more insight into structural and functional roles of each individual sterol molecule, one approach is the use of simple, well-defined in vitro membrane models (liposomes). A large body of work has thus been devoted to interactions of cholesterol with a vast array of lipid species, and information about the structural and dynamic properties of cholesterol in biological membranes has been obtained by a wide range of physical techniques (e.g. fluor-escence depolarization of suitable probes, 2
H-NMR, electron spin resonance spectroscopy and differential scanning calorimetry). Phospholipids from plant membranes are generally characterized by a higher proportion of unsaturated fatty acyl chains than those from animal or fungal membranes. It is therefore of crucial impor-tance to investigate the behaviour of plant sterols with lipids of plant origin. A series of experiments has recently been performed with large unilamellar vesicles prepared from soybean phospha-tidylcholine, a representative plant phospholipid, and various plant sterols in different molar ratios. Effects of sterols on membrane fluidity were investigated by diphenyl hexatriene fluorescence polarization measurements25
and 2
H-NMR spectroscopy with per-deuderiated dimyristoylphosphatidylcholine on the sn-2 acyl chain as a probe26,27
. Permeability changes triggered by plant sterols were monitored by measuring the swelling rates of vesicles following an osmotic shock in a stopped-flow spectrophotometer26
. Liposomes are also very useful for studying the roles of sterols on the activity of membrane-bound enzymes in reconstitution experiments. A recent study has been devoted to effects of plant sterols on the plasma membrane H+
-ATPase (Ref. 28).
Mutants and transgenic plants
Sterol auxotrophs are very convenient tools for investigating the roles of sterols in membranes. The yeast Saccharomyces cere-visiaehas been the most widely used organism. A wide range of mutants defective in ergosterol biosynthesis has been isolated using polyene antibiotics as selective agents. In particular, many studies have been devoted to the mutant GL7, and valuable infor-mation about the structural features of the sterol molecule required for yeast membrane functions is readily available29–31
. Various investigations have established multiple roles for sterol during yeast growth31
. All relevant genes of the ergosterol pathway have been cloned32
, allowing isolation of mutants by gene disruption. In contrast, most of the genes encoding enzymes involved in the plant sterol pathway are unknown33
. However, major advances in this field are expected, mainly because the large international sequencing programmes with Arabidopsis have made available many expressed sequence tag (EST) clones. A plant sterol mutant,
STE1, has recently been isolated in Arabidopsis34
. This mutant is defective in the D7
-sterol C5-desaturase and thus accumulates D7
-sterols34
, but displays no particular phenotype.
Information is also expected from studies using transgenic plants in which genes encoding enzymes of sterol biosynthesis can be overexpressed. For example, in tobacco plants overexpressing the HMG-CoA reductase gene, the free sterol content of membranes was found to be unchanged, whereas the excess metabolic inter-mediates are diverted from the main pathway and accumulated as steryl esters in lipid droplets35
Structural roles of sterols
Sterol molecules are incorporated into membranes with the 3b -OH facing the water interface and the side chain extending into the hydrophobic core to interact with fatty acyl chains of phospho-lipids and proteins. Thus, they modulate the physical state of bi-layers by restricting the motion of fatty acyl chains (ordering effect), which at physiological temperatures are in the liquid-crystalline phase. Previous investigations led to the identification of several structural features of the sterol molecule required for a membrane function: a free 3b-hydroxyl group; a planar tetracyclic skeleton; and an aliphatic side chain with 8–10 carbon atoms29
. The typical plant sterols (sitosterol, stigmasterol and 24-methylcholesterol) all satisfy these requirements. Experiments with soybean phosphati-dylcholine bilayers indicate that all the plant sterols tested are able to regulate membrane fluidity, but with different efficiency25–27
. Sitosterol and 24-methylcholesterol are the most efficient sterols for restricting the mobility of phospholipid fatty acyl chains. The intro-duction of a trans-oriented double bond at C22 in the side chain of the sterol molecule significantly reduces its ordering ability, as attested by the comparison between sitosterol and stigmasterol. Such a reduced ordering ability was observed only in the presence
of an alkyl group in the side chain, as cholesterol and D22
-dehy-drocholesterol have similar efficiencies27
. The stereochemistry at C24 was also shown to interfere with the ordering ability of the sterol36
. The ability of two unusual sterols, 24-methylpollinastanol (a 9b,19-cyclopropylsterol) and 14a,24-dimethylcholest-8-en-3b -ol (a 14a-methyl D8
-sterol), in regulating membrane fluidity was examined. These two sterols are isomers. As they accumulate in plants treated with fungicides used in agriculture19
, the functional consequences of their incorporation into the membranes of such plants were investigated. Whereas the 9b,19-cyclopropylsterol ex-hibits a high ability to order soybean phosphatidylcholine bilayers, similar to that of sitosterol, the 14a-methyl D8
-sterol appears to be a far less efficient compound27
.
Effects triggered by the same range of sterols on the water permeability of soybean phosphatidylcholine bilayers have also been investigated26
. Sitosterol and 24-methylcholesterol appeared to be very active in reducing membrane permeability. In contrast, stigmasterol was very inefficient. Of two unusual sterols tested previously, 24-methylpollinastanol was found to be functionally equivalent to sitosterol, and the 14a-methyl D8
-sterol was found not to be very effective.
Fig. 3.Use of sterol biosynthesis inhibitors to manipulate the sterol profile of plant membranes in vivo. The main pathway from acetyl-CoA to end products (sitosterol, stigmasterol and 24-methylcholesterol) via mevalonic acid (MVA) is indicated by open arrows. Downstream of cycloartenol, the first cyclic intermediate, two enzymes, the cycloeucalenol obtusifoliol isomerase (COI) and the obtusifoliol 14-demethylase (OBT14DM), are the targets of two classes of fungicide widely used in agriculture, N-alkylmorpholines and triazole derivatives, respectively. The inhibition of COI leads to an accumulation of 9b,19-cyclopropylsterols (e.g. 24-methylpollinastanol); the inhibition of OBT14DM triggers an accumulation of 14a-methyl D8
-sterols (e.g. 14a,24-dimethylcholest-8-en-3b-ol) at the expense of the naturally occurring D5
In conclusion, with soybean phosphatidylcholine bilayers, there is an excellent correlation between sterol effects on acyl chain ordering and membrane permeability to water. Although these results have been obtained with a model membrane system, they suggest that the most efficient sterols for regulating both functions are able to influence the properties of plant membranes. Stigmasterol might be required for a different function, although the interaction of this sterol with another class of phospholipid (e.g. phosphatidylethanolamine) remains to be determined.
Metabolic and regulatory functions of sterols Cellular proliferation and differentiation
Active sterol synthesis occurs following seed germination to meet the needs for new membranes, as attested by the several-fold increase in free sterol concentration3
. The rate of sterol synthesis then gradually decreases with seed maturity.
Evidence that sterols may play an important metabolic role in the cell proliferation process, in addition to the purely structural function of controlling the physical state of membranes, has been emerging recently. For such a function, only a small fraction of specific sterol molecules might be necessary. A more specific requirement for stigmasterol has been shown in the case of celery cells treated by an inhibitor of the obtusifoliol 14-demethylase37
. Cholesterol at 50–100 mMrestored growth to 40–50% of that of
the control, but full growth was only achieved in the presence of stigmasterol (50 mM). Lower concentrations of stigmasterol
(0.05–0.5 mM) were unable to restore growth, but a combination of a low concentration of stigmasterol together with a high con-centration of cholesterol (50 mM) was as effective as stigmasterol alone (50 mM). However, most plant cells exhibit a very low
abil-ity for exogenous sterol uptake.
Sterols as effectors of membrane-bound enzymes
Recent evidence suggests that plant sterols are able to modulate the activity of the plasma membrane H+
-ATPase from maize roots28
. In particular, cholesterol and stigmasterol were found to stimulate proton pumping, especially at a low concentration (5 mol %), whereas all the other sterols tested (e.g. sitosterol and 24-methylcholesterol) behaved as inhibitors at any concentration. When given at a concentration of 3 mMto intact maize roots, these
two sterols are also able to stimulate H+
secretion38
. They might therefore be considered as effective ligands in much the same way that cholesterol is for the Na+
/K+
ATPase of animal cells39
. In the same context, specific sterol molecules might be required by sur-face receptors and participate in some signal transduction events as in mammalian cells.
Conclusions
As is the case for animal and yeast cells, sterols play many roles in higher plants. One role is as a bulk sterol for new membranes syn-thesized during plant development, and this function can be satis-fied by a variety of sterol compounds. Most of the free sterols of the cell reside in the plasma membrane, where they contribute to the maintenance of an intermediate state of fluidity consistent with optimal functioning of integral enzymes and surface recep-tors. The plant sterols sitosterol and 24-methylcholesterol prob-ably play a role similar to that of cholesterol in mammalian cells. Stigmasterol, another major plant sterol, which differs from sito-sterol only by an additional double bond at C22 in the side chain, appears not to be involved in the regulation of membrane proper-ties. The ability of 9b,19-cyclopropylsterols to replace D5
-sterols as effectors of membrane fluidity and permeability is quite remarkable. Small amounts of specific D5
-sterols also appear to be required to promote cell proliferation and to regulate metabolic
events. Stigmasterol, but also cholesterol, might be involved in such processes. The lack of plant mutants auxotrophic to sterols has been a serious handicap to research in this field, but progress in cloning genes encoding enzymes of sterol biosynthesis as well as in gene-targeted disruption techniques will undoudtedly help to clarify the functions of plant sterols.
References
01Yokota, T. (1997) The structure, biosynthesis and function of brassinosteroids,
Trends Plant Sci.2, 137–143
02Osbourn, A. (1996) Saponins and plant defence – a soap story, Trends Plant Sci.1, 4–9
03Guo, D.A., Venkatramesh, M. and Nes, W.D. (1995) Developmental regulation of sterol biosynthesis in Zea mays, Lipids30, 203–219
04Moss, G.P. (1989) The nomenclature of steroids: recommendations by the IUPAC–IUB joint commission on biochemical nomenclature, Eur. J. Biochem. 186, 429–458
05Parker, S.R. and Nes, W.D. (1992) Regulation of sterol biosynthesis and its phylogenetic implications, Am. Chem. Soc. Symp. Ser.497, 110–145
06Nes, W.R. (1977) The biochemistry of plant sterols, Adv. Lipid Res. 15, 233–324
07Goad, L.J. (1991) Phytosterols, in Methods in Plant Biochemistry(Vol. 7) (Charlwood, B.V. and Banthorpe, D.V., eds), pp. 369–434, Academic Press
08Akihisa, T., Kokke, W.C.M.C. and Tamura, T. (1991) Naturally occurring sterols and related compounds from plants, in Physiology and Biochemistry of Sterols (Patterson, G.W. and Nes, W.D., eds), pp. 172–228, American Oil Chemists’ Society
09Patterson, G.W. (1991) Sterols of algae, in Physiology and Biochemistry of Sterols(Patterson, G.W. and Nes, W.D., eds), pp. 118–157, American Oil Chemists’ Society
10Douglas, H.H. et al. (1996) Changes in lipid composition during floral development of Brassica campestris, Phytochemistry42, 335–339
11Noda, M. et al. (1988) Occurrence of cholesterol as a major sterol component in leaf surface lipids, Lipids23, 439–444
12Wojciechowski, Z.A. (1991) Biochemistry of phytosterol conjugates, in
Physiology and Biochemistry of Sterols(Patterson, G.W. and Nes, W.D., eds), pp. 361–395, American Oil Chemists’ Society
13Benveniste, P. (1986) Sterol biosynthesis, Annu. Rev. Plant Physiol. 37, 275–308
14Nes, W.D. and Venkatramesh, M. (1997) Enzymology of phytosterol transformations, in Biochemistry and Function of Sterols(Parish, E.J. and Nes, W.D., eds), pp. 111–122, CRC Press
15Hartmann, M-A. and Benveniste, P. (1987) Plant membrane sterols: isolation, identification and biosynthesis, Methods Enzymol. 148, 632–650
16Duxbury, C.L. et al. (1991) Alterations in membrane protein conformation in response to senescence-related changes, Phytochemistry 30, 63–68
17Yoshida, S. and Uemura, M. (1986) Lipid composition of plasma membranes and tonoplasts isolated from etiolated seedlings of mung bean (Vigna radiata
L.), Plant Physiol. 82, 807–812
18Méance, J., Dupéron, P. and Dupéron, R. (1976) Répartition des substances stéroliques à l’intérieur des mitochondries de l’inflorescence de Chou-fleur,
Physiol. Vég.59, 745–756
19Köller, W. (1992) Antifungal agents with target sites in sterol functions and biosynthesis, in Target Sites in Fungicide Action (Köller, W., ed.), pp. 119–205, CRC Press
20Burden, R.S., Cooke, D.T. and Carter, G.A. (1989) Inhibitors of sterol biosynthesis and growth in plants and fungi, Phytochemistry28, 1791–1804
21Mercer, E.I. (1993) Inhibitors of sterol biosynthesis and their applications,
Prog. Lipid Res.32, 357–416
22Rahier, A. and Taton, M. (1997) Fungicides as tools in studying postsqualene sterol synthesis in plants, Pestic. Biochem. Physiol. 57, 1–27
24 Grandmougin, A. et al. (1989) Cyclopropyl sterols and phospholipid composition of membrane fractions from maize roots treated with fenpropimorph, Plant Physiol.90, 591–597
25 Schuler, I. et al. (1990) Soybean phosphatidylcholine vesicles containing plant sterols: a fluorescence anisotropy study, Biochim. Biophys. Acta 1028, 82–88
26 Schuler, I. et al. (1991) Differential effects of plant sterols on water permeability and on acyl chain ordering of soybean phosphatidylcholine bilayers, Proc. Natl. Acad. Sci. U. S. A. 88, 6926–6930
27 Krajewsky-Bertrand, M-A., Milon, A. and Hartmann, M-A. (1992) Deuterium-NMR investigation of plant sterol effects on soybean phosphatidylcholine acyl chain ordering, Chem. Phys. Lipids 63, 235–241
28 Grandmougin-Ferjani, A., Schuler-Muller, I. and Hartmann, M-A. (1997) Sterol modulation of the plasma membrane H+-ATPase activity from corn
roots reconstituted into soybean lipids, Plant Physiol.113, 163–174
29 Bloch, K.E. (1983) Sterol structure and membrane function, Crit. Rev. Biochem. 14, 47–82
30 Nes, W.D. et al.(1993) The structural requirement of sterols for membrane function in Saccharomyces cerevisiae, Arch. Biochem. Biophys.300, 724–733
31 Rodriguez, R.J. et al. (1985) Multiple functions for sterols in Saccharomyces cerevisiae, Biochim. Biophys. Acta837, 336–343
32 Lees, N.D. et al. (1995) Cloning of the late genes in the ergosterol biosynthetic pathway of Saccharomyces cerevisiae– a review, Lipids30, 221–226
33 Bach, T.J. and Benveniste, P. (1997) Cloning of cDNAs or genes encoding enzymes of sterol biosynthesis from plants and other eukaryotes: heterologous expression and complementation analysis of mutations for functional characterization, Prog. Lipid Res., 36, 197–226
34 Gachotte, D., Meens, R. and Benveniste, P. (1995) An Arabodopsismutant deficient in sterol biosynthesis: heterologous complementation by ERG3
encoding a D7-sterol-C5-desaturase from yeast, Plant J.8, 407–416 35 Schaller, H. et al. (1995) Expression of the Hevea brasiliensis (H.B.K.) Müll.
Arg. 3-hydroxy-3-methylglutaryl coenzyme A reductase 1 in tobacco results in sterol overproduction, Plant Physiol.109, 761–770
36 Marsan, M.P., Muller, I. and Milon, A. (1996) Ability of clionasterol and poriferasterol (24-epimers of sitosterol and stigmasterol) to regulate membrane lipid dynamics, Chem. Phys. Lipids 84, 117–121
37 Goad, L.J. (1990) Application of sterol synthesis inhibitors to investigate the sterol requirements of protozoa and plants, Biochem. Soc. Trans. 18, 63–65
38 Cerana, R. et al. (1984) Regulating effects of brassinosteroids and of sterols on growth and H+secretion in maize roots, Z. Pflanzenphysiol.114, 221–225 39 Cornelius, F. (1995) Cholesterol modulation of molecular activity of
reconstituted shark Na+,K+-ATPase, Biochim. Biophys. Acta1235, 205–212
Note added in proof
M. Havaux has recently discussed the absence of sterols in thylakoid membranes (Trends Plant Sci.3, 147–151). In such membranes the function played by sterols might be fulfilled by other isoprenoids, such as xanthophyll carotenoids (e.g. zeaxanthin) and a-tocopherol.
C
ytoplasmic male sterility (CMS) has been observed in over 150 plant species1–3. CMS systems make excellent models in which to study the interaction between nuclear and cyto-plasmic factors, because fertility restoration relies on nuclear genes that suppress cytoplasmic dysfunction (Fig. 1). Analyses of the molecular mechanisms by which fertility loss and restoration occur can also help elucidate pollen development and normal mitochondrial function.
Nuclear restoration allows the commercial exploitation of CMS systems in the production of hybrid seed. This is because, in com-bination with CMS, it eliminates the need for hand emasculation and yet ensures the production of male-fertile, first-generation (F1)
progeny. For example, prior to the epidemic of southern corn leaf
blight in 1970, male-sterile T (Texas)-cytoplasm maize system was used to produce approximately 85% of hybrid seed in the USA. Breeders produce hybrid seed using a CMS system by developing female lines that carry CMS cytoplasm but lack restorer genes and by developing male lines that carry the appro-priate restorer genes. F1hybrid seed produced by the female lines
carry the CMS cytoplasm but yield fertile plants because of the action of the paternally contributed nuclear restorers.
Origin of cytoplasmic male sterility
Because of their value in hybrid seed production, CMS systems have been identified and characterized in many crop species, in-cluding Phaseolus vulgaris, beet, carrot, maize, onion, petunia,
Marie-Andrée Hartmann is at the Institut de Biologie Moléculaire des Plantes du Centre National de la Recherche Scientifique (CNRS), 28 rue Goethe, 67083 Strasbourg, France (tel +33 3 88 35 83 70; fax +33 3 88 35 84 84; e-mail [email protected]).