Improvement of in vitro gynogenesis induction in onion (
Allium
cepa
L.) using polyamines
Liliana E. Martı´nez
a,*, Cecilia B. Agu¨ero
a, Marı´a E. Lo´pez
a,
Claudio R. Galmarini
baCa´tedra de Fisiologı´a Vegetal,Facultad de Ciencias Agrarias,CC No. 7,5505,Chacras de Coria,Mendoza, Argentina bEstacio´n Experimental INTA La Consulta,CC 8,5567,La Consulta,Mendoza, Argentina
Received 20 September 1999; received in revised form 15 March 2000; accepted 16 March 2000
Abstract
The effects of polyamines on gynogenetic embryogenesis and regeneration of plantlets in onion were studied. Whole flowers from two onion genotypes, ‘Valcatorce INTA’ cultivar and ‘Torrentina’ population, were used as initial explants. Embryo induction was greatest with a combined treatment of 2 mM putrescine and 0.1 mM spermidine. Addition of putrescine alone, with a few exceptions, did not have any significant effect on either embryo induction or haploid plantlet production for both onion genotypes. ‘Torrentina’ showed a higher embryo generation capacity (9.5%) than ‘Valcatorce INTA’ (2.8%). Fast regeneration of embryos was achieved (from 60 to 90 days) as compared to a previously reported time of 46 – 152 days. The use of spermidine (0.1 mM) after 15 days of culture promoted further embryo maturation and plantlet formation. ‘Torrentina’ produced more haploid plants (1.90%). This is the first report of successful use of polyamines for induction of gynogenic embryos and regeneration of onion plantlets. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Allium cepaL.; Onion; Haploids; In vitro culture; Gynogenesis; Polyamines; Breeding
www.elsevier.com/locate/plantsci
1. Introduction
The onion crop is of great importance in Ar-gentina and is one of the main exported fresh vegetables. For decades there have been well-es-tablished onion breeding and seed production pro-grams in Argentina, developing short, intermediate and long-day cultivars. Today almost 90% of the cultivars used come from local breed-ing programs. The most successful cultivar is ‘Val-catorce INTA’, representing 80% of the onion area in the country: it is also cropped in Chile and Uruguay. The main onion breeding program is
located at La Consulta Experimental Station (INTA). The breeding goals are early harvesting and good storage quality in long-day cultivars, and introduction of pink-root resistance and high dry matter into cultivars for the dehydration in-dustry [1]. In the breeding program classical meth-ods, like mass selection, pedigree, recurrent selection and hybrid production are used, together with in vitro culture techniques (e.g. ovule and ovary culture) to hasten the production of ho-mozygous lines. At present several haploid plants have been obtained [2].
The use of haploidization methods in breeding hybrid cultivars is of particular interest in long cycle species [3], since at least 6 – 10 years are required to produce inbred lines [4]. Treatment of haploid plants with colchicine can produce supe-rior homozygous diploid lines, which can be used to create hybrids with desirable features in consid-erably shorter time.
Abbre6iations:BA, 6-benzylaminopurine; BDS, Dunstan and Short medium; B5, Gamborg medium; MS, Murashige and Skoog medium; NAA, naphtalenacetic acid.
* Corresponding author. Tel.: 54-261-4960004, ext. 2036; fax: + 54-261-4960469.
E-mail address:[email protected] (L.E. Martı´nez).
Different techniques have been used to obtain haploid plants: induced parthenogenesis by inter-specific or intergeneric crosses, use of inactivated pollen and in vitro culture of male or female gametophytes [5].
The induction of onion haploid plants has re-cently been achieved in vitro via gynogenesis [2,6 – 15], and even in situ after crossing onion plants with irradiated pollen [16]. Gynogenic onion embryos have been obtained from ovules, ovaries and whole flowers, using various media and culture conditions. However, in spite of many efforts trying to improve the haploid induction technique, the yield of gynogenic embryos and haploid plants obtained is frequently very low [2,6 – 15].
Cellular and morphogenetic events during so-matic embryogenesis are controlled by an array of culture conditions (medium composition and physical environment) and by genetic effects [17,18]. The genotype of donor plants has a big influence on onion haploid induction ability [4,14,15]. Inbreds and synthetics tend to have higher rates of embryo production and plant re-generation, compared to open populations where the response is generally lower [14].
Polyamines (agmatine, putrescine, spermidine, spermine, cadaverine, etc.) are normal plant growth regulators involved in all growth or devel-opmental process in plants [19,20]. They occur in high amounts in the flowers of various plants [20 – 24]. An increase in polyamine biosynthesis has been shown to precede or accompany calloge-nesis [24], organogecalloge-nesis [25] and somatic embryo-genesis [26] in a number of plant cell cultures [27 – 30]. Danin et al. [28] suggested that the onset of embryogenesis in celery is characterized by a high content of putrescine and cytokinin, while a decrease in putrescine synthesis and cytokinin con-tent and an increase in spermidine and spermine content accompany further embryo development and plantlet formation. Despite several experi-ments in a wide range of species with these growth regulators, the role of polyamines in different spe-cies, in in vitro gynogenesis has not been eluci-dated, not even in onion.
The present research was designed to determine, for the first time, the effects of different polyamines in the production of embryos and haploid onion plants through in vitro gynogene-sis.
2. Material and methods
2.1. Plant material
Experiments were carried out during the growth period of November, 1995 and August, 1996. The genotypes used were a long-day cultivar ‘Valca-torce INTA’ and an intermediate-day population, ‘Torrentina’. Young flowers were collected from the umbels 3 – 5 days before anthesis, surface-ster-ilized with 10% sodium hypochlorite solution con-taining a few drops of Tween 20 for 15 min, and rinsed three times with sterile distilled water.
2.2. Media and culture conditions
Flowers were cultured on induction medium consisting of Dunstan and Short medium (BDS) macro [32], Gamborg medium (B5) microelements [33] and Murashige and Skoog medium (MS) vita-mins [34], supplemented with 100 g/l sucrose (medium A). Putrescine treatments consisted of 0 mM (control P0), 0.1 mM (P1), 0.5 mM (P2), 1 mM (P3) and 2 mM (P4) concentrations. After 15 days of culture, flowers were transferred to a regeneration medium: medium A with 0.1 mM of spermidine (S1) or without spermidine (control S0).
Gynogenic embryos breaking through the ovary wall were transferred to embryo culture medium composed of macro, microelements and vitamins according to MS [34], without growth regulators and supplemented with 40 g/l sucrose and 7.5 g/l agar (medium B). The explants were grown until complete plant development was reached. After 40 days of culture on the previous medium, the regen-erated plantlets were removed and cultured on micropropagation medium: medium B plus 2 mg/l naphtalenacetic acid (NAA) and 2 mg/l 6-benzy-laminopurine (BA).
All media were adjusted to pH 5.8, solidified with 0.75% agar and sterilized by autoclaving at 121°C for 20 min. Petri dishes containing 30 ml of medium and 30 flowers each were placed under a 16-h photoperiod (30mmol/s per m−2, supplied by
Phillips cool-white fluorescent bulbs), at 25 – 209
2°C.
‘Val-catorce INTA’ and ‘Torrentina’, respectively. One experiment was conducted. Data obtained were statistically processed with analysis of variance followed (when required) by Tukey’s test using an SAS 6.04 program (SAS Institute, Cary, NC 27512-8000, USA, licensed to Instituto Nacional de Tecnologı´a Agropecuaria, site 14759001).
2.3. Karyotype analysis
Chromosome numbers were determined in root-tip cells obtained from plantlets after treatment in 0.1% colchicine for 3 h, fixation in 3:1 ethanol: glacial acetic acid, digestion in HCl 1 N at 60°C for 8 min and squashing in 45% acetic acid. For microscopic inspection of the karyotype, the root tips were stained with 1% haematoxiline.
3. Results and discussion
After 30 days of culture, ovaries were two to three times their original size. Some of them broke spontaneously and showed black ovules, which looked like fully developed seeds.
Anther dehiscence was not observed, so it was assumed that in vitro pollination did not occur. These findings support the observations of Smith et al. [9].
The gynogenic embryos were easily distin-guished, coming out from the ovules. They ap-peared 60 – 90 days after inoculation of the flowers on induction medium (Fig. 1). This result suggests
an extremely fast regeneration of embryos, which is a desired characteristic to reduce the total time required for in vitro gynogenesis.
In some cases, direct plantlet regeneration was observed from the septal nectaries region. The ploidy level of these plantlets was diploid (2n=16) due to their somatic origin, so they were not taken into account for further studies.
For ‘Valcatorce INTA’, the embryo yield ob-tained in media containing spermidine along with increasing concentration of putrescine was signifi-cantly higher than in putrescine media or polyamines-free media (control) (Table 1). ‘Tor-rentina’ showed similar behavior. The maximum yield (9.5%) was achieved with 2 mM putrescine and subsequent culture on 0.1 mM spermidine (Table 2). Lower putrescine concentration signifi-cantly reduced embryogenesis in this population compared to the P4-S1 medium. ‘Torrentina’ showed higher embryo generation capacity com-pared to the ‘Valcatorce INTA’.
This is the first report showing a positive effects of polyamines for embryo induction in onion or in other species.
Previous reports have shown a lesser percentage of gynogenic embryos from different genotypes, organs and culture media, ranging from 0.27 to 7.6% [2,4,7,8,11,13,12]. However, recently Bo-hanec and Jakse [15] found average embryo yields of 18.6 – 22.6% for line and 51.7% for individual plants, indicating that gynogenesis is strongly af-fected by the genotype.
Table 1
Effects of putrescine (P) and spermidine (S) on the gynogenetic embryo and plantlet yields of ‘Valcatorce INTA’ cultivara
Treatments No. of flowers Gynogenic embryos Total regenerated haploid plantlets
No. (%) No.
3a
Control P0-control S0 301 (0.9) 1a
4ab
Control P0-S1 330 (1.2) 2a
7b (2.3)
aMeans within each column followed by the same letter are not significantly different atP50.05 (Tukey’s test).
Table 2
Effects of putrescine (P) and spermidine (S) on the gynogenetic embryo and plantlet yields of ‘Torrentina’ populationa
Treatments No. of flowers Gynogenic embryos Total regenerated haploid plantlets
No. (%) No.
173
Control P0-control S0 1b (0.6) 1b
2b (1.1)
P4-control S0 108 1b
10c (9.5)
105 9c
P4-S1
Total 813 16 (2.0) 15
aMeans within each column followed by the same letter are not significantly different atP50.05 (Tukey’s test).
Several studies demonstrate a role for polyamines in carrot somatic embryogenesis [27] and in celery embryo differentiation and plantlet development [28]. In the present work, the addi-tion of putrescine to the medium during the first 15 days of culture followed by addition of 0.1 mM spermidine induced the onset of embryogenesis and increased the number of gynogenic embryos obtained.
The embryo production phase is crucial to im-prove the yield of regenerated plantlets, as the higher the gynogenic embryo number, the higher probability of obtaining haploid plants. There were significant differences in plantlet regeneration between the two onion populations. The mean percentage of embryos which developed into whole plantlets (haploids and diploids) in ‘Tor-rentina’ was 82% while in ‘Valcatorce INTA’ it was 33%. This supports the findings of Phillips
and Luteyn [31], Bohanec et al. [4] and Gioffriau et al. [14], who emphasized that the production of onion haploid plants was strongly affected by the population.
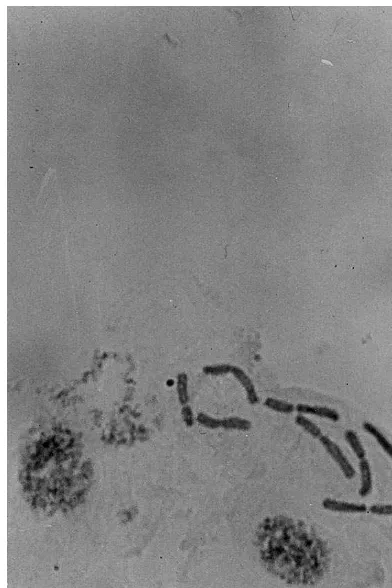
Spontaneous diploidization in onion occurs in the roots [6] and shoot apices [13]. In the present study, the ploidy of the regenerants was estab-lished by counting the chromosomes and gave only n=8 (Fig. 2) for haploid plantlets. Diploid plantlets were observed, but they probably origi-nated from diploid somatic tissue and not from spontaneous duplication in haploids.
Fig. 2. Eight chromosomes in root tip cells of haploid regen-erated plantlet.
Fig. 4. Effect of polyamines on the production of haploid plantlets of ‘Torrentina’ population.
According to these results, the use of spermidine in the media should promote further embryo mat-uration and plantlet formation in onion. More-over, the percentage of haploid plants obtained with ‘Valcatorce INTA’ and ‘Torrentina’ repre-sents an important improvement in the gynogene-sis technique used in onion, for the production of haploid and doubled haploid plants. ‘Torrentina’ appeared to be the most responsive to produce haploid plants.
The percentage of haploid plantlets obtained using polyamines allows use of this methodology in onion breeding programs; consequently large number of accessions could be used in a more efficient way. Further experiments are in progress to duplicate the chromosome number of haploid plants to hasten the production of homozygous lines for hybrid production.
Acknowledgements
This research was supported by a grant from CIUNC (Consejo de Investigaciones de la Univer-sidad Nacional de Cuyo). The authors thank Dr K. Bradford for critical review of the manuscript.
References
[1] C.R. Galmarini, Onion breeding in Argentina, Acta Hor-tic. 358 (1994) 205 – 209.
[2] L. Martı´nez, C. Agu¨ero, C. Galmarini, Obtention of haploid plant by ovaries and ovules cultured in vitro, Acta Hortic. 433 (1997) 447 – 453.
[3] J. Cohat, Obtention chez l%e´chalote (Allium cepa L. var aggregatum) de plantes haploı¨des gynoge´ne´tiques par culture in vitro de boutons floraux, Agronomie 14 (1994) 299 – 304.
Fig. 3. Effect of polyamines on the production of haploid plantlets of ‘Valcatorce INTA’ cultivar.
[4] B. Bohanec, M. Jakse, A. Ihan, B. Javornik, Studies of gynogenesis in onion (Allium cepaL.): induction proce-dures and genetic analysis of regenerants, Plant Sci. 104 (1995) 215 – 224.
[5] C.J. Jensen, Haploid induction and production in crops plants, in: W. Horn, C.J. Jensen, W. Odenbach, O. Schieder (Eds.), Genetic Manipulation in Plant Breed-ing, De Gruyter, Berlin, 1986, pp. 231 – 256.
[6] B. Campion, M.T. Azzimonti, Evolution of ploidy level in haploid plants of onion (Allium cepa L.) obtained through in vitro gynogenesis, in: Eucarpia 4th
AlliumSymposium, Institute of Horticultural Research, Wellesbourne, Warwick, UK, 6 – 9 September, 1988, pp. 85 – 89.
[7] R. Muren, Haploid plant induction from unpollinated ovaries in onion, HortScience 24 (1989) 833 – 834. [8] J. Keller, Culture of unpollinated ovules, ovaries, and
flower buds in some species of the genus Allium and haploid induction via gynogenesis in onion (Allium cepa
L.), Euphytica 47 (1990) 241 – 247.
[9] B.M. Smith, R.M. Godwin, E. Harvey, C.P. Werner, Gynogenesis from whole flower buds in bulb onions (Allium cepaL.) and leeks (Allium porrumL.), J. Genet. Breeding 45 (1991) 353 – 358.
[10] B. Campion, C. Alloni, Induction of haploid plants in onion (Allium cepa L.) by in vitro culture of unpolli-nated ovules, Plant Cell Tiss. Org. Cult. 20 (1990) 1 – 6. [11] B. Campion, M.T. Azzimonti, E. Vicini, M. Schiavi, A. Falavigna, Advances in haploid plant induction in onion (Allium cepaL.) through in vitro gynogenesis, Plant Sci. 86 (1992) 97 – 104.
[12] B. Campion, E. Perri, M.T. Azzimonti, E. Vicini, M. Schiavi, Spontaneous and induced chromosome dou-bling in gynogenic lines of onion (Allium cepaL.), Plant Breeding 114 (1995) 243 – 246.
[13] M. Jakse, B. Bohanec, A. Ihan, Effect of media compo-nents on the gynogenic regeneration of onion (Allium cepaL.) cultivars and analysis of regenerants, Plant Cell Rep. 15 (1996) 934 – 938.
[14] E. Gioffriau, R. Kahane, M. Rancillac, Variation of gynogenesis ability in onion (Allium cepaL.), Euphytica 94 (1997) 37 – 44.
[15] B. Bohanec, M. Jakse, Variation of gynogenic response among long-day onion (Allium cepaL.) accessions, Plant Cell Rep. 18 (1999) 737 – 742.
[16] C. Dore´, F. Marie, Production of gynogenetic plants of onion (Allium cepa L.) after crossing with irradiated pollen, Plant Breeding 111 (1993) 142 – 147.
[17] A. Altman, M. Schwartz, Y. Cohen, T. Arzee, A role for polyamines in differentiation and growth of plant roots, in: S.H. Goldenberg, A. Algranati (Eds.), Biology and Chemistry of Polyamines, CRC Press, Oxford, 1990, pp. 147 – 157.
[18] P.V. Ammirato, in: D.A. Evans, W.R. Sharp, P.V. Am-mirato, Y. Yamada (Eds.), Embryogenesis. In: Hand-book of Plant Cell Culture, vol. 1, MacMillan, New York, 1983, pp. 82 – 123.
[19] J.P. Couillerot, E. Decout, F. Warnot, J. Dubois, J. Vasseur, E´ volution des polyamines libres en relation avec la source carbone´e et l%embryogene`se somatique chez un Cichoriumhybride, C. R. Acad. Sci. Paris 316 (3) (1993) 299 – 305.
[20] A. Kumar, T. Altabella, M.A. Taylor, A.F. Tiburcio, Recent advances in polyamine research, Trends Plant Sci. 2 (1997) 124 – 130.
[21] N. Bangi, P. Torrigiani, Polyamines: a new class of growth substances, in: C.M. Karseen, L.C. Van Loon, D. Vreugdenhil (Eds.), Progress in Plant Growth Regu-lation, Kluwer, Dordrecht, 1992, pp. 264 – 275.
[22] J.G. Buta, R.R. Izac, Caffeoylputrescine in Nicotiana tabacum, Phytochemistry 11 (1972) 1188 – 1189.
[23] P.T. Evans, R.L. Malmberg, Do polyamines have roles in plant development?, Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 40 (1989) 235 – 269.
[24] M. Ponchet, J. Martin-Tanguy, A. Marais, C. Martin, Hydroxycinnamoyl acid amides and aromatic amines in the inflorescence of some Araceae species, Phytochem-istry 21 (1982) 2865 – 2869.
[25] M. Aribaud, M. Carre´, J. Martin-Tanguy, Polyamine metabolism and in vitro cell multiplication and differen-tiation in leaf explants of Chrysanthemum morifolium
Ramat, Plant Growth Regul. 15 (1994) 143 – 155. [26] Z.H. Liu, W.C. Wang, S.Y. Yan, Effect of hormone
treatment on callus formation and endogenous indole-acetic acid and polyamine contents of soybean hypocotyl cultivated in vitro, Bot. Bull. Acad. Sin. 38 (1997) 171 – 176.
[27] S.C. Minocha, N.S. Papa, A.J. Khan, A. Samuelsen, Polyamines and somatic embryogenesis in carrot: III. Effects of methylglyoxal bis(guanylhydrazone), Plant Cell Physiol. 32 (3) (1991) 395 – 402.
[28] M. Danin, S.J. Upfold, N. Levin, B.L. Nadel, A. Alt-man, J. Van Staden, Polyamines and cytokinins in celery embryogenic cell cultures, Plant Growth Regul. 12 (1993) 245 – 254.
[29] M. Santos, N. Boget, J.M. Torne, Endogenous polyamine content during in vivo maturation and in vitro culture of maize pollen, Plant Growth Regul. 16 (1995) 19 – 26.
[30] S. Helleboid, J.P. Couillerot, J.L. Hilbert, J. Vasseur, Inhibition of direct somatic embryogenesis by alfa-difl-uoromethylarginine in a Cichorium hybrid: effects on polyamine content and protein patterns, Planta 196 (1995) 571 – 576.
[31] G.C. Phillips, K.J. Luteyn, Effects of picloram and other auxins on onion tissue cultures, J. Am. Soc. Hort. Sci. 108 (1983) 948 – 953.
[32] D.I. Dunstan, K.C. Short, Improved growth of tissue cultures of the onion, Allium cepa, Physiol. Plant. 41 (1977) 70 – 72.
[33] O.L. Gamborg, R.A. Miller, K. Ojima, Nutrient require-ments of suspension culture of soybean root cells, Exp. Cell Res. 50 (1968) 157 – 158.
[34] T. Murashige, F. Skoog, A revised medium for rapid growth and bioassays with tobacco tissue cultures, Phys-iol. Plant. 15 (1962) 473 – 497.