SUPPLEMENTARY MATERIAL
(Tables 6-8, 10, 12, 14, 19, 20, 24, 25, 28, 29)
MOLECULAR MECHANICS STUDIES (MM4) OF SULFIDES AND MERCAPTANS
Norman L. Allinger and Yi Fan
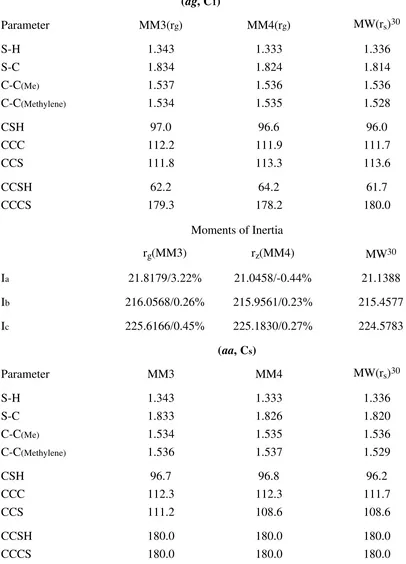
Table 6. Comparison of Calculated and Experimental Geometries of 1-Propanethiol
Bond Length ( Å ) and Bond Angles ( )
(ag, C1)
Parameter MM3(rg) MM4(rg) MW(rs)30
S-H 1.343 1.333 1.336
S-C 1.834 1.824 1.814
C-C(Me) 1.537 1.536 1.536
C-C(Methylene) 1.534 1.535 1.528
CSH 97.0 96.6 96.0
CCC 112.2 111.9 111.7
CCS 111.8 113.3 113.6
CCSH 62.2 64.2 61.7
CCCS 179.3 178.2 180.0
Moments of Inertia
rg(MM3) rz(MM4) MW30
Ia 21.8179/3.22% 21.0458/-0.44% 21.1388
Ib 216.0568/0.26% 215.9561/0.23% 215.4577
Ic 225.6166/0.45% 225.1830/0.27% 224.5783
(aa, Cs)
Parameter MM3 MM4 MW(rs)30
S-H 1.343 1.333 1.336
(Table 6 continued)
Moments of Inertia
rg(MM3) rz(MM4) MW30
Ia 21.1514/-1.89% 21.0812/-0.53% 21.1940
Ib 216.8275/2.69% 211.8501/0.33% 211.1483
Table 7. Comparison of Calculated and Experimental Geometries of 2-Propanethiola
Bond Length ( Å ) and Bond Angles ( )
Parameter MM3 MM4 MW(rz)31
S-H 1.343 1.333 1.345
S-C 1.841 1.827 1.849
C-C 1.537 1.535 1.520
CSH 97.4 96.4 96.5
CCC 110.7 110.9 113.6
CCS 110.2 111.1 111.2
CCSH 61.2 62.0 61.0
Moments of Inertia(Cs)
rg(MM3) rz(MM4) MW31
Ia 64.9556/1.45% 64.2363/0.29% 64.0515
Ib 115.0802/0.51% 114.2347/-0.13% 114.3780
Ic 159.9976/-0.02% 159.2697/-0.65% 160.3185
Moments of Inertia(C1)
rg(MM3) rz(MM4) MW31
Ia 64.5926/0.72% 64.3422/0.33% 64.1331
Ib 114.6337/2.63% 111.7507/0.05% 111.6954
Ic 162.0758/1.64% 158.8753/-0.36% 159.4571
_____________________________
Table 8. Comparison of Calculated and Experimental Geometries of Diisopropyl Sulfide (I, C2)
Bond Length ( Å ) and Bond Angles ( )
Parameter MM3 MM4 MOCED(rg)a
S-C 1.832 1.830 1.829(3)
C1-C4 1.538 1.538 1.528(2)
C1-C5 1.537 1.535 1.532(2)
C-H 1.114 1.112 1.118(3)
CSC 100.9 102.0 102.9(17)
C4C1S2 110.5 112.5 112.0(7)
C5C1S2 109.0 106.0 106.5(7)
CCC 110.1 110.2 110.5(9)
CSCH 42.0 53.0 59(7)
___________________________________
a This structure was investigated by the molecular orbital constrained electron
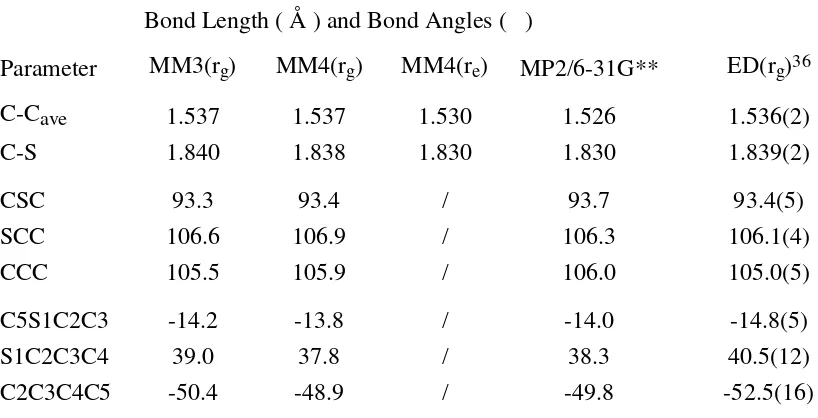
Table 10. Comparison of Calculated and Experimental Geometries of Thiacyclopentane(C2)
Bond Length ( Å ) and Bond Angles ( )
Parameter MM3(rg) MM4(rg) MM4(re) MP2/6-31G** ED(rg)36
C-Cave 1.537 1.537 1.530 1.526 1.536(2)
C-S 1.840 1.838 1.830 1.830 1.839(2)
CSC 93.3 93.4 / 93.7 93.4(5)
SCC 106.6 106.9 / 106.3 106.1(4)
CCC 105.5 105.9 / 106.0 105.0(5)
C5S1C2C3 -14.2 -13.8 / -14.0 -14.8(5)
S1C2C3C4 39.0 37.8 / 38.3 40.5(12)
Table 12. Comparison of Calculated and Experimental Geometries of Thiane (Cs)
Bond Length ( Å ) and Bond Angles ( )
Parameter MM3 MM4 MW39 ED40
C-C 1.536 1.531 1.533 1.528(3)
C-S 1.813 1.814 1.832 1.811(4)
C-H 1.114 1.113 1.095 1.114(2)
CSC 97.5 97.4 99.2 97.6(8)
C2C3C4 112.7 112.7 107.9 112.3(4)
C3C4C5 112.6 112.9 109.2 113.6(8)
HCH 106.4 106.4 108.5 105.9(9)
CCS 111.3 112.8 114.1 112.7(2)
Moments of Inertia
rg(MM3) rz(MM4) MW39
Ia 127.673/0.87% 125.9488/-0.50% 126.575
Ib 168.229/1.08% 168.9888/0.51% 168.134
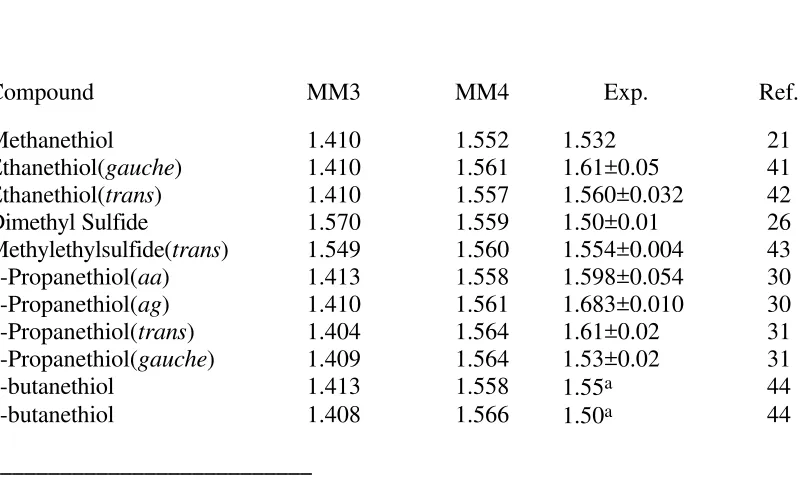
Table 14. Dipole Moments(Debye)
Compound MM3 MM4 Exp. Ref.
Methanethiol 1.410 1.552 1.532 21
Ethanethiol(gauche) 1.410 1.561 1.61±0.05 41
Ethanethiol(trans) 1.410 1.557 1.560±0.032 42
Dimethyl Sulfide 1.570 1.559 1.50±0.01 26
Methylethylsulfide(trans) 1.549 1.560 1.554±0.004 43
1-Propanethiol(aa) 1.413 1.558 1.598±0.054 30
1-Propanethiol(ag) 1.410 1.561 1.683±0.010 30
2-Propanethiol(trans) 1.404 1.564 1.61±0.02 31
2-Propanethiol(gauche) 1.409 1.564 1.53±0.02 31
1-butanethiol 1.413 1.558 1.55a 44
2-butanethiol 1.408 1.566 1.50a 44
___________________________
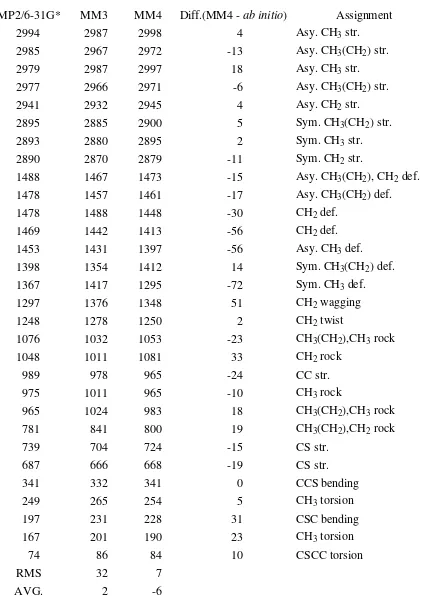
Table 19. Vibrational Spectrum of trans-Methyl Ethyl Sulfide(Cs) (cm-1)
MP2/6-31G* MM3 MM4 Diff.(MM4 - ab initio) Assignment
2994 2987 2998 4 Asy. CH3 str.
1297 1376 1348 51 CH2 wagging
1248 1278 1250 2 CH2 twist
341 332 341 0 CCS bending
249 265 254 5 CH3 torsion
197 231 228 31 CSC bending
167 201 190 23 CH3 torsion
74 86 84 10 CSCC torsion
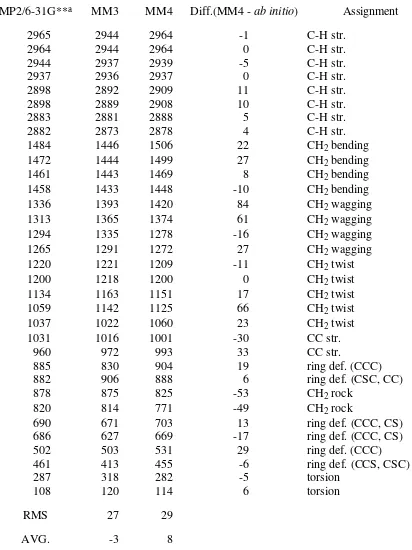
Table 20. Vibrational Spectrum of Thiacyclopentane(C2) (cm-1)
MP2/6-31G**a MM3 MM4 Diff.(MM4 - ab initio) Assignment
2965 2944 2964 -1 C-H str.
2964 2944 2964 0 C-H str.
2944 2937 2939 -5 C-H str.
2937 2936 2937 0 C-H str.
2898 2892 2909 11 C-H str.
2898 2889 2908 10 C-H str.
2883 2881 2888 5 C-H str.
2882 2873 2878 4 C-H str.
1484 1446 1506 22 CH2 bending
1472 1444 1499 27 CH2 bending
1461 1443 1469 8 CH2 bending
1458 1433 1448 -10 CH2 bending
1336 1393 1420 84 CH2 wagging
1313 1365 1374 61 CH2 wagging
1294 1335 1278 -16 CH2 wagging
1265 1291 1272 27 CH2 wagging
1220 1221 1209 -11 CH2 twist
1200 1218 1200 0 CH2 twist
1134 1163 1151 17 CH2 twist
1059 1142 1125 66 CH2 twist
1037 1022 1060 23 CH2 twist
1031 1016 1001 -30 CC str.
960 972 993 33 CC str.
885 830 904 19 ring def. (CCC)
882 906 888 6 ring def. (CSC, CC)
878 875 825 -53 CH2 rock
____________________________
aThe wavenumbers for C-H stretching are scaled down to 92% of original values,
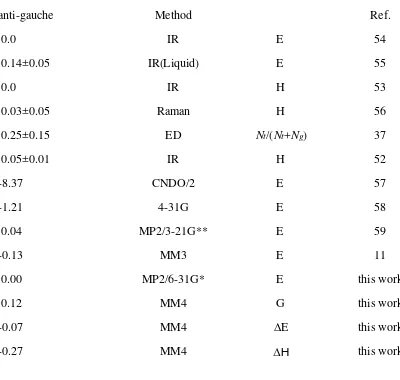
Table 24. Relative Stability of Methyl Ethyl Sulfide Conformers
anti-gauche Method Ref.
0.0 IR E 54
0.14±0.05 IR(Liquid) E 55
0.0 IR H 53
0.00 MP2/6-31G* E this work
0.12 MM4 G this work
-0.07 MM4 ∆E this work
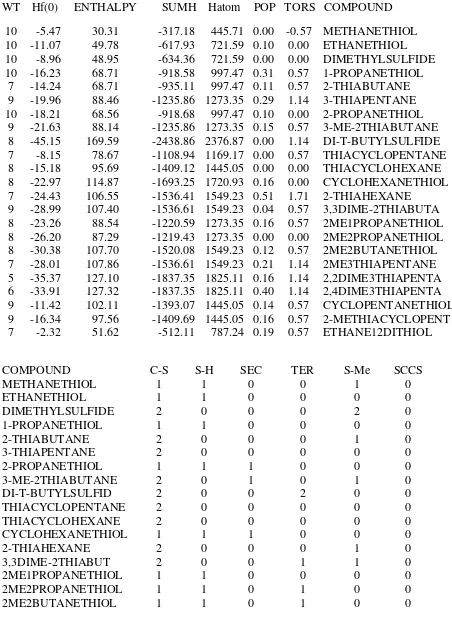
Table 25. Input for the Heat Calculations of Sulfides and Mercaptans
WT Hf(0) ENTHALPY SUMH Hatom POP TORS COMPOUND 10 -5.47 30.31 -317.18 445.71 0.00 -0.57 METHANETHIOL 10 -11.07 49.78 -617.93 721.59 0.10 0.00 ETHANETHIOL 10 -8.96 48.95 -634.36 721.59 0.00 0.00 DIMETHYLSULFIDE 10 -16.23 68.71 -918.58 997.47 0.31 0.57 1-PROPANETHIOL
7 -14.24 68.71 -935.11 997.47 0.11 0.57 2-THIABUTANE 9 -19.96 88.46 -1235.86 1273.35 0.29 1.14 3-THIAPENTANE 10 -18.21 68.56 -918.68 997.47 0.10 0.00 2-PROPANETHIOL
9 -21.63 88.14 -1235.86 1273.35 0.15 0.57 3-ME-2THIABUTANE 8 -45.15 169.59 -2438.86 2376.87 0.00 1.14 DI-T-BUTYLSULFIDE 7 -8.15 78.67 -1108.94 1169.17 0.00 0.57 THIACYCLOPENTANE 8 -15.18 95.69 -1409.12 1445.05 0.00 0.00 THIACYCLOHEXANE 8 -22.97 114.87 -1693.25 1720.93 0.16 0.00 CYCLOHEXANETHIOL 7 -24.43 106.55 -1536.41 1549.23 0.51 1.71 2-THIAHEXANE
9 -28.99 107.40 -1536.61 1549.23 0.04 0.57 3,3DIME-2THIABUTA 8 -23.26 88.54 -1220.59 1273.35 0.16 0.57 2ME1PROPANETHIOL 8 -26.20 87.29 -1219.43 1273.35 0.00 0.00 2ME2PROPANETHIOL 8 -30.38 107.70 -1520.08 1549.23 0.12 0.57 2ME2BUTANETHIOL 7 -28.01 107.86 -1536.61 1549.23 0.21 1.14 2ME3THIAPENTANE 5 -35.37 127.10 -1837.35 1825.11 0.16 1.14 2,2DIME3THIAPENTA 6 -33.91 127.32 -1837.35 1825.11 0.40 1.14 2,4DIME3THIAPENTA 9 -11.42 102.11 -1393.07 1445.05 0.14 0.57 CYCLOPENTANETHIOL 9 -16.34 97.56 -1409.69 1445.05 0.16 0.57 2-METHIACYCLOPENT 7 -2.32 51.62 -512.11 787.24 0.19 0.57 ETHANE12DITHIOL
COMPOUND C-S S-H SEC TER S-Me SCCS
METHANETHIOL 1 1 0 0 1 0
ETHANETHIOL 1 1 0 0 0 0
DIMETHYLSULFIDE 2 0 0 0 2 0
1-PROPANETHIOL 1 1 0 0 0 0
THIACYCLOPENTANE 2 0 0 0 0 0
THIACYCLOHEXANE 2 0 0 0 0 0
CYCLOHEXANETHIOL 1 1 1 0 0 0
2-THIAHEXANE 2 0 0 0 1 0
3,3DIME-2THIABUT 2 0 0 1 1 0
2ME3THIAPENTANE 2 0 1 0 0 0
2,2DIME3THIAPENT 2 0 0 1 0 0
2,4DIME3THIAPENT 2 0 2 0 0 0
CYCLOPENTANETHIOL 1 1 1 0 0 0
2-METHIACYCLOPEN 2 0 1 0 0 0
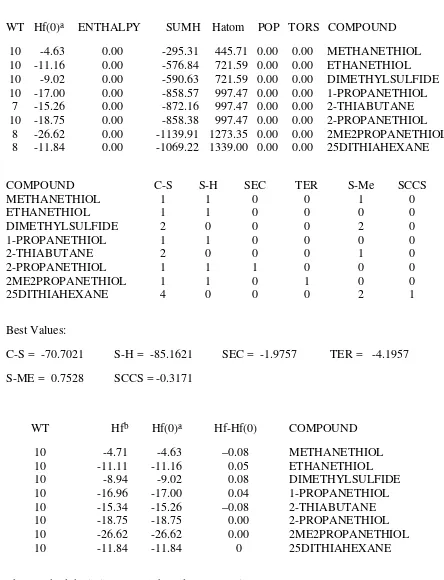
Table 28. Strainless Heats of Formation of Sulfides and Mercaptans
WT Hf(0)a ENTHALPY SUMH Hatom POP TORS COMPOUND
10 -4.63 0.00 -295.31 445.71 0.00 0.00 METHANETHIOL 10 -11.16 0.00 -576.84 721.59 0.00 0.00 ETHANETHIOL 10 -9.02 0.00 -590.63 721.59 0.00 0.00 DIMETHYLSULFIDE 10 -17.00 0.00 -858.57 997.47 0.00 0.00 1-PROPANETHIOL
7 -15.26 0.00 -872.16 997.47 0.00 0.00 2-THIABUTANE 10 -18.75 0.00 -858.38 997.47 0.00 0.00 2-PROPANETHIOL
8 -26.62 0.00 -1139.91 1273.35 0.00 0.00 2ME2PROPANETHIOL 8 -11.84 0.00 -1069.22 1339.00 0.00 0.00 25DITHIAHEXANE
COMPOUND C-S S-H SEC TER S-Me SCCS
METHANETHIOL 1 1 0 0 1 0
ETHANETHIOL 1 1 0 0 0 0
DIMETHYLSULFIDE 2 0 0 0 2 0
1-PROPANETHIOL 1 1 0 0 0 0
2-THIABUTANE 2 0 0 0 1 0
2-PROPANETHIOL 1 1 1 0 0 0
2ME2PROPANETHIOL 1 1 0 1 0 0
25DITHIAHEXANE 4 0 0 0 2 1
Best Values:
C-S = -70.7021 S-H = -85.1621 SEC = -1.9757 TER = -4.1957 S-ME = 0.7528 SCCS = -0.3171
WT Hfb Hf(0)a Hf-Hf(0) COMPOUND
10 -4.71 -4.63 –0.08 METHANETHIOL
10 -11.11 -11.16 0.05 ETHANETHIOL
10 -8.94 -9.02 0.08 DIMETHYLSULFIDE
10 -16.96 -17.00 0.04 1-PROPANETHIOL
10 -15.34 -15.26 –0.08 2-THIABUTANE
10 -18.75 -18.75 0.00 2-PROPANETHIOL
10 -26.62 -26.62 0.00 2ME2PROPANETHIOL
10 -11.84 -11.84 0 25DITHIAHEXANE
________________________
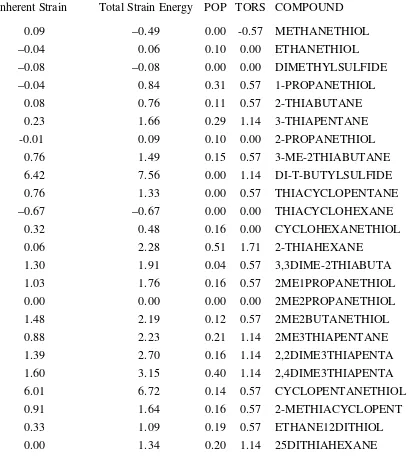
Table 29. Strain Energies of Sulfides and Mercaptans
Inherent Strain Total Strain Energy POP TORS COMPOUND
0.09 –0.49 0.00 -0.57 METHANETHIOL
–0.04 0.06 0.10 0.00 ETHANETHIOL
–0.08 –0.08 0.00 0.00 DIMETHYLSULFIDE
–0.04 0.84 0.31 0.57 1-PROPANETHIOL
0.08 0.76 0.11 0.57 2-THIABUTANE
0.23 1.66 0.29 1.14 3-THIAPENTANE
-0.01 0.09 0.10 0.00 2-PROPANETHIOL
0.76 1.49 0.15 0.57 3-ME-2THIABUTANE
6.42 7.56 0.00 1.14 DI-T-BUTYLSULFIDE
0.76 1.33 0.00 0.57 THIACYCLOPENTANE
–0.67 –0.67 0.00 0.00 THIACYCLOHEXANE
0.32 0.48 0.16 0.00 CYCLOHEXANETHIOL
0.06 2.28 0.51 1.71 2-THIAHEXANE
1.30 1.91 0.04 0.57 3,3DIME-2THIABUTA
1.03 1.76 0.16 0.57 2ME1PROPANETHIOL
0.00 0.00 0.00 0.00 2ME2PROPANETHIOL
1.48 2.19 0.12 0.57 2ME2BUTANETHIOL
0.88 2.23 0.21 1.14 2ME3THIAPENTANE
1.39 2.70 0.16 1.14 2,2DIME3THIAPENTA
1.60 3.15 0.40 1.14 2,4DIME3THIAPENTA
6.01 6.72 0.14 0.57 CYCLOPENTANETHIOL
0.91 1.64 0.16 0.57 2-METHIACYCLOPENT
0.33 1.09 0.19 0.57 ETHANE12DITHIOL