L
Journal of Experimental Marine Biology and Ecology 243 (2000) 81–94
www.elsevier.nl / locate / jembe
Estimation of the life history parameters of Mytilus
galloprovincialis (Lamarck) larvae in a coastal lagoon
(Ria Formosa — south Portugal)
*
´ ´
Luıs M.Z. Chıcharo , M. Alexandra Chicharo
ˆ ´
Unidade de Ciencias e Tecnologias dos Recursos Aquaticos — CCMAR, Universidade do Algarve,
Campus de Gambelas, 8000 Faro, Portugal
Received 5 February 1999; received in revised form 22 July 1999; accepted 23 July 1999
Abstract
The life history parameters of early stages of marine invertebrates, particularly field estimations, have received relatively little attention. The aim of this research was to estimate in situ abundance and growth of Mytilus galloprovincialis planktonic larvae. Plankton samples were filtered through gauze of 63 mm mesh and identified, counted and measured using an inverted microscope. Short-term fluctuations in Mytilus galloprovincialis larvae abundance and environmental parame-ters (water temperature, salinity, wind velocity direction, tidal amplitude and chlorophyll a) were monitored (two to three times a week). Larval cohorts were identified using size–frequency distributions and age estimates compared with larval shell growth lines. Data were fitted to the linear, exponential, von Bertalanffy and Laird–Gompertz growth models. Larval growth adjusted
21 2
better to the Laird–Gompertz model (0.52560.073mm d ; r 50.768; P,0.05). Reduction of shell growth after 1 to 1.5 months in the plankton suggests the occurrence, in this period, of a ‘delay of metamorphosis’ phase. The results of our study indicate that advection and availability of settlement substratum may be the key factors for the life history parameters of this species in a coastal system such as the Ria Formosa. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Bivalve larvae; Growth; Mussel; Mytilus galloprovincialis
1. Introduction
The high productivity of shallow coastal lagoons, such as the Ria Formosa, is the result of contact of the water column and the sediment with good light conditions
*Corresponding author. Tel.:1351-89-800-900; fax:1351-89-818-353. ´
E-mail address: [email protected] (L.M.Z. Chıcharo)
(Sprung, 1994). The Ria Formosa is a highly dynamic system and is characterised by ´
high larval abundance, especially of Mytilus galloprovincialis (Chıcharo, 1996). The Ria Formosa has a long tradition of bivalve culture, especially Ruditapes decussatus, contributing 90% of Portuguese production. However, the importance of diversification of cultured species is recognised. The high availability of mussel larvae in the plankton offers an important alternative for bivalve production, if adequate research on the subject is implemented.
In situ Mytilus galloprovincialis larval growth and mortality have not received much attention, despite their ecological importance. This is probably because their life history variables are difficult to assess due to methodological problems. The most accurate estimations of field growth and mortality result from continuously sampling the larvae in the plankton from the same water column and detecting their variation in length and in number. However, identifying and following larval cohorts in the plankton is very difficult (Morgan, 1994, 1995). Due mainly to this limitation, estimates of mortality and growth of bivalve larvae based on larval cohorts in the plankton have only been made by Korringa (1941), Quayle (1964), Jørgensen (1981) and You and Ryu (1985). A comparatively larger number of studies have analysed laboratory reared bivalve larvae growth and mortality (e.g., Bayne, 1965; Walne, 1966; Pechenik et al., 1990; Rumrill, 1991; Beiras et al., 1994).
Several environmental factors, such as temperature and salinity (Calabrese, 1969; Kingston, 1974; Hrs-Brenko, 1978), food concentration (Sprung, 1984; Pechenik et al., 1990), physical mechanisms (Morgan, 1994; Belgrano et al., 1995; Richards et al., 1995), or predation (Young and Chia, 1987) may affect bivalve larval growth and mortality rates. Whereas in laboratory experiments some of these factors can be controlled, their influence in natural habitats is impossible to ascertain.
The dynamics of larvae from planktonic populations have important implications for the life histories of marine benthic invertebrates (Vance, 1973a,b; Strathmann, 1985; Hines, 1986). Thorson (1950, 1966) highlighted the impact of larval mortality in the recruitment of benthic populations. In fact, rates of growth and natural mortality can vary among populations of larvae, and the availability of settling larvae may influence patterns of recruitment.
Specific objectives of the present study were the estimation of abundance and growth during the planktonic life of Mytilus galloprovincialis in a coastal lagoon, Ria Formosa. The abundance results were compared with tidal amplitude, water temperature, salinity, wind velocity and direction and a food availability indicator (chlorophyll a).
2. Methods
2.1. Study site
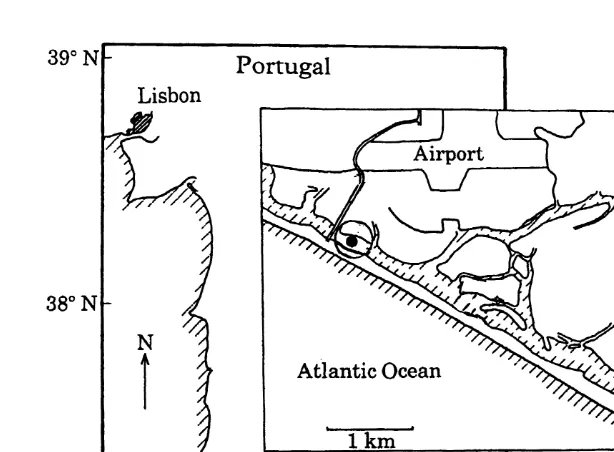
M. galloprovincialis larvae were collected from the plankton of the Ria Formosa lagoon (south Portugal), a tidal lagoon including salt marshes, creeks and tidal flats, extending for about 55 km in length and up to 6 km in width, with an average depth of 3
˜
Fig. 1. Location of sampling station (d). This station was sampled over the period May–August 1990.
communication with the Atlantic Ocean. The exchange of water between the Ria and the ´
adjacent ocean is about 50–75% in each tidal cycle (Aguas, 1986). The water temperature varies from about 12–138C in winter to 27–288C in summer. Variations of ˜ salinity are small, ranging between 25.5 and 36.9 PSU (practical salinity units) (Falcao et al., 1985).
2.2. Environmental parameters
Environmental parameters were measured two to three times per week, between May 4 and August 31, 1990, at the location indicated in Fig. 1. Water temperature and salinity were measured with a Kent Eil 5005 MC5 probe. Wind velocity and direction data were obtained weekly from the Meteorological Service of Faro Airport (Fig. 1), which is directly adjacent to the study site. Chlorophyll a was determined spectrophotometrically and corrected for phaeopigments (Lorenzen and Jeffrey, 1980). Tidal amplitude was calculated by subtracting the low tide (m) from the high tide level (m).
2.3. Sampling strategy and laboratory procedures
hypochlorite solution. Bivalve larvae were identified (according Le Pennec, 1978), counted and measured to the nearest micrometer at 400–1000 magnification, using a Zeiss IM35 inverted microscope, after subsampling using a Stempel pipette. Shell growth lines were counted on photographs.
2.4. Growth models
Length–frequency distributions were analysed by the Bhattacharya (1967) method, and calculations were performed with the Complete Elefan package (Gayanilo et al., 1988). This method assumes that the components are normally distributed. These underlying distributions can be identified as a series of two or more points defining a regression line with negative slope when the logarithms of the ratios of successive frequencies are plotted against the corresponding midpoints. Two criteria were used to identify the modes: (a) the separation index, a ratio based on the difference between the means of the components and their standard deviations — components showing a separation index greater or equal to 2 were considered meaningfully separated; and (b) the confidence interval of the correlation coefficients of the regression lines referred to above. The observed and expected distributions were compared by the Chi-square method (Sokal and Rohlf, 1981).
The age of each modal class was attributed considering that age-0 corresponds to the length of the smallest larvae caught, with 0–1 growth lines (‘D’ veligers, according to Le Pennec, 1978). The modal length of different larval cohorts was plotted separately to follow their progression over the sampling period. Series of estimated mean lengths-at-age, considered to represent growth in time, were used to estimate the daily growth rate. The age attribution was compared with the number of fine growth lines present in the
Mytilus galloprovincialis larval shell.
Following previous literature (Bayne, 1976; Zweifel and Lasker, 1976; Cerrato, 1990; Campana and Jones, 1992) larval growth was examined using several models: the von Bertalanffy (1938) model:
(2K (t2t ))c 0
Lt5L [1` 2e ]
the Laird–Gompertz model (Gompertz, 1825; Laird et al., 1965):
2G t0
where L refers to the shell length at time t in days from the larva appearing in thet plankton, L` is the maximum or asymptotic length, L the theoretical length corre-0
sponding to age-0, G the specific growth rate at t50, G the rate of exponential decay,0
By solving the Laird–Gompertz equation it is possible to calculate the length, corresponding to the inflexion point of the sigmoid curve, at which the growth rate is maximal (L ):i
Li5L / e`
as well as the maximal growth rate (GCM):
GCM5G L0 i
and the maximum length for the species (L ):`
Kc
L`5L e0
3. Results
3.1. Environmental factors and Mytilus galloprovincialis larval abundance
During the sampling period, between May 4 and August 31, 1990, spring tides occurred on May 23–25, June 23–25, July 23–26 and August 22–24 (Fig. 2a). Wind
21
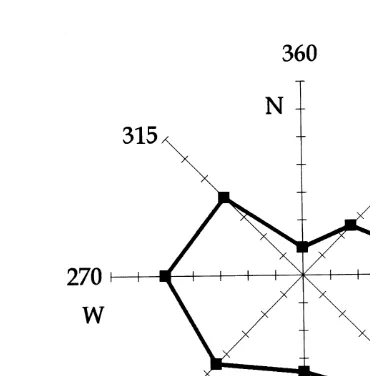
velocity ranged between 2 and 6.7 m s (Fig. 2b). The highest values were registered in July. The wind direction was variable (Fig. 3). A seasonal pattern of Chlorophyll a was
21
observed (Fig. 2b), with the highest value during spring (11 June, 5mg l ) and lowest 21
during late summer (27 August, 1.8mg l ). Water temperature and salinity ranged from 19 to 27.58C, and from 35.1 to 36.5 PSU, respectively (Fig. 2c).
Mytilus galloprovincialis larvae were present in the plankton throughout the entire
sampling period (Fig. 2a). The abundance began to increase the first day of May, when 23
the maximal value was recorded, 4721 individuals m . The lowest value was observed 23
by the middle of June with 37 individuals m , however an important variation in the abundance was observed between successive samples. A great reduction was noted during spring tides.
3.2. Growth rate
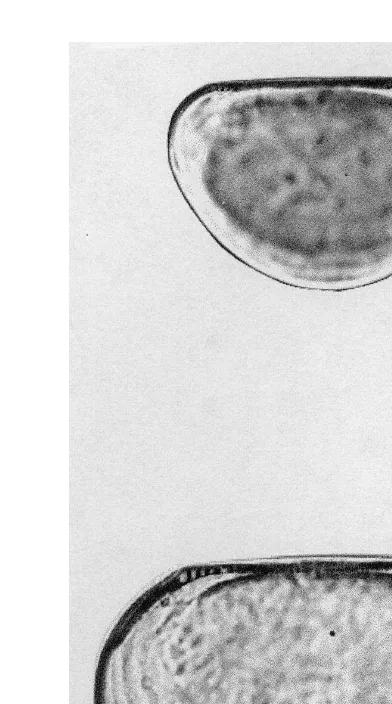
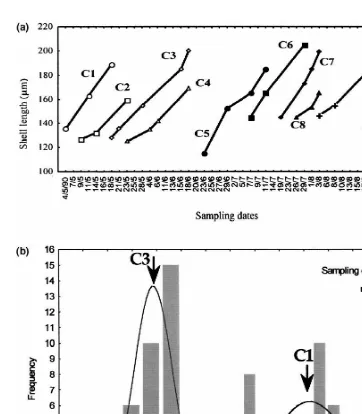
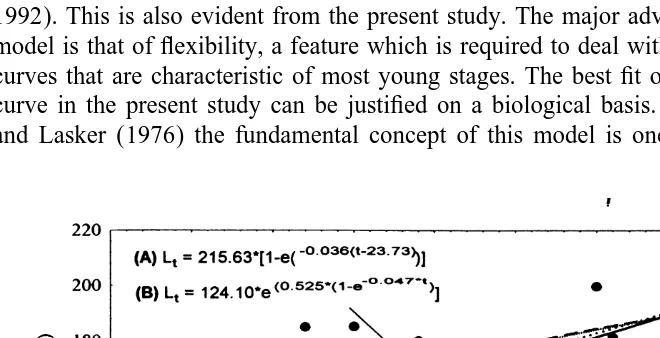
Ten larval cohorts of M. galloprovincialis were identified. The progression of the modal classes considered for each cohort is plotted in Fig. 4. Fine growth lines in larval shells (Fig. 5) were assumed to be deposited daily and suggested development of an age–length relationship. Fitted parameters for all the growth models are given in Table 1. Growth curves (Fig. 6) for larval shell length were significant at P,0.05. The Laird–Gompertz model yielded the best fit to length data, as shown by the correlation coefficients and P values for the overall growth parameters (Table 1). Based on the Laird–Gompertz equation, the maximum length for the larvae of M. galloprovincialis
21
Fig. 3. Wind direction registered near the sampling station (Airport — see Fig. 1). Frequency distribution over the entire study period (4 May–31 August 1990).
4. Discussion
The abundance, growth and mortality of larvae during the planktonic life are interdependent and affected by similar factors. In fact, slower growth results in a longer planktonic life with an inherently longer exposure to predators and to possible adverse environmental conditions, leading to reduced abundance. Consequently, larval life history parameters in the plankton are generally affected by the same factors, namely temperature, food quality and quantity (Sprung, 1984), abundance of predators (Young and Chia, 1987; Rumrill, 1991), salinity (Richmond and Woodin, 1996), physical mechanisms such as advection, diffusion or water turbulence caused by wind (Belgrano et al., 1995) or currents (Morgan, 1994, 1995; Richards et al., 1995) and the availability of settlement substratum (Bayne, 1965, 1983).
Food quality or quantity were not expected to be determining factors for M.
galloprovincialis larval growth or mortality because this coastal lagoon is considered to
be a very productive system (Sprung, 1994). The biomass of the phytoplankton of the lagoon is usually dominated by small flagellates (von Brockel, personal communication), which is an appropriate food source for bivalve larvae. Moreover, bivalve larvae have morphological and behavioural adaptations that allow them to tap alternative food resources ranging from omnivory to DOM (Williams, 1980; Manahan and Richardson, 1982; Manahan, 1983; Lucas et al., 1986; His et al., 1989; Manahan et al., 1989).
Fig. 4. Growth lines in M. galloprovincialis larval shell. (a) Right valve, 135395 mm; (b) left valve, 1603120mm.
Salinity and wind velocity did not show major variations. Salinity values were close to typical sea salinity and wind velocities were relatively low. Moreover, the duration of wind was relatively short and the orientation of the wind with respect to the long axis of the embayment was not constant. Thus, this factor did not have a great influence on water exchange between the lagoon and the sea, the main role being played by tides.
Fig. 5. (a) Change in modal length (derived from length–frequency histogram and growth lines) of cohorts from May to August 1990. C1–C10: cohorts 1 to 10. (b) Typical example sample showing the data and cohort structure.
Besides the losses of larvae to the adjacent ocean, the availability of substrata for settlement may also influence the life history parameters. Despite the high abundance of
´
Table 1
Growth parameters of the von Bertalanffy, Laird–Gompertz, linear and exponential models fitted to the length-at-age data for Mytilus galloprovincialis larvae (n536; r — correlation coefficient)
Laird–Gompertz von Bertalanffy Linear Exponential
R 0.876; P,0.001 0.877; P,0.001 0.857; P,0.001 0.845; P,0.001
L (0 mm); (*L )` 124.1065.17 215.63624.9* 132.2163.37 135.0763.12
P,0.001 P,0.001 P,0.001 P,0.001
21
K (c mm d ) 0.52560.073 0.03660.018 1.68960.174 0.01060.001
P,0.001 P,0.1 P,0.001 P,0.001
asymptotic for larger larvae, as supported by the best fit yielded by the von Bertalanffy and Laird–Gompertz growth models. Also, mortality caused by predators is very important, especially for smaller larvae (Lebour, 1933; Thorson, 1946; Mileikovsky, 1974; Brewer and Kleppel, 1986; Young and Chia, 1987), and this can result in a higher survival of older larvae, with comparatively smaller growth rates. Also, the growth rates are probably underestimated due to the preferential advection of small larvae with potentially high growth rates, but probably with retention strategies that are not yet developed (Bayne, 1963, 1964; Mileikovsky, 1973).
The selection of an appropriate growth model is generally based on goodness of fit and convenience; however, some were developed on the basis of perceived growth processes. The curvilinear growth models tend to be well suited for the description of marine animal larval stages growth (Bayne, 1976; Cerrato, 1990; Campana and Jones, 1992). This is also evident from the present study. The major advantage of this class of model is that of flexibility, a feature which is required to deal with the S-shaped growth curves that are characteristic of most young stages. The best fit of the Laird–Gompertz curve in the present study can be justified on a biological basis. According to Zweifel and Lasker (1976) the fundamental concept of this model is one of change in animal
mass with time, being primarily due to self-multiplication of cells and genetically determined limitations on growth parameters.
The maximum growth rate calculated from the Laird–Gompertz equation parameters
21 21
(4.5 day ) is near the lower value of the interval from 4.1 to 11.8 day determined by Sprung (1984) for the daily growth of M. edulis larvae under laboratory conditions, with a temperature of 188C. This difference may be due to the high food availability in the laboratory, which allows faster growth. Also, the eventual presence of a higher number of larger larvae of M. galloprovincialis in the plankton of the Ria Formosa lagoon may be due to an increased planktonic life depending on the few available substrata for settlement.
It can be concluded from this study that advection may be an important factor for abundance, and availability of settlement substratum may be a key factor for growth. In addition, it can also be concluded that, following cohorts of larvae in the plankton, with a sampling of fine temporal resolution, can provide estimates of growth. However, it is not possible to ensure that larvae are collected from a continuous population throughout the entire sampling since fluctuations in growth may have resulted from immigration or emigration processes.
In addition to sampling difficulties, the determination of larval growth using the modal progression analysis of size–frequency distributions is a methodology that is difficult and susceptible to errors. Nevertheless, the use of the fine growth lines present in
Mytilus galloprovincialis larval shell helped with age attribution. Since no laboratory
calibration exists for Mytilus galloprovincialis larvae, we assumed that these were daily, following previous results with other species (Wilbur and Simkiss, 1968; Kennish, 1984). Thus, effort must be made in age assessment, especially with studies under controlled conditions, to validate the periodicity of growth ring deposition in this species.
Further studies on the growth and natural mortality of planktonic larval populations will contribute to a better understanding of the dynamics of benthic populations of
Mytilus galloprovincialis in the area. If adequate availability of substrata for settlement
is implemented, the high availability of mussel larvae in the plankton may constitute an alternative for bivalve production.
Acknowledgements
The authors are indebted to Prof. Martin Sprung for his criticism to the team of the German–Portuguese research project ‘Die Biologie der Ria Formosa’ and to Dr. Ana Barbosa for the phytoplankton information. We are also grateful to Dr. John Nascimento and Prof. Karim Erzini for improving the English.
References
´ ˜ ˜ ˆ
Bhattacharya, G.K., 1967. A simple method of resolution of a distribution into Gaussian components. Biometrics 23 (1), 115–135.
Bayne, B.L., 1963. Response of larvae of Mytilus edulis L. to increase in hydrostatic pressure. Nature 198, 406–407.
Bayne, B.L., 1964. Response of Mytilus edulis L. larvae to light and gravity. Oikos 15, 162–174. Bayne, B.L., 1965. Growth and the delay of metamorphosis of the larvae of Mytilus galloprovincialis L.
Ophelia 2, 1–47.
Bayne, B.L., 1976. The biology of mussel larvae. In: Bayne, B.L. (Ed.), Marine Mussels: Their Ecology and Physiology, Cambridge University Press, Cambridge, pp. 81–120.
Bayne, B.L., 1983. Physiological ecology of marine molluscan larvae. In: Verdonk, K. (Ed.), Development, Mollusca, Vol. 3, Academic Press, New York, pp. 299–343.
Beiras, R., Camacho, A.P., Albentosa, M., 1994. Influence of temperature on the physiology of growth in
Ruditapes decussatus (L) larvae. J. Shellfish Res. 13 (1), 77–93.
Belgrano, A., Legendre, P., Dewarumez, J.M., Frontier, S., 1995. Spatial structure and ecological variations of meroplankton on the French–Belgian coast of the North Sea. Mar. Ecol. Prog. Ser. 128, 43–50. Bertalanffy, L. von, 1938. A quantitative theory of organic growth. Hum. Biol. 10, 181–213.
Brewer, G.D., Kleppel, G.S., 1986. Diel vertical distribution of fish larvae and their prey in nearshore waters of southern California. Mar. Ecol. Prog. Ser. 27 (3), 217–226.
Calabrese, A., 1969. Individual and combined effects of salinity and temperature on embryos and larvae of the coot clam Mulinia lateralis (Say). Biol. Bull. 137 (3), 417–428.
Campana, S.E., Jones, C., 1992. Analysis of otolith microstructure data. In: Stevenson, D.K., Campana, S.E. (Eds.), Otolith Microstructure Examination and Analysis. Can. Spec. Pub. Fish. Aquat. Sci. 117, 73–100. Cerrato, R.M., 1990. Interpretable statistical test for growth comparisons using parameters in von Bertalanflly
equation. Can. J. Fish. Aquat. 47, 1416–1426.
´ ´ ˆ ´
Chıcharo, L.M.Z., 1996. Sistematica ecologia e dinamica de larvas e pos-larvas de bivalves na Ria Formosa (Systematic, ecology and dynamic of bivalve larvae and post-larvae in the Ria Formosa, PhD thesis). Tese de Doutoramento, Universidade do Algarve, Faro, 450 pp.
Hines, A.H., 1986. Larval problems and perspectives in life histories of marine invertebrates. Bull. Mar. Sci. 39, 506–525.
His, E., Robert, R., Dinet, A., 1989. Combined effects of temperature and salinity on fed and starved larvae of the Mediterranean mussel Mytilus galloprovincialis and the Japanese oyster Crassostrea gigas. Mar. Biol. 100 (4), 455–463.
Hrs-Brenko, M., 1978. The relationship of temperature and salinity to larval development in mussels (Mytilus
galloprovincialis Lamarck). In: McLusky, D.S., Berry, A.T. (Eds.), Proceedings of the 12th European
Symposium on Marine Biology, Stirling, Physiology and Behaviour of Marine Organisms, pp. 359–365.
˜ ´ ´ ´ ˜
Falcao, M., Pissarra, J., Cavaco, M., 1985. Caracterısticas fısicas e quımicas da Ria de Faro–Olhao: 1984. Rel INIP 61, 1–24.
Gayanilo, F.C., Sohriano, M., Pauly, D., 1988. A Draft Guide to the Complete Elefan (ICLARM Software, 2), International Center for Living Aquatic Resources Management, Manila, Philippines.
Gompertz, B., 1825. On the nature of the function expressive of the law of human mortality and on a new mode of determining the value of life contingencies. Philos. Trans. R. Soc. London 115, 515–585. Jørgensen, C.B., 1981. Mortality, growth and grazing impact of a cohort of bivalve larvae Mytilus
galloprovincialis L. Ophelia 20, 185–192.
Kennish, M.J., 1984. The use of shell microgrowth patterns in age determinations of the hard clam, ´
Mercenaria mercenaria (Linne). In: Mitchell, D.H., Johnson, T.E. (Eds.), Invertebrate Models in Aging
Research, CRC Press, Boca Raton, FL, pp. 143–164.
Kingston, P., 1974. Some observations on the effect of temperature and salinity upon the growth of Cardium
edule and Cardium glaucum larvae in the laboratory. J. Mar. Biol. Assoc. UK 54, 309–317.
Korringa, P., 1941. Experiments and observations on swarming pelagic life and settling in the European flat oyster Ostrea galloprovincialis L. Arch. Neerl. Zool. 5, 1–249.
Laird, A.K., Tyler, S.A., Barton, A.D., 1965. Dynamics of normal growth. Growth 29, 233–248. ´
´ ´
Le Pennec, M., 1978. Genese de la coquille larvaire et post-larvaire chez divers bivalves marins. These d’Etat, ´
Universite de Bretagne Occidentale, Laboratoire de Zoologie, Brest, 229 pp.
Lorenzen, C.J., Jeffrey, S.W., 1980. Determination of chlorophyll in seawater. UNESCO Tech. Pap. Mar. Sci. 35, 3–21.
`
Lucas, A., Cherbab-Chalabi, L., Aldana Aranda, D., 1986. Passage de l’endotrophie a l’exotrophie chez des larves de Mytilus galloprovincialis. Oceanol. Acta 9 (1), 97–103.
Manahan, D.T., 1983. The uptake and metabolism of dissolved aminoacids by bivalve larvae. Biol. Bull. 164, 243–250.
Manahan, D.T., Richardson, K., 1982. Competition studies on the uptake of dissolved organic nutrients by bivalve larvae (Mytilus galloprovincialis) and marine bacteria. Mar. Biol. 75, 241–247.
Manahan, D.T., Jaeckle, W.B., Nourizadeh, S.D., 1989. Ontogenetic changes in the rates of amino acid transport from sea water by marine invertebrate larvae (Echinodermata, Echiura, Mollusca). Biol. Bull. (Woods Hole) 176 (2), 161–168.
Mileikovsky, S.A., 1973. Speed of active movement of pelagic larvae of marine invertebrates and their ability to regulate their vertical position. Mar. Biol. 23, 11–17.
Mileikovsky, S.A., 1974. On predation of pelagic larvae and early juveniles of marine bottom invertebrates and their passing alive through their predators. Mar. Biol. 26, 303–311.
Morgan, S., 1994. Influence of tidal regime on timing of larval release: implications for recruitment. In: Dyer, J., Orth, M. (Eds.), Proceedings of ECSA22 / ERF Symposium, Changes in Fluxes in Estuaries — Implications from Science to Management, University of Plymouth, pp. 323–329.
Morgan, S., 1995. The timing of larval release. In: McEdward, L. (Ed.), Ecology of Marine Invertebrate Larvae, CRC Press, Boca Raton, FL, pp. 323–329.
Pechenik, J.A., Eyster, L.S., Widdows, J., Bayne, B.L., 1990. The influence of temperature on growth and morphological differentiation of blue mussel larvae Mytilus galloprovincialis L larvae. J. Exp. Mar. Biol. Ecol. 136, 47–64.
Quayle, D.B., 1964. Distribution of introduced marine molluscs in British Columbia waters. Fish. Res. Board Can. 21, 1155–1181.
Richards, S., Possingham, H., Noye, B., 1995. Larval dispersion along a straight coast with tidal currents: complex distribution patterns from a simple model. Mar. Ecol. Prog. Ser. 122, 59–71.
Richmond, C.E., Woodin, S.A., 1996. Short-term fluctuations in salinity: effects on planktonic invertebrate larvae. Mar. Ecol. Prog. Ser. 133, 167–177.
Rumrill, S., 1991. Natural mortality of marine invertebrate larvae. Ophelia 32 (1 / 2), 163–198.
Sprung, M., 1984. Physiological energetics of mussel larvae (Mytilus galloprovincialis). I. Shell growth and biomass. Mar. Ecol. Prog. Ser. 17, 283–293.
Sprung, M., 1994. Macrobenthic secondary production in the intertidal zone of the Ria Formosa — a lagoon in southern Portugal. Estuarine Coastal Shelf Sci. 38, 539–558.
Sokal, R.R., Rohlf, F.J., 1981. Biometry, WH Freeman, New York, 859 pp.
Strathmann, R.R., 1985. Feeding and non-feeding larval development and life history evolution in marine invertebrates. Annu. Rev. Ecol. Syst. 16, 339–361.
Thorson, G., 1946. Reproduction and larval development of Danish marine bottom invertebrates. Meddr. Komm. Damn. Fisk-og Havunders. Ser. Plankton 4, 1–523.
Thorson, G., 1950. Reproductive and larval ecology of marine bottom invertebrates. Biol. Rev. 25, 1–45. Thorson, G., 1966. Some factors influencing the recruitment and establishment of marine benthic communities.
Neth. J. Sea Res. 3 (2), 267–293.
Vance, R.R., 1973a. On reproductive strategies in marine benthic invertebrates. Am. Nat. 107 (955), 339–352. Vance, R.R., 1973b. More on reproductive strategies in marine benthic invertebrates. Am. Nat. 107 (955),
353–361.
¨ Walne, P.R., 1966. Experiments in the large scale culture of the larvae of Ostrea galloprovincialis. Helgolander
Wiss. Meeresunter. 20, 514–525.
Wilbur, R.T., Simkiss, D., 1968. Calcified shells. In: Florkin, G., Stotz, A.D. (Eds.), Comprehensive Biochemistry, Elsevier, Amsterdam, pp. 229–295.
Williams, J.G., 1980. The influence of adults on the settlement of spat of the clam Tapes japonica. J. Mar. Res. 38 (4), 729–741.
Young, C.M., Chia, F.S., 1987. Abundance and distribution of pelagic larvae as influenced by predation, behaviour and hydrographic factors. In: Giese, A.C., Pearse, J.S. (Eds.), Reproduction of Marine Invertebrates, Vol. 9, Academic Press, New York, pp. 385–463.