www.elsevier.com / locate / bres
Short communication
A di-synaptic projection from the lateral cerebellar nucleus to the
laterodorsal part of the striatum via the central lateral nucleus of the
thalamus in the rat
a ,
*
b aNoritaka Ichinohe
, Fumiaki Mori , Kazuhiko Shoumura
a
Department of Anatomy(1st Division), Hirosaki University, School of Medicine, 5 Zaifucho, Hirosaki 036-8562, Japan
b
Department of Neuropathology, Hirosaki University, School of Medicine, 5 Zaifucho, Hirosaki 036-8562, Japan
Accepted 25 July 2000
Abstract
We have examined a cerebello–thalamo–striatal pathway from the lateral cerebellar nucleus (LCN) to the laterodorsal part of the striatum (LDS) through the central lateral nucleus (CL) using light and electron microscopy through the employment of a combination of anterograde and retrograde tracing techniques. Biotinylated dextran amine (BDA) was injected into the unilateral LCN, and used as an anterograde tracer. Cholera toxin B subunit (CTb), used for light microscopy, and wheat germ agglutinin–horseradish peroxidase (WGA-HRP), used for electron microscopy, were injected into the contralateral LDS as retrograde tracers. Light microscopic analysis showed a good overlap of the distribution of BDA-labeled axon terminals and CTb-labeled neurons in the middle third of the CL in both dorsoventral and rostrocaudal axes on the LDS injection side. Electron microscopy confirmed the presence of direct synaptic contacts between BDA-labeled terminals and WGA-HRP-labeled dendrites in the CL. 2000 Elsevier Science B.V. All rights reserved.
Theme: Motor systems and sensorimotor integration
Topic: Thalamus
Keywords: Lateral cerebellar nucleus; Striatum; Central lateral nucleus of thalamus; Axonal transport; Synapse; Rat
The neurons of the lateral cerebellar nucleus (LCN) closely related to the control of motor function by the receive a large afferent input from the motor association LCN. However, no study has yet appeared to determine areas of the cerebral cortex through various brainstem whether the CL neurons that send their axons to the LDS nuclei. Efferent fibers from the LCN terminate predomi- receive monosynaptic inputs from the LCN. We therefore nantly on the thalamic nuclei that in turn project to the address this issue by using a combination of anterograde motor cortex [21,22,27,28]. Through the neural circuit and retrograde tracing techniques.
composed of these pathways, the LCN is considered to be The experiments were carried out on male Wistar rats critically involved in voluntary motor control, contributing weighing 280–340 g. All surgical procedures were per-to the programming, initiation and execution of somatic formed under general anesthesia with intraperitoneal in-movement [7]. The central lateral nucleus of the thalamus jection of pentobarbital (70 mg / kg). For light microscopic (CL), which receives input from the LCN, projects not analysis, six rats received stereotaxic injections of cholera only to the motor cortex but also to the striatum toxin B (CTb, Sigma) into the left LDS and biotinylated [1,2,4,11,12]. In the rat, the CL projects strongly to the dextran amine (BDA, Molecular Probes) into the right laterodorsal part of the striatum (LDS), which receives LCN. Injection of CTb was performed iontophoretically input from the motor cortex [6,8,10]. Thus, the neuronal through a fine glass pipette (15–30mm) filled with a 1% circuit involving the LCN, CL and LDS is considered to be solution of CTb dissolved in a buffer of 0.5 M Tris containing 2 M NaCl, 0.03 M NaN and 0.01 M EDTA3
(pH 7.5). Positive ejecting current (5–7 mA, 7 s on / 7 s *Corresponding author. Fax:181-172-37-1223.
E-mail address: [email protected] (N. Ichinohe). off) was delivered for 20–30 min. After CTb injection,
pressure injection of 10% BDA in 0.01 M phosphate buffer formed as described above. Three days after BDA in-(pH 7.2) was made through a fine glass pipette attached to jection, a 4% solution of WGA-HRP in buffered salt was a 10 ml microsyringe (Hamilton). A total volume of 0.2– injected into the LDS contralateral to the BDA injection by 0.3ml of BDA solution was delivered over a period of 15 the same method as CTb injection described above. After a min. After 5 to 7 days survival, the rats were re-anes- further 1 day survival, the rats were re-anesthetized and thetized and perfused transcardially with 100 ml of saline perfused transcardially with 100 ml of saline followed by followed by 500 ml of a solution composed of 4% 500 ml of a solution composed of 2% paraformaldehyde paraformaldehyde and 0.1% glutaraldehyde in 0.1 M and 1% glutaraldehyde in PB. After perfusion, the brains phosphate buffer (pH 7.4; PB). After perfusion, the brains were removed, postfixed for 1 h in the same fixative, and were removed and placed in a solution of 30% sucrose in placed in PB overnight at 48C. Serial frontal sections were the same buffer for 2 days at 48C. Serial frontal sections cut at 50mm on a microslicer (Dosaka EM). The sections were cut at 40mm on a freezing microtome. After washed were incubated for visualization of WGA-HRP with in 0.1 M phosphate-buffered saline (PBS), the sections tetramethyl benzidine and ammonium paratungstate, and were incubated in PBS containing avidin–biotin–peroxi- reaction products were stabilized with cobalt chloride-DAB dase complex (1:100–200 dilution; Vector) and 0.25% [17]. Subsequently, the sections were treated to visualize Triton X-100. The sections were again washed in PBS, and BDA as described above, and postfixed with a solution of then incubated in PB containing 0.1% 3,39-diaminoben- 1% osmium tetroxide in PB for 30 min. After washing in zidine dihydrochloride (DAB, Sigma) and 0.003% H O .2 2 PB, the sections were dehydrated in a graded series of BDA-labeled axons were visualized as a brown stain. The dilutions of ethanol, cleared in propylene oxide and then sections were then washed in PBS and incubated overnight embedded flat in Epon. Where there was a good overlap in PBS containing 1% normal rabbit serum, 0.25% Triton between WGA-HRP-labeled neurons and BDA-labeled X-100 and goat anti-CTb primary antibody (List Biology axon terminals, the specimens were trimmed from the Labs; 1:5,000). Subsequently, the sections were washed in thalamic region. Thin sections were serially cut on an PBS, incubated in PBS containing rabbit anti-goat IgG ultramicrotome, collected on formvar-coated, single-slot (Zymed Lab, 1:100) for 2 h, washed in PBS, incubated in grids, stained with lead citrate and uranyl acetate, and PBS containing goat peroxidase anti-peroxidase soluble examined with an electron microscope (JEM 2000-EX, complex (DAKO, 1:200) for 2 h, washed in PBS, and then JEOL).
incubated in the VIP complex (Vector) diluted in 0.01 M Electron microscopic analysis was performed on three phosphate-buffered saline (pH 7.4) according to the in- sets of serial sections from three rats. In these sections, all structions included in the VIP kit. CTb-labeled neurons labeled terminals making synaptic contacts were recon-were stained purple. The sections recon-were mounted on structed and analyzed. Data were accumulated from all the gelatinized slides. BDA-labeled axons and CTb-labeled animals. Although each set consisted of more than 100 neurons were plotted. Coverslips were then removed and serial sections, because of the limited penetration of ABC the sections were counterstained with thionin for cyto- complex, BDA-labeled terminals were only found in up to architectural landmarks. 40 sections from the surface of the block. For the same For the electron microscopic study, horseradish per- reason, most reconstructed labeled terminals were already oxidase conjugated to wheat germ agglutinin (WGA-HRP: partly cut during microslicer sectioning. Thus, the quantita-Vector) was used as a retrograde tracer instead of CTb tive data shown in Table 2 are from a limited portion of because HRP histochemistry was superior to CTb immuno- the tissue stained. Because of the difficulty of retrogradely histochemistry in maintaining the ultrastructure. BDA labeling the distal parts of dendrites with WGA-HRP [13], injection into the unilateral LCN of three rats was per- morphometric data of BDA-labeled terminals are shown
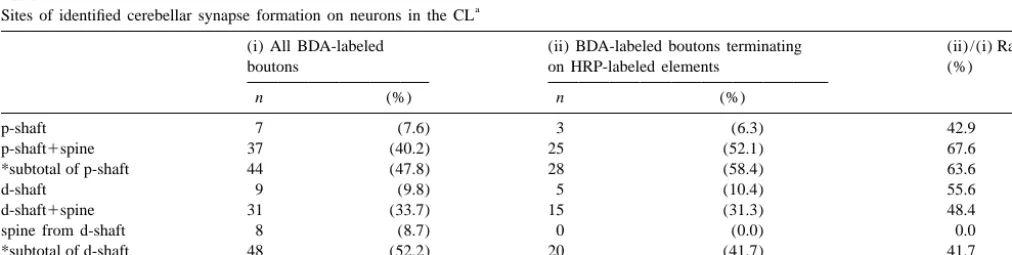
Table 1
a
Sites of identified cerebellar synapse formation on neurons in the CL
(i) All BDA-labeled (ii) BDA-labeled boutons terminating (ii) /(i) Ratio
boutons on HRP-labeled elements (%)
n (%) n (%)
p-shaft 7 (7.6) 3 (6.3) 42.9
p-shaft1spine 37 (40.2) 25 (52.1) 67.6
*subtotal of p-shaft 44 (47.8) 28 (58.4) 63.6
d-shaft 9 (9.8) 5 (10.4) 55.6
d-shaft1spine 31 (33.7) 15 (31.3) 48.4
spine from d-shaft 8 (8.7) 0 (0.0) 0.0
*subtotal of d-shaft 48 (52.2) 20 (41.7) 41.7
**total 92 (100.0) 48 (100.0) 52.2
a
Table 2
Morphometric data of BDA-labeled boutons in the CL
(i) All BDA-labeled boutons (ii) BDA-labeled boutons terminating on HRP-labeled elements
Mean6S.D. Range Mean6S.D. Range
Number of terminal specializations
of each bouton 4.664.0 (1–15) 4.563.5 (1–13)
Number of spines which are
contacted by each bouton 2.062.0 (0–8) 2.262.4 (0–8)
Short axis diameter of target
dendrites(mm) 1.286042 (0.42–1.79) 1.3660.39 (0.71–1.79)
Short axis diameter of
BDA-labeled boutons(mm) 1.1760.44 (0.30–2.08) 1.2160.43 (0.48–1.79)
not only for those contacting WGA-HRP-labeled targets as proximal dendrites. Small dendrites with a lower density but also for all labeled terminals (Tables 1 and 2, Fig. 4). of these organellae were considered distal dendrites. However, apparent differences were not found between the Representative short-axis diameters of boutons and de-two sets of data. Dendrites were classified into proximal ndrites were determined by choosing the maximum figures and distal according to size and density of cytoplasmic from short-axis diameters measured in reconstructed serial organellae. Large dendrites with a higher density of sections.
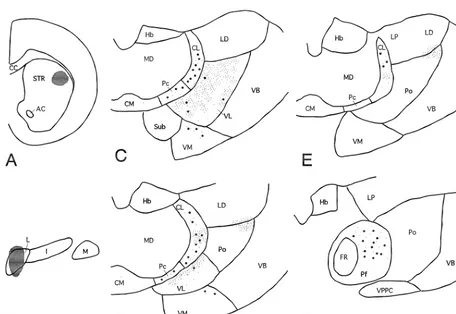
ribosomes, endoplasmic reticulum, Golgi apparatus, mito- When the BDA injection site was localized within the chondria, microtubules and neurofilaments were classified LCN, the labeled terminal distribution in the dorsal
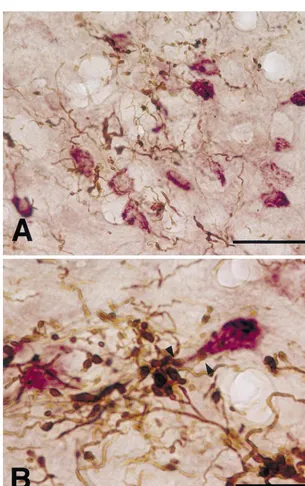
thalamus was similar to that described in a previous report labeled terminals and CTb-labeled neurons in the middle by Aumann et al. [4]. Labeled terminals were found in the third of the CL in both dorsoventral and rostrocaudal axes caudal two-thirds of the CL, contralateral to the BDA (Figs. 1 and 2). Occasionally, BDA-labeled boutons were injection. Within this region, labeled terminals were denser in close apposition to CTb-labeled dendrites in this region and larger in size in the middle third of the dorsoventral (Fig. 2B). Besides in the CL, an overlap of the dis-axis of the CL than in its other regions (Figs. 1 and 2). tributions of BDA-labeled terminals and CTb-labeled CTb-labeled neurons were predominant ventrally in the neurons was found in the ventrolateral nucleus (VL), rostral third of the CL, while in the caudal third, they were ventromedial nucleus (VM), paracentral nucleus (Pc) and dorsally predominant. In the rostrocaudal middle third, parafascicular nucleus (Pf). However, cerebellar terminals they were distributed throughout in the dorsoventral axis. were smaller and less dense in the VM, Pc and Pf than in Thus, there was a good overlap in the extent of BDA- the overlapping region of the CL. CTb-labeled neurons
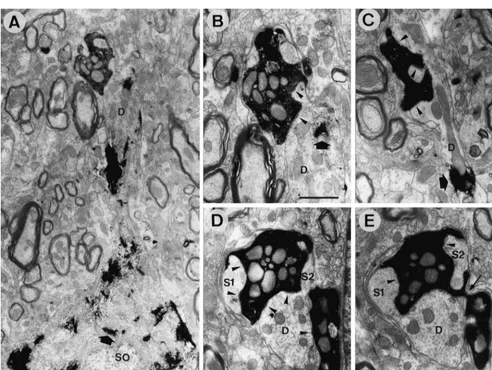
were less dense in the VL even though BDA-terminals terminal frequently made small (up to 0.5mm) but multiple were densest among the thalamic nuclei described above. (up to 15) synaptic specializations with a dendritic shaft Electron microscopic analysis was performed in the CL and its spine-like appendages (Fig. 3B–E; Tables 1 and 2). area where WGA-HRP-labeled neurons and BDA-labeled Occasionally two or three labeled boutons connected by terminals overlapped well (i.e. in the middle third of the thin short axons shared a common target dendrite (Fig. 3D, CL in the rostrocaudal and dorsoventral axes, like the CTb E). Only 3 out of 92 reconstructed terminals made synaptic injection cases mentioned above). WGA-HRP-labeled cell contacts with two dendritic profiles, suggesting that each bodies and dendrites contained electron-dense peroxidase LCN terminal usually makes contact with a single target reaction product, while BDA-labeled terminals were recog- dendrite. The synaptic sites of a substantial proportion of nized by the presence of electron-dense amorphous re- BDA-labeled terminals were judged to be proximal de-action product that filled the spaces between the synaptic ndrites (47.8% of all labeled terminals and 58.4% of vesicles and mitochondria (Fig. 3). About half the BDA- labeled terminals contacting WGA-HRP-labeled dendrites: labeled terminals made synaptic contacts with dendritic Figs. 3A, B and 4; Table 1). In fact, the target dendrites of shafts and spine-like appendages labeled with WGA-HRP 9 out of 92 labeled terminals could be followed to their reaction product (Fig. 3A–C; Table 1). BDA-labeled cell bodies (Fig. 3A). In these cases, the distances between terminals were large in size and made asymmetrical the terminal contact site and cell body-dendrite border synaptic contacts with their targets (Fig. 3 and Table 2). were less than 60mm.
Reconstruction from serial sections showed that each In this study, we have demonstrated the overlapping
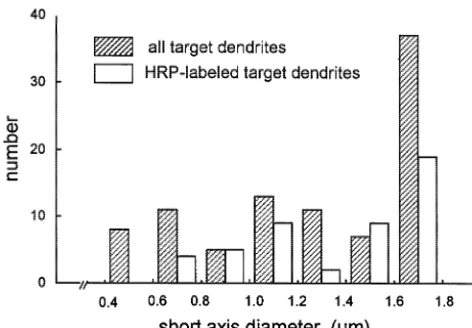
neurons (projection neurons) of the striatum. Cortical fibers synapse mainly with the heads of spines, whereas thalamic terminals preferentially synapse with dendritic shafts [9,23]. Furthermore, cortical or thalamic afferents have complementary input upon subclasses of striatal interneurons. That is, cholinergic interneurons of the striatum receive only a sparse input from the cerebral cortex, while they receive robust glutamatergic input of thalamic origin [15,18,19]. Other populations of striatal interneurons immunoreactive for somatostatin / neuropep-tide Y or parvalbumin / GABA receive cortical but not thalamic input [5,14,16,20,26]. Motor cortical input is also modulated by the LCN through several thalamic nuclei (mainly the VL). Thus, the LCN could modulate LDS function simultaneously at least in two different ways, Fig. 4. Histogram illustrating the size distribution of dendrites
postsynap-through the cerebello-thalamo (mainly VL)-motor cortico-tic to BDA-labeled terminals in the middle third of the CL in rostrocaudal
striatal pathway and the cerebello-thalamo (mainly CL)-and dorsoventral axes of the rat.
striatal pathway including the di-synaptic LCN-CL-LDS link shown in this study. The physiological significance of distribution of cerebellar terminals from the LCN and the this LCN-CL-LDS link awaits elucidation.
thalamic neurons sending their axons to the LDS in the CL by simultaneous visualization methods. Furthermore, we have shown monosynaptic connections between LCN
Acknowledgements terminals and CL-LDS neurons.
The morphological features of cerebellar terminals in the
This study was supported by Karouji Memorial Founda-CL were very similar to those previously reported for the
tion for Medical Research. The authors are grateful to Paul VL, red nucleus (RN), Pf, central medial nucleus (CM)
Hollister M.D., Medical English Consultant, Hirosaki and zona incerta (ZI) in the rat [3]. Cerebellar terminals in
University School of Medicine, for his thorough review of these brain regions were large and made multiple
English. asymmetric synaptic contacts with dendritic shafts and
spine-like appendages. Aumann et al. [3] also pointed out that cerebellar terminals predominately contacted proximal
dendrites in the main target nuclei (the VL and RN) of the References cerebellum. In the VL, 55% of these inputs occur on
proximal dendrites and only 3% on somata; in the RN, [1] P. Angaut, F. Cicirata, F. Serapide, Topographic organization of the cerebellothalamic projections in the rat. An autoradiographic study, 65% are found on proximal dendrites. By contrast, neurons
Neuroscience 15 (1985) 389–401. in the minor target nuclei such as the Pf, CM and ZI
[2] C. Asanuma, W.T. Thach, E.G. Jones, Anatomical evidence for receive only a small portion of cerebellar inputs on their
segregated focal groupings of efferent cells and their terminal proximal dendrites; in the Pf 15% occur on proximal ramifications in the cerebellothalamic pathway of the monkey, Brain dendrites while in the CM there are 8%. In the ZI, 13% are Res. Rev. 5 (1983) 267–298.
found on proximal dendrites. This may mean that cerebel- [3] T.D. Aumann, M.K. Horne, A comparison of the ultrastructure of synapses in the cerebello-rubral and cerebello-thalamic pathways in lar synaptic transmission is very secure through such
the rat, Neurosci. Lett. 211 (1996) 175–178. favorably positioned contacts on the VL and RN neurons.
[4] T.D. Aumann, J.A. Rawson, D.I. Finkelstein, M.K. Horne, Projec-In fact, electrophysiological studies have shown that tions from the lateral and interposed cerebellar nuclei to the cerebellar input can evoke large unitary excitatory post- thalamus of the rat: a light and electron microscopic study using synaptic potentials having a fast rising phase on VL and single and double anterograde labelling, J. Comp. Neurol. 349
(1994) 165–181. RN neurons [24,25]. We have shown that the proportion of
[5] B.D. Bennett, J.P. Bolam, Synaptic input and output of parvalbumin-cerebellar boutons contacting proximal dendrites of CL
immunoreactive neurons in the neostriatum of the rat, Neuroscience neurons is similar to that of neurons in the cerebellar main 62 (1994) 707–719.
target nuclei (see above). Thus, potent excitatory cerebello- [6] H.W. Berendse, H.J. Groenewegen, Organization of the thalamos-thalamic transmission can occur in the CL, suggesting the triatal projections in the rat, with special emphasis on the ventral
striatum, J. Comp. Neurol. 299 (1990) 187–228. CL is a significant relay site for the information from the
[7] V.B. Brooks, W.T. Thach, Cerebellar control of posture and move-LCN to the LDS.
ment, in: V.B. Brooks (Ed.), Handbook of Physiology, Section 1: The LDS receives input from the motor cortex as well as The Nervous System, Motor Control, Vol. 2, American Physiological from the thalamus. Cortical and thalamic fibers are known Society, Bethesda, 1981, pp. 877–946.
in-dividual cortical fields conform to histochemically distinct striatal neurons in nucleus accumbens of the rat: a light and electron compartments in the rat, Brain Res. 365 (1986) 397–403. microscopic study, J. Comp. Neurol. 296 (1990) 204–221. [9] L. Dube, A.D. Smith, J.P. Bolam, Identification of synaptic terminals [20] T.M. Rudkin, A.F. Sadikot, Thalamic input to
parvalbumin-im-of thalamic or cortical origin in contact with distinct medium-size munoreactive GABAergic interneurons: organization in normal spiny neurons in the rat neostriatum, J. Comp. Neurol. 267 (1988) striatum and effect of neonatal decortication, Neuroscience 88
455–471. (1999) 1165–1175.
[10] A. Ebrahimi, R. Pochet, M. Roger, Topographical organization of [21] G.R. Schell, P.L. Strick, The origin of thalamic inputs to the arcuate the projections from physiologically identified areas of the motor premotor and supplementary motor areas, J Neurosci. 4 (1984) cortex to the striatum in the rat, Neurosci. Res. 14 (1992) 39–60. 539–560.
[11] A.J. Haroian, L.C. Massopust, P.A. Young, Cerebellothalamic pro- [22] Y. Shinoda, M. Kano, T. Futami, Synaptic organization of the jections in the rat: an autoradiographic and degenerative study, J. cerebello-thalamo-cerebral pathway in the cat. I. Projection of Comp. Neurol. 197 (1981) 217–236. individual cerebellar nuclei to single pyramidal tract neurons in [12] S.H.C. Hendry, E.G. Jones, J. Graham, Thalamic relay nuclei for areas 4 and 6, Neurosci. Res. 2 (1985) 133–156.
cerebellar and certain related fiber systems in the cat, J. Comp. [23] P. Somogyi, A.J. Hodgson, A.D. Smith, An approach to tracing Neurol. 185 (1979) 679–714. neuron networks in the cerebral cortex and basal ganglia. Combina-[13] N. Ichinohe, K. Shoumura, A di-synaptic projection from the tion of Golgi staining, retrograde transport of horseradish peroxidase superior colliculus to the head of the caudate nucleus via the and anterograde degeneration of synaptic boutons in the same centromedian-parafascicular complex in the cat: an anterograde and material, Neuroscience 4 (1979) 1805–1852.
retrograde labeling study, Neurosci. Res. 32 (1998) 295–303. [24] N. Tsukahara, K. Toyama, K. Kosaka, Electrical activity of red [14] P. Kachidian, J. Vuillet, A. Nieoullon, G. Lafille, L. Kerkerian-Le nucleus neurones investigated with intracellular microelectrodes,
Goff, Striatal neuropeptide Y neurons are not a target for thalamic Exp. Brain Res. 4 (1967) 18–33.
afferent fibres, NeuroReport 7 (1996) 1665–1669. [25] M. Uno, M. Yoshida, I. Hirota, The mode of cerebello-thalamic [15] S.R. Lapper, J.P. Bolam, Input from the frontal cortex and the relay transmission investigated with intracellular recordings from parafascicular nucleus to cholinergic interneurons in the dorsal cells of the ventrolateral nucleus of cat’s thalamus, Exp. Brain Res. striatum of the rat, Neuroscience 51 (1992) 533–545. 10 (1970) 121–139.
[16] S.R. Lapper, Y. Smith, A.F. Sadikot, A. Parent, J.P. Bolam, Cortical [26] J. Vuillet, L. Kerkerian, P. Kachidian, O. Bosler, A. Nieoullon, input to parvalbumin-immunoreactive neurons in the putamen of the Ultrastructural correlates of functional relationships between nigral squirrel monkey, Brain Res. 580 (1992) 215–224. dopaminergic or cortical afferent fibers and neuropeptide Y-con-[17] I.J. Llewellyn-Smith, P. Pilowsky, J.B. Minson, Retrograde tracers taining neurons in the rat striatum, Neurosci. Lett. 100 (1989)
for light and electron microscopy, in: J.P. Bolam (Ed.), Experimental 99–104.
Neuroanatomy: A Practical Approach, Oxford University Press, [27] R. Wiesendanger, M. Wiesendanger, Cerebello-cortical linkage in New York, 1992, pp. 31–59. the monkey as revealed by transcellular labeling with the lectin [18] G.E. Meredith, H.T. Chang, Synaptic relationships of enkephaliner- wheat germ agglutinin conjugated to the marker horseradish
per-gic and cholinerper-gic neurons in the nucleus accumbens of the rat, oxidase, Exp. Brain Res. 59 (1985) 105–117.
Brain Res. 667 (1994) 67–76. [28] M. Yoshida, K. Yajima, M. Uno, Different activation of the 2 types [19] G.E. Meredith, F.G. Wouterlood, Hippocampal and midline thalamic of the pyramidal tract neurones through the