www.elsevier.com / locate / bres
Research report
Electroconvulsive seizures modulate levels of thyrotropin releasing
hormone and related peptides in rat hypothalamus, cingulate and
q
lateral cerebellum
a,c ,
*
e a,b,dA. Eugene Pekary
, James L. Meyerhoff , Albert Sattin
a
Research, VA Greater Los Angeles Healthcare System, Los Angeles, CA 90073, USA
b
Psychiatry Services, VA Greater Los Angeles Healthcare System, Los Angeles, CA 90073, USA
c
Department of Medicine, UCLA School of Medicine, Los Angeles, CA 90073, USA
d
Department of Psychiatry and Biobehavioral Sciences and Brain Research Institute, UCLA School of Medicine, Los Angeles, CA 90073, USA
e
Department of Neuroendocrinology and Neurochemistry, Div. Neuroscience, Walter Reed Army Institute of Research, Washington, DC 20307, USA
Accepted 29 August 2000
Abstract
We have studied the neuroanatomic extent of electroconvulsive (ECS)-responsive prepro-TRH and TRH-related gene expression and its possible interaction with forced swimming. Young adult male Wistar rats were treated in a 232 Latin square protocol of swimming, no swimming, three daily ECS or sham ECS. Sixteen different brain regions were dissected and immunoreactivity measured for TRH ( pGlu–His–Pro–NH ); TRH–Gly, a TRH precursor; Ps4, a prepro-TRH-derived TRH-enhancing decapeptide, and EEP ( pGlu–Glu–2 Pro–NH ). ECS, in addition to elevating TRH–immunoreactivity (TRH–IR), TRH–Gly–IR, Ps4–IR and EEP–IR levels in the limbic2 regions, as we have previously reported, also significantly increased Ps4–IR levels in hypothalamus, posterior cingulate and lateral cerebellum, and increased TRH–Gly–IR levels in hypothalamus. Interestingly, the combination of ECS and swimming significantly reduced the levels of TRH–Gly–IR in the anterior cingulate compared to the sham ECS-no swim group. The combined use of high-pressure liquid chromatography and the EEP radioimmunoassay (RIA) revealed that pGlu–Tyr–Pro–NH and / or pGlu–Phe–Pro–2 NH occur in amygdala, anterior cingulate, frontal cortex, entorhinal cortex, lateral cerebellum and striatum and make a substantial2 contribution to the EEP–IR and TRH–IR. We conclude that ECS can alter the expression and secretion of TRH-related peptides in the hypothalamus, cingulate and lateral cerebellum. Such effects have not previously been reported in these limbic and extra-limbic regions which are increasingly implicated in the autonomic, behavioral and volitional changes which accompany severe depression and its treatment. 2000 Elsevier Science B.V. All rights reserved.
Theme: Neural basis of behavior
Topic: Neuropeptides and behavior
Keywords: Depression; Limbic system; High-pressure liquid chromatography; Radioimmunoassay
1. Introduction
Electroconvulsive therapy (ECT) is an effective method q
Research was conducted in compliance with the Animal Welfare Act, for the treatment of severe unipolar and bipolar depression and other Federal statutes and regulations relating to animals and
as well as schizophrenia and Parkinson’s disease [42,47].
experiments involving animals and adheres to principles stated in the
Understanding the mechanism by which ECT ameliorates
Guide for the Care and Use of Laboratory Animals, NIH publication
86-23, 1985 edition. these serious mental disorders should lead to safer and
The views of the authors do not purport to reflect the position of the more cost-effective treatments. Evidence has accumulated Department of the Army or the Department of Defense (para 4-3, AR that electroconvulsive seizures (ECS) modify the expres-360-5).
sion of several genes in various regions of the brain
*Corresponding author. Tel.: 11-310-268-4430; fax: 1
1-310-268-associated with regulation of mood, including the
4982.
E-mail address: [email protected] (A.E. Pekary). amygdala, hippocampus, entorhinal cortex, and pyriform
cortex, important components of the limbic system efficacy of ECT for a variety of mental disorders including [3,14,17,34,43,44]. Genes which undergo a sustained depression.
increase in expression include those for thyrotropin releas-ing hormone (TRH, pGlu–His–Pro–NH ) and a closely2
related peptide, pGlu–Glu–Pro–NH (EEP) [31].2 2. Materials and methods
Increased transcription, translation and post-translational
processing of the prepro-TRH gene leads to the production 2.1. Peptides of not only more TRH, but also other prepro-TRH-derived
peptides which may have a role in alleviating depressive Ps4 (Ser–Phe–Pro–Trp–Met–Glu–Ser–Asp–Val–Thr) symptoms. These include Ps4, a TRH-enhancing decapep- was obtained from Bachem California (Torrance, CA,
2
tide and Ps5, corticotropin releasing hormone (CRH) USA), [Phe ]–TRH and Tyr –Ps4 were prepared by0
inhibiting factor (CRIF, a 22 amino acid residue peptide) Peninsula Laboratories (Belmont, CA, USA). EEP ( pGlu– [32,37–39]. Ps4 greatly enhances the pituitary TSH [5] and Glu–Pro–NH ) and TRH ( pGlu–His–Pro–NH ) were2 2
gastric acid releasing activity [48] of TRH but lacks any purchased from Sigma Chemical Co. (St. Louis, MO).
2
TRH-like activity itself. It is likely that Ps4 also enhances [Tyr ]–TRH was custom synthesized by the UCLA Pep-the CNS effects of TRH since it is a necessary byproduct tide Synthesis Laboratory.
of TRH biosynthesis and would be cosecreted with TRH
by TRH-containing synaptic vesicles. Ps5 inhibition of 2.2. Immunization procedure release of CRH, should also contribute to the
antidepres-sant effect of ECT, because excessive CRH may contribute Ps4 and EEP were conjugated to keyhole limpet to anxiety components of depressive syndromes [26]. hemocyanin (KLH, Sigma Chemical Co., St. Louis, MO, TRH has been reported to alleviate depressive symp- USA) by the carbodiimide method [1]. TRH was conju-toms [20], but the necessity of intrathecal administration gated to KLH by the dinitrodifluorobenzene method [48]. to minimize rapid inactivation by serum enzymes, is a New Zealand white rabbits (Universal Animal Care, serious limitation for clinical use of this peptide. Numer- Bloomington, CA) were immunized subcutaneously with a ous analogs of TRH with greatly increased in vivo stable emulsion prepared from equal volumes of conjugate stability and enhanced CNS versus TSH-stimulating effect (1.5 mg / ml saline) and Freund’s complete adjuvant. After exist [11]. Interestingly, EEP, an endogenous TRH-like shaving a 10310-cm area of the back or flank, rabbits peptide, is not metabolized by thyroliberrinase, a blood received 10–12 subcutaneous injections, equalling 1.0 ml
enzyme which specifically inactivates TRH [25]. EEP of emulsion prepared with the Ps4– and EEP–KLH
does not bind to TRH receptors isolated from the pituitary conjugates, as previously described [31,32]. Three weeks of mammals, as evidenced by the inability of high dose later, all rabbits received injections of immunogen identi-EEP to release T from the thyroid gland [19]. Neverthe-3 cal to that received initially. The rabbits were bled from an less, EEP does have antidepressant activity similar to that ear vein prior to the second and subsequent immunizations. for TRH in the Porsolt Forced Swim Test [19].
Inter-action of EEP with an EEP-specific receptor is a possible 2.3. Experimental design mechanism of action.
The symptomatology which is included in depressive Male Wistar rats weighing 160–180 g on arrival syndromes might involve pathophysiologic changes in (Simonsen Laboratories, Gilroy, CA, USA), maintained in brain areas outside the classical limbic structures. For a temperature and light-controlled environment (lights on: example, insomnia, anorexia, and alterations in normal 6 am to 6 pm) were provided water and standard rodent lab diurnal rhythms alter neuroendocrine functions associated chow (Purina[5001 and water ad libitum. After a 1-week
paradigms are not directly comparable [44]. Their effect on ous phase was lyophilized. After dissolving the residue in swim immobility time in the Porsolt forced swim test was 0.1% trifluoroacetic acid (TFA), each pooled extract was
measured 24 h after the last ECS. loaded onto a C18 Sep-Pak cartridge (Waters, Milford,
MA) which had been previously activated by washing with methanol and water. The cartridges were eluted with 3 ml 2.4. Forced swim test
of methanol and the combined sample pass-through and methanol eluates were dried completely on a heater block The Porsolt forced swim test involves two phases. In the
and redissolved in 0.1% TFA. Particulates were removed first phase (pre-swim), the rats are individually placed in a
with a HPLC-certified Acro LC3A filter (Pall Gelman tank of water for 15 min, from which they cannot escape.
Laboratories, Ann Arbor, MI). These pooled extracts were During the second phase (swim test), the animals are
then injected into a programmable HPLC system
returned to the tank for 5 min, and the duration of
(Shimadzu Corp, Kyoto) equipped with a 4.63150 mm immobility is recorded. Antidepressant treatments (ECS)
Econosphere, 3 mm C18 reverse phase column (Alltech are administered between the first (pre-swim) and second
Associates, Deerfied, IL) previously equilibrated with 0.1% (swim test) phases of the Porsolt procedure. The
antide-TFA. At the time of injection, a 0.33% / min gradient of pressant treatments typically increase the swimming
activi-acetonitrile at a flow-rate of 1 ml / min was started. At 30 ty (decreased immobility). Decreases in mean immobility
min the gradient was increased to 1% / min and at 70 min time (during swim test), relative to sham-treated animals,
the gradient was further increased to 5% / min. The 1 ml are predictive of antidepressant effects of drugs and ECS
fractions collected were dried completely and reconstituted [6,31,32,35,44]. Twenty-four hours before the first ECS,
with 1 ml of 0.02% NaN3 just before EEP RIA. The all rats were placed in a cylindrical polycarbonate tank of
optical density profile for a mixture of EEP, TRH, pGlu– water (2560.58C) for 15 min (pre-swim). The tank was 22
Tyr–Pro–NH , and pGlu–Phe–Pro–NH (252 2 mg / ml each, cm in diameter and filled to a depth of 30 cm. At this depth
0.5 ml injected volume,1.0 absorbance units full scale) was the rat is not able to contact the bottom of the cylinder
used to determine the HPLC retention times for these four while floating. One day after the third (final) ECS, all rats
peptides (results not shown). were placed into the tank for 300 s (swim test). All
conditions were the same. The cumulative duration of immobility (absence of swimming movements) was
re-2.7. RIA procedure corded [31,32,44].
125
TRH, Tyr –Ps4 and TRH–Gly were labeled witho I
2.5. Dissection and extraction using the chloramine-T method [30–32]. Using a small
column of 2% BSA-treated Sephadex G10 packed in a
125
Olfactory bulbs, vermis, lateral cerebellum, medulla, 5-ml pipet, the tracer was separated from unreacted I by pons, interpeduncular nucleus, frontal cortex, anterior and eluting with phosphate-buffered saline (PBS), pH 7.5. It posterior cingulate, septum, ventral striatum (nucleus ac- was then diluted in 0.325% normal rabbit serum, 0.025 M cumbens and olfactory tubercle), striatum, pyriform cortex, EDTA, and 0.02% sodium azide (NaN ) to 15 000 cpm /
3
hypothalamus, hippocampus, entorhinal cortex, amygdala, 0.2 ml. Ps4, EEP, TRH or TRH–Gly standards and thalamus, rest of cortex and midbrain were hand dissected samples were diluted in 0.02% NaN . Standards and tissue
3
as previously described [21,22], weighed rapidly, and then extracts or HPLC fractions, in duplicate 100-ml aliquots, boiled in 1.0 M acetic acid at 958C for 15 min in glass test tracer (200 ml), Ps4 antiserum 1128B7 diluted 1:2000, tubes [31,32,44]. After Polytron (Brinkman Instruments, EEP antiserum 580B4 diluted 1:100, TRH antiserum Westbury, NY, USA) homogenization, the samples were 465B12 diluted 1:5000 or TRH–Gly antiserum 898B6 lyophilized to a damp pellet, redispersed in 2.0 ml metha- diluted 1:200 in 0.02% NaN in PBS to give 20%
3
nol, centrifuged, and the supernatant transferred to glass specifically bound counts / total counts (B /T ), and goat
o
test tubes and dried completely. Dried samples from a antirabbit IgG in 0.02% NaN in PBS (100ml) were added
3
given experiment were stored at2208C and reconstituted together, vortex mixed, and incubated at 48C for 24 h. with 1.0 ml 0.02% NaN3 prior to Ps4, EEP, TRH and Tubes were centrifuged at 1000 g for 30 min, aspirated, TRH–Gly radioimmunoassay (RIA) and high-pressure and counted for 2 min. The minimum detectable dose was
liquid chromatography (HPLC). 20 pg / ml. Each of these RIAs has previously been
described in detail [30–33,44]. All samples from an
2.6. HPLC experiment were run in the same assay. pGlu–Tyr–Pro–
2 2
NH (Tyr –TRH) and pGlu–Phe–Pro–NH (Phe –TRH)2 2
2.8. Statistical analysis shown). Post-ECS hindlimb paralysis reduced the number of ECS animals. This was most likely due to the use of Statistical comparisons were made with the aid of 65-day-old, 320 g body weight, instead of 55-day-old, 250 Statview (Abacus Concepts, Inc., Berkeley, CA), a statisti- g animals. The three rats that received both ECS and a cal software package for the Macintosh computer. All swim score were not sufficient for meaningful correlation multigroup comparisons were carried out by one way of swimming behavior with levels of TRH and TRH-analysis of variance using post-hoc Scheffe contrasts with related peptides in various brain regions.
the control group. Nevertheless, highly significant increases in the levels of
Ps4–IR, TRH–IR, and TRH–Gly–IR were observed in a variety of brain regions, not only in the limbic system as
3. Results we have previously reported [31,32,44], but also in a
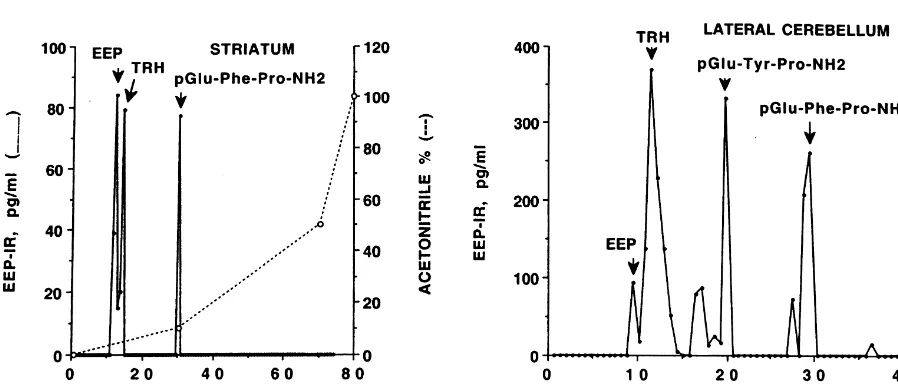
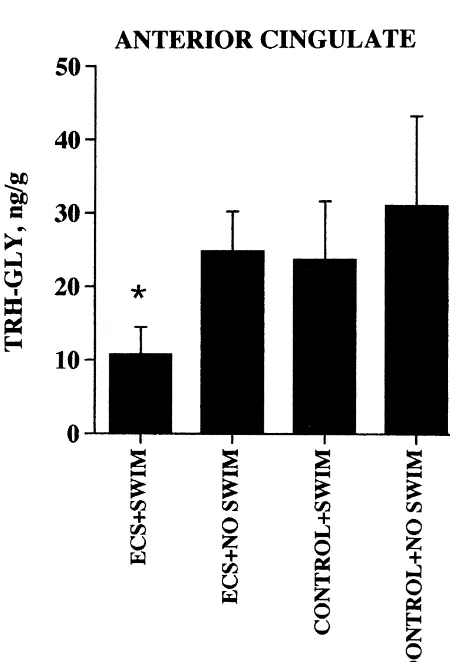
number of non-limbic areas as summarized in Table 1. The combined use of HPLC and EEP RIA (see Fig. 1 Consistent with our previous reports [31,32,44], ECS for representative EEP–IR profiles following HPLC of increased Ps4–IR, TRH–IR and TRH–Gly–IR levels in
2
pooled tissue extracts) demonstrated that EEP, TRH, Tyr – pyriform cortex, hippocampus, entorhinal cortex, and
2
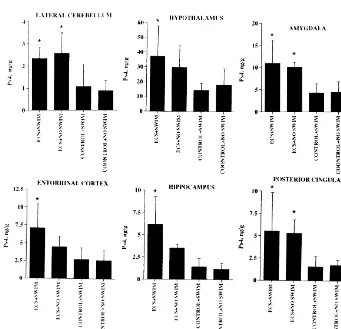
TRH, and Phe –TRH all occur in rat brain but their amygdala. TRH–Gly–IR levels in the amygdala were at relative concentrations vary markedly between specific least 10-fold higher than the corresponding levels in any brain regions. A minor EEP–IR peak with a retention time other brain region studied. In addition, we found that ECS of about 15 min has not yet been identified. It is apparent increased Ps4–IR levels in hypothalamus, posterior cingu-that in lateral cerebellum and striatum (Fig. 1) and in late and lateral cerebellum and raised the TRH–Gly–IR amygdala, anterior cingulate, entorhinal cortex, and frontal concentration in the hypothalamus.
cortex (results not shown), the EEP–IR (and TRH–IR) The combined effect of ECS and swimming was to
consists of a mixture of two or more different TRH-like decrease the level of TRH–Gly–IR in the anterior
cingu-2
peptides. Following ECS the peak corresponding to Phe – late (Fig. 2), an observation that has recently been TRH in lateral cerebellum increased six-fold (results not confirmed in a similar protocol using vagal nerve stimula-shown). We have not been successful in our attempts to tion instead of ECS (S. Krahl, A.E. Pekary, A. Sattin,
2
produce more specific antibodies to EEP, Tyr –TRH and unpublished results). This result is consistent with
in-2
Phe –TRH using currently available methodologies [28]. creased conversion of TRH–Gly–IR (for anterior
cingu-2 2 2
This is the first report of Tyr –TRH and Phe –TRH late, a mixture of EEP–Gly and Tyr –TRH–Gly) to EEP
2
occurring in mammalian brain. and Tyr –TRH, respectively, the major EEP–IR peaks
Three ECS administered on consecutive days with found after HPLC and EEP RIA of this tissue (result not progressively increasing current, with or without forced shown), and release of these peptides [4,30]. This com-swimming, did not affect total body weights or tissue bined suppressive effect of ECS and forced swimming on weights in the brain regions that were dissected (results not TRH–Gly–IR concentration in the anterior cingulate is in
Table 1
Effect of electroconvulsive seizure (ECS; three swim plus three non-swim) versus sham ECS (control; six swim plus six non-swim) treatment on levels of Ps4 immunoreactivity (Ps4–IR); TRH–IR, and TRH–Gly–IR, (ng / g wet weight), levels in various brain regions of male Wistar rats, 300 to 350 g body
a
weight
Brain region Pyriform cortex Hypothalamus Hippocampus Entorhinal cortex Amygdala Posterior cingulate Lateral cerebellum
TRH–IR
P-values are for the two-tailed Student’s t-test.
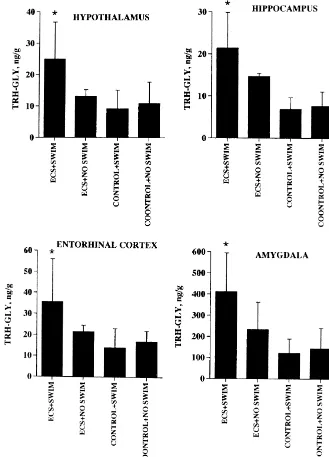
marked contrast with the observed increase in TRH–Gly– IR, TRH–IR and Ps4–IR levels in other brain regions, summarized in Figs. 3–5.
TRH, TRH–Gly and Ps4 are all prepro-TRH-derived
2
peptides. The TRH-like peptides, EEP, Tyr –TRH, and
2
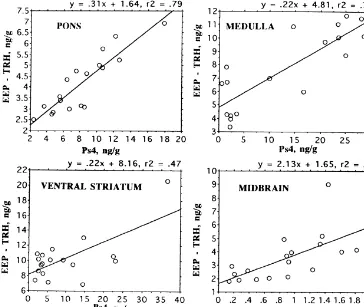
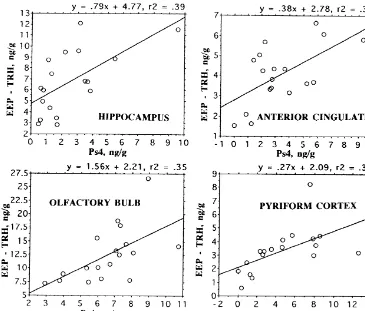
Phe –TRH are the products of other, as yet uncharacter-ized, precursor proteins [31]. Nevertheless, expression of these peptides appears to vary in a similar manner to the prepro-TRH-derived peptides as evidenced by the signifi-cant correlation of EEP–IR and Ps4–IR levels in a variety of brain regions as displayed in Figs. 6 and 7.
4. Discussion
Previous studies clearly indicate that ECS increases the expression, in rat limbic system, not only of the prepro-TRH gene [17] but also the enzymes [3] responsible for the processing of the corresponding protein to a variety of prepro-TRH-derived peptides including TRH–Gly, TRH, Ps4, and Ps5 [31,32,44]. In this and our previous studies, ECS consistently increases the levels of TRH–Gly–IR, TRH–IR and Ps4–IR in rat pyriform cortex, hippocampus, entorhinal cortex, and amygdala [31,32,44]. We report for the first time that ECS also increases Ps4–IR levels in the lateral cerebellum, posterior cingulate and hypothalamus. TRH–Gly–IR levels are also increased in the
hypo-2
thalamus and Phe –TRH concentration increased in lateral cerebellum.
The cerebellum receives its inputs from the sensory,
Fig. 2. Effect of electroconvulsive seizures (ECS), sham ECS, forced motor and parietal cortex by way of the relay nuclei in the swimming (swim) and no swim on TRH–Gly levels in the anterior
pons. From the cerebellar hemispheres (lateral cerebellum)
cingulate of male Wistar rats. The number of animals in each group (in
the output is projected to the cerebellar deep nuclei then to
parentheses) were: ECS1SWIM (3); ECS1NO SWIM (3); CONTROL1
Fig. 3. Effect of electroconvulsive seizures (ECS), sham ECS, forced swimming (swim) and no swim on TRH–Gly levels in hypothalamus and three limbic regions of male Wistar rats.
Fig. 4. Effect of electroconvulsive seizures (ECS), sham ECS, forced swimming (swim) and no swim on TRH levels in three limbic regions of male Wistar rats.
Fig. 6. Correlation of EEP levels and Ps4 concentration in three posterior brain regions and ventral striatum of male Wistar rats. All correlations are
P,0.01. EEP–IR calculated as the difference between EEP RIA results, which gives sum of EEP and TRH levels [31], and the specific TRH RIA.
well as mood-regulating biogenic amines and other neuro- neurosecretory neurons projecting to the hypothalamic– peptides or the responsiveness of the cognate receptor and pituitary portal circulation [45]. The occurrence of less
second messenger systems [6,16,24]. abundant TRH-containing neurons within the
hypo-Interneuronal signalling by biogenic amines, amino thalamus that are less efficient in converting TRH–Gly to acids, neuropeptides and protein hormones is mediated form TRH but are susceptible to upregulation of prepro-primarily by G-protein-coupled receptors [10]. The great TRH expression by ECS, is evidenced by the ECS-induced diversity and specificity of signalling between cells is due increase in TRH–Gly–IR, and Ps4–IR. These cells do not, to the variety of somatic and dendritic couplings between however, contribute enough TRH to alter the high level of neurons [8], the large number of receptor subtypes with this tripeptide produced for neurosecretion into the portal varying G-protein coupling preferences [2,9,10,12,15,18] system, which is primarily under the control of thyroid and the heterogeneous distribution of G-proteins within hormone negative feedback [4].
different brain regions, cell types and subcellular domains TRH injected intrathecally almost immediately reduces [2,23]. ECS, as discussed above, alters prepro-TRH gene depressive symptoms in 50% of severely depressed pa-transcription, translation and post-translational processing. tients [20]. The therapeutic potential of this tripeptide, Levels of the TRH receptor [17] and G-proteins [2,14] however, is limited by the necessity of intrathecal injection within a variety of limbic and extra-limbic regions of the to minimize rapid degradation by TRH-degrading enzymes mammalian brain are also modified by ECS. Given this in the circulation and to maximize restricted transport pleotropic action of ECS, it should not be surprising that in across the blood–brain barrier by a non-saturable, enzyme the anterior cingulate, at least, an atypical reduction in degradation-coupled mechanism [25,50]. These limitations TRH–Gly–IR level was observed following the combined would not apply, of course, to ECT-induced intracerebral treatment with ECS and the depressogenic forced swim synthesis of TRH and other prepro-TRH-derived peptides
test. that might augment the antidepressant action of TRH. For
Fig. 7. Correlation of EEP levels and Ps4 concentration in four limbic brain regions, of male Wistar rats. All correlations are P,0.02. EEP–IR calculated as the difference between EEP RIA results, which gives sum of EEP and TRH levels [31], and the specific TRH RIA.
W.H. Daughaday (Eds.), Principles of Competitive Protein-Binding
In summary, corneal ECS increases the expression of
Assays, Lippincott, Philadelphia, 1971, pp. 134–139.
prepro-TRH-derived and other TRH-like peptides in
lim-[2] C.K. Bauer, I. Davidson, I. Kubasov, J.R. Schwarz, W.T. Mason,
bic, including hypothalamic and cingulate, regions and Different G-proteins are involved in the baphasic response of clonal lateral cerebellum of the rat brain in addition to its rat pituitary cells to thyrotropin-releasing hormone, Pfugers Arch.
previously demonstrated effects in multiple limbic regions. 428 (1994) 17–25.
[3] R.V. Bhat, F.A. Tausk, J.M. Baraban, R.E. Mains, B.A. Eipper,
The levels of prepro-TRH-derived and related peptides
Rapid increases in peptide processing enzyme expression in
hip-vary proportionately in all brain regions studied, whether
pocampal neurons, J. Neurochem. 61 (1993) 1315–1322.
or not their expression is inducible by ECS. The levels of [4] T.O. Bruhn, J.H. Taplin, I.M. Jackson, Hypothyroidism reduces TRH–Gly–IR in the amygdala are at least 10-fold higher content and increases in vitro release of pro-thyrotropin-releasing than in the other brain regions studied. We conclude that hormone peptides from the median eminence, Neuroendocrinology
53 (1991) 511–515.
ECS induction of TRH and TRH homolog expression in
[5] M. Bulant, J.P. Roussel, H. Astier, P. Nicolas, H. Vaudry, Processing
rat limbic and extra-limbic regions contributes to the
of thyrotropin-releasing hormone prohormone (Pro-TRH) generates
accumulating evidence for TRH and TRH-like peptides as a biologically active peptide, prepro-TRH (160–169), which reg-mediators of the antidepressant effects of ECT [43]. ulates TRH-induced thyrotropin secretion, Proc. Natl. Acad. Sci.
USA 87 (1990) 4439–4443.
[6] M.J. Detke, M. Rickels, I. Lucki, Active behaviours in the rat forced swimming test differentially produced by serotonergic and norad-Acknowledgements
renergic antidepressants, Psychopharmacology 121 (1995) 66–72. [7] O. Devinsky, M.J. Morrell, B.A. Vogt, Contributions of anterior
This work was supported by the Research Service of the cingulate cortex to behaviour, Brain 118 (1995) 279–306. US Department of Veterans Affairs. [8] R.S. Feldman, J.S. Meyer, L.F. Quenzer, Principles of
Neuro-psychopharmacology, Sinauer Associates, Inc, Sunderland, MA, 1997.
[9] M. Gollasch, C. Kleuss, J. Hescheler, B. Wittig, G. Schultz, Gi2 and
References protein kinase C are required for thyrotropin-releasing
hormone-21
induced stimulation of voltage-dependent Ca channels in rat [1] G.E. Abraham, P.K. Grover, Covalent Linkage of Hormonal Haptens pituitary GH cells, Proc. Natl. Acad. Sci. USA 90 (1993) 6265–3
[10] T. Gudermann, F. Kalkbrenner, E. Dippel, K.L. Laugwitz, G. Hershman, Testosterone increases TRH biosynthesis in epididymis Schultz, Specificity and complexity of receptor–G-protein inter- but not heart of zinc-deficient rats, Peptides 14 (1993) 315–324. action, Adv. Second Messenger Phosphoprotein Res. 31 (1997) [31] A.E. Pekary, A. Sattin, R.L. Lloyd, Electroconvulsive seizures 253–262. increase levels of pGlu–Glu–Pro–NH (EEP) in rat brain, Peptides2
[11] A. Horita, An update on the CNS actions of TRH and its analogs, 20 (1999) 107–119.
Life Sci. 62 (1998) 1443–1448. [32] A.E. Pekary, A. Sattin, R.L. Lloyd, Electroconvulsive seizures [12] K.P. Hsieh, T.F. Martin, Thyrotropin-releasing hormone and gona- increase levels of Ps4, the TRH-enhancing peptide [Prepro-TRH dotropin-releasing hormone receptors activate phospholipase C by (160–169)], in rat brain, Neuroendocrinology 65 (1997) 377–384. coupling to the guanosine triphosphate-binding proteins Gq and [33] A.E. Pekary, R. Stephens, M. Simard, X.-P. Pang, V. Smith, J.J. G11, Mol. Endocrinol. 6 (1992) 1673–1681. DiStefano III, J.M. Hershman, Release of thyrotropin and prolactin [13] D.H. Ingvar, The will of the brain: cerebral correlates of willful acts, by a thyrotropin-releasing hormone (TRH) precursor, TRH–Gly: J. Theor. Biol. 171 (1994) 7–12. conversion to TRH is sufficient for in vivo effects, Neuroendocrinol-[14] H. Iwasa, S. Hasegawa, S. Kikuchi, K. Watanabe, K. Koseki, K. ogy 52 (1990) 618–625.
3
Kodama, T. Sato, Kindling-induced changes of [ H]GTP binding in [34] J.M. Perlin, C.M. Gerwin, D.M. Panchision, R.S. Vick, E.R. Jakoi, the cerebral cortical membrane, Jpn. J. Psychiatry 45 (1991) 303– R.J. DeLorenzo, Kindling produces long-lasting and selective
307. changes in gene expression of hippocampal neurons, Proc. Natl.
[15] U.B. Kaiser, R.A. Katzenellenbogen, P.M. Conn, W.W. Chin, Evi- Acad. Sci. USA 90 (1993) 1741–1745.
dence that signalling pathways by which thyrotropin-releasing [35] R.D. Porsolt, G. Anton, N. Blavel, M. Jalfre, Behavioral despair in hormone and gonadotropin-releasing hormone act are both common rats: a new model sensitive to antidepressant treatments, Eur. J. and distinct, Mol. Endocrinol. 8 (1994) 1038–1048. Pharmacol. 47 (1978) 379–391.
[16] M.L. Koenig, D.L. Yourick, J.L. Meyerhoff, Thyrotropin-releasing [36] G. Rajkowska, J.J. Miguel-Hidalgo, J. Wei, G. Dilley, S.D. Pittman, hormone (TRH) attenuates glutamate-stimulated increases in cal- H.Y. Meltzer, J.C. Overholser, B.L. Roth, C.A. Stockmeier, Mor-cium in primary neuronal cultures, Brain Res. 730 (1996) 143–149. phometric evidence for neuronal and glial prefrontal cell pathology [17] M.J. Kubek, S.M. Knoblach, N.A. Sharif, D.R. Burt, G.G. Buter- in major depression, Biol. Psychiatry 45 (1999) 1083–1084.
baugh, K.S. Fuson, Thyrotropin-releasing hormone gene expression [37] E. Redei, H. Hilderbrand, F. Aird, Corticotropin release–inhibiting and receptors are differentially modified in limbic foci by seizures, factor is encoded within prepro-TRH, Endocrinology 136 (1995)
Ann. Neurol. 33 (1993) 70–76. 1813–1816.
[18] D. Lipinsky, M.C. Gershengorn, Y. Oron, Ga11 and Gaq guanine [38] E. Redei, H. Hilderbrand, F. Aird, Corticotropin release–inhibiting nucleotide regulatory proteins differentially modulate the response factor is prepro-thyrotropin-releasing hormone-(178–199), Endo-to thyrotropin-releasing hormone in Xenopus oocytes, FEBS Lett. crinology 136 (1995) 3557–3563.
307 (1992) 237–240. [39] E. Redei, P.A. Rittenhouse, S. Revskoy, F. McGiven, F. Aird, A [19] R.L. Lloyd, A.E. Pekary, A. Sattin, Antidepressant effects of a novel endogenous corticotropin release inhibiting factor, Ann. New
stable analog of thyrotropin releasing hormone in a rodent model of York Acad. Sci. 840 (1998) 456–469.
depression, Soc. Neurosci. [Abstract] 23 (Part 2) (1997) 1661. [40] S. Reichlin, Neuroendocrinology, in: J.D. Wilson, D.W. Foster [20] L.B. Marangell, M.S. George, A.M. Callahan, T.A. Ketter, P.J. (Eds.), Williams Testbook of Endocrinology, W.B. Saunders Co.,
Pazzaglia, T.A. L’Herrou, G.S. Leverich, R.M. Post, Effects of Philadelphia, 1992, pp. 135–219, Chapter 5.
intrathecal thyrotropin-releasing hormone (protirelin) in refractory [41] M.A. Rogers, J.L. Bradshaw, C. Pantelis, J.G. Phillips, Frontostriatal depressed patients, Arch. Gen. Psychiatry 54 (1997) 214–222. deficits in unipolar major depression, Brain Res. Bull. 47 (1998) [21] J.L. Meyerhoff, V.E. Bates, M.J. Kubek, Increases in brain thyrot- 297–310.
ropin-releasing hormone (TRH) following kindled seizures, Brain [42] H.A. Sackeim, Central issues regarding the mechanisms of action of Res. 525 (1990) 144–148. electroconvulsive therapy: directions of future research, Psycho-[22] J.L. Meyerhoff, K.J. Koller, D.D. Walczak, J.T. Coyle, Brain pharmacol. Bull. 30 (1994) 281–308.
regional levels of N-acetlaspartylglutamate (NAAG): the effect of [43] A. Sattin, The role of TRH and related peptides in the mechanism of kindled seizures, Brain Res. 346 (1985) 392–396. action of ECT, JECT 15 (1999) 76–92.
[23] G. Murer, C. Adelbrecht, I. Lauritzen, F. Lesage, M. Lazdunski, Y. [44] A. Sattin, A.E. Pekary, R.L. Lloyd, TRH gene products are Agid, R. Raisman-Vozari, An immunocytochemical study on the implicated in the antidepressant mechanisms of seizures, Ann. New distribution of two G-protein-gated inward rectifier potassium York Acad. Sci. 739 (1994) 135–153.
channels (GIRK2 and GIRK4) in the adult rat brain, Neuroscience [45] W.J. Sheward, A.J. Harmar, H.M. Fraser, G. Fink G, Thyrotropin-80 (1997) 345–357. releasing hormone in rat pituitary stalk blood and hypothalamus: [24] D. Ongur, W.C. Drevets, J.L. Price, Glial reduction in the subgenual studies with high-performance liquid chromatography,
Endocrinolo-prefrontal cortex in mood disorders, Proc. Natl. Acad. Sci. USA 95 gy 113 (1983) 1865–1869.
(1998) 13290–13295. [47] M.E. Thase, A.J. Rush, When at first you don’t succeed: sequential [25] G. O’Cuinn (Ed.), Metabolism of Brain Peptides, CRC Press, Boca strategies for antidepressant non-responders, J. Clin. Psychiatry 58
Raton, 1995. (Suppl. 13) (1997) 23–29.
[26] M.J. Owens, C.B. Nemeroff, The role of corticotropin-releasing [48] T.J. Visser, W. Klootwijk, R. Docter, G. Hennemann, A new factor in the pathophysiology of affective and anxiety disorders: radioimmunoassay of thyrotropin-releasing hormone, FEBS Lett. 83 laboratory and clinical studies, Ciba Found. Symp. 172 (1993) (1977) 37–40.
296–316. [49] H. Yang, Y. Tache, Prepro-TRH (160–169) potentiates gastric acid [27] A.E. Pekary, Is Ps4 (prepro-TRH[160–169]) more than an enhancer secretion stimulated by TRH microinjected into the dorsal motor
for thyrotropin-releasing hormone?, Thyroid 8 (1998) 963–968. nucleus of the vagus, Neurosci. Lett. 174 (1994) 43–46.