www.elsevier.com / locate / bres
Research report
Diffusion- and perfusion-weighted magnetic resonance imaging of
focal cerebral ischemia and cortical spreading depression under
conditions of mild hypothermia
a,b,d ,
*
a,d c,d c,d ,1Midori A. Yenari
, David Onley
, Maj Hedehus
, Alexander deCrespigny
,
a,d c,d a,d
Guo Hua Sun
, Michael E. Moseley
, Gary K. Steinberg
a
Department of Neurosurgery, Stanford University Medical Center, 120 Welch Road, HSLS Bldg. P304, Stanford, CA 94305-5487, USA
b
Department of Neurology, Stanford University Medical Center, Stanford, CA, USA
c
Department of Radiology, Stanford University Medical Center, Stanford, CA, USA
d
Stanford Stroke Center, Stanford University Medical Center, Stanford, CA, USA
Accepted 29 August 2000
Abstract
In a model of experimental stroke, we characterize the effects of mild hypothermia, an effective neuroprotectant, on fluid shifts, cerebral perfusion and spreading depression (SD) using diffusion- (DWI) and perfusion-weighted MRI (PWI). Twenty-two rats underwent 2 h of middle cerebral artery (MCA) occlusion and were either kept normothermic or rendered mildly hypothermic shortly after MCA occlusion for 2 h. DWI images were obtained 0.5, 2 and 24 h after MCA occlusion, and maps of the apparent diffusion coefficient (ADC) were generated. SD-like transient ADC decreases were also detected using DWI in animals subjected to topical KCl application (n54) and ischemia (n56). Mild hypothermia significantly inhibited DWI lesion growth early after the onset of ischemia as well as 24 h later, and improved recovery of striatal ADC by 24 h. Mild hypothermia prolonged SD-like ADC transients and further decreased the ADC following KCl application and immediately after MCA occlusion. Cerebral perfusion, however, was not affected by temperature changes. We conclude that mild hypothermia is neuroprotective and suppresses infarct growth early after the onset of ischemia, with better ADC recovery. The ADC decrease during SD was greater during mild hypothermia, and suggests that the source of the ADC is more complex than previously believed. 2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Ischemia
Keywords: Mild hypothermia; Rat; Magnetic resonance imaging; Stroke; Spreading depression
1. Introduction the extent of protection seen with only modest reductions
in brain temperature [14]. Diffusion-weighted magnetic While mild hypothermia has been shown to be neuro- resonance imaging (DWI) is capable of non-invasively protective against cerebral ischemia, the precise mecha- detecting fluids shifts following experimental stroke with nisms are not well known. Alterations in cerebral metabo- quantitative estimates determined by computing the appar-lism and blood flow are known to play some role in this ent diffusion coefficient (ADC) [38,40]. ADC abnor-protective effect [33,39], although this cannot fully explain malities detected by DWI have been correlated with ATP
1 1
depletion, tissue acidosis, decreases in Na ,K -ATPase
1
activity and K concentration [2,38]. These metabolic
*Corresponding author. Tel.: 11-650-723-4448; fax: 11-650-723- disturbances occur when the ADC decreases 35–50% of 4451.
normal or more. If mild hypothermia’s protective effects
E-mail address: [email protected] (M.A. Yenari).
1 are, in part, due to preservation of ion gradients, DWI
Present address: NMR Center, Massachusetts General Hospital, Boston,
MA, USA. should be capable of detecting this. Perfusion-weighted
MRI (PWI) can be used to evaluate cerebral perfusion. within cerebral cortex and striata. Brain temperature was Although PWI does not precisely reflect regional cerebral reduced to 358C, 338C, 308C and 288C by placing the blood flow (rCBF), it is a non-invasive technique which animal on a cooling blanket and spraying ethanol onto the can provide information regarding microvascular patency body, then applying cool air from a hair dryer hose. [41,62]. As these techniques [3,58,60] and hypothermic Temperature was maintained for 15–20 min prior to therapies [35,51] are beginning to gain interest at the cooling to the next level. To determine the effects of clinical level, we utilize DWI and PWI to study the temperature changes during ischemia, temperature probes temporal and regional evolution of mild hypothermia in an were stereotaxically placed into each striata and a third experimental model of transient focal cerebral ischemia. probe was placed in the rectum. Temperatures were DWI can also be used to detect transient declines in monitored during conditions of ischemic normothermia ADC which correspond electrophysiologically with the (n51) and mild hypothermia (n51) as described sub-transient depolarizations or spreading depressions (SD) sequently.
observed following ischemia [5,6,17,20,31,48–50,54]. SDs
which occur during cerebral ischemia are thought to 2.2. Effects of temperature on ADC contribute to infarct growth by altering ion gradients or
increasing extracellular glutamate [20]. The extent and As micromolecular diffusion is altered with temperature distribution of SDs also appear to be correlated with changes, the relationship between brain ADC and tempera-expression of various genes, such as immediate early genes ture was established in our model. Using the correlation [19], cyclooxygenase [37] and protein kinase C (PKC) data from the above experiment, we monitored rectal [29]. While the SDs may result in worsening of ischemic temperature during scanning in this experiment and all injury, pre-insult induction of SDs by KCl application may subsequent experiments. Three anesthetized non-ischemic actually protect the brain from subsequent ischemic events animals underwent DWI imaging with brain temperatures (ischemic tolerance) [26,27,36]. An earlier report showed varied from 308C to 408C. Rectal temperatures were varied that mild hypothermia slows the propagation of the SDs as described above and DWI images were performed at within cortex, as detected by DC potential measurements each temperature. Trace DWI images were generated as [59]. We now show complementary results using DWI, described subsequently in the MRI protocol section. Re-which has the advantage of being non-invasive and offers gions of interest (ROIs) were identified within regions of anatomical resolution. To our knowledge, this is the first cortex, striatum and thalamus, and ADCs from fitted maps report characterizing the temperature dependence of SD- were measured within these ROIs. Correction curves were like ADC changes in ischemia and KCl models using determined for each of the structures as a function of
DWI. temperature.
2.3. Ischemia model
2. Materials and methods
approxi-mately 19–20 mm from the bifurcation in order to occlude mic (n53), or were cooled to 308C (rectal temperature, the ostium of the MCA. A temporary aneurysm clip was n53) immediately prior to suture insertion.
also placed on the CCA. Animals were either kept normothermic (normo, rectal5378C, n511) or rendered
mildly hypothermic (hypo, rectal5308C, n511) within 2.6. MRI scanning 15–20 min after MCA occlusion, and maintained for 2 h.
After 2 h of ischemia, the suture was withdrawn with the DWI and PWI images were obtained intra-ischemically animal remaining in the MRI scanner. The animal was (30 min following MCAO), post-reperfusion (within 5–10 rewarmed within 15–20 min using a warming blanket and min of reperfusion) and 24 h later. Imaging was performed by blowing warm air into the scanner bore. Following the on a Bruker CSI 2.0 Tesla system. The complete protocol final scan, the animal was returned to the operating table. (for each DWI and PWI scan set) took approximately 30 The aneurysm clip was removed and the surgical incisions min to complete. Because of the prolonged time under closed. The animal was allowed to recover, then trans- halothane anesthesia, many animals were incapable of ported to the intensive care unit at the animal facility for surviving the full 24 h. Only four normothermic and three post-operative monitoring. At the completion of the experi- mildly hypothermic animals were able to complete imag-ment, the animal was euthanized with a barbiturate over- ing at all three time points. Therefore, subsequent animals
dose. were randomized into two groups. In the first group (three
normothermics, four mildly hypothermics), animals were imaged intra-ischemically and early into reperfusion. In the 2.4. KCl-induced SD experiments
second group (four normothermics and four mildly hypo-thermics), the animal was removed from the scanner after In separate, non-ischemic, halothane anesthetized
ani-obtaining the intra-ischemic images and the mild hypo-mals (n54), a KCl chamber was placed 2 mm anterior to
thermia was completed. The suture was removed and the the lambdoidal suture and 2 mm lateral to the sagittal
animal was allowed to recover. The following day, a suture over the right hemisphere. A DC electrode was
second set of scans at 24 h was performed. For all groups, placed 2 mm anterior to the coronal suture and 2 mm
the total duration under anesthesia was similar (4.5 h). A lateral to the sagittal suture. KCl (3 M) was applied during
total of 22 animals, 11 normothermic and 11 mildly imaging acquisition and a DC shift was confirmed by
hypothermic, were imaged for this part of the study. simultaneous recordings from the electrode. Rectal
tem-perature was varied and images were obtained at 308C, 338C and 378C, corresponding to brain temperatures of
2.7. DWI protocols 338C, 358C and 388C, respectively. DWI imaging
(de-scribed below) commenced immediately before and up to
Spin echo echo planar images (SE EPI) was used with
20 min after KCl application. 2
the following imaging parameters: FOV540340 mm and resolution564364. For the ischemia and correlative ex-2.5. Ischemia-induced SD experiments periments, a 5-cm diameter bird-cage radio frequency coil was used. For the SD experiments, a surface coil was used. In six separate animals, MCA occlusion was induced Both isotropic diffusion-weighted (IDW) and DWI trace remotely, with the animal inside of the MRI scanner. This images were obtained. Imaging parameters were: TE / TR5
method has been published previously [49], but some 66 ms / 4 s, no. of slices54, coronal plane, slice thickness5
modifications have been made. PE-50 tubing 10 mm in 2.0 mm. For IDW scans, 16 images with varying gradient 2 length was tapered on one end. The opposite end of the amplitudes (16 b-values between 100 and 1200 s / mm in tubing was inserted approximately 5 mm into PE-90 tubing random order) were acquired for each slice. For the trace which extended the length of the MR cradle (|30 cm). The DWI images, eight images were acquired from each slice
2
tapered tip of the PE-50 tubing was inserted 5 mm into the (eight b-values between 0 and 1400 s / mm ), and repeated ECA stump and secured. A 30-mm monofilament suture with the gradients applied in the x, y and z directions. was attached to another segment of PE-50 tubing (45 cm Trace images were derived by averaging the images in length) and fed through the P-90 tubing and into the obtained from each direction. T2-Weighted (T2W) images ECA approximately 15 mm from the CCA bifurcation. were obtained using b-values50.
2.8. PWI protocols Maps of bolus arrival delays were fitted from the PWI scans using a fitting routine previously reported by our PWI scans were performed using a T2-weighted gra- group (MRVision, Menlo Park, CA) [62]. We and others dient echo EPI to track the contrast bolus [48,49,62]. Three have found the delay maps to be the most sensitive coronal slices were repeatedly acquired during rapid perfusion index compared to relative cerebral blood vol-intravenous injection of a contrast agent (0.2 mmol / kg ume (rCBV) and bolus peak effect [41,62]. Regions of gadopentitate dimeglumine). Imaging parameters were the significant delay were mapped out using a thresholding same as for DWI imaging. All three slices were acquired routine. Only areas where bolus arrival delays were greater every 2 s prior to and during contrast injection, and than 2 s compared to the contralateral hemisphere were continued for a total of 32 s. included and expressed as a percentage of the total
ipsilateral hemisphere area [62].
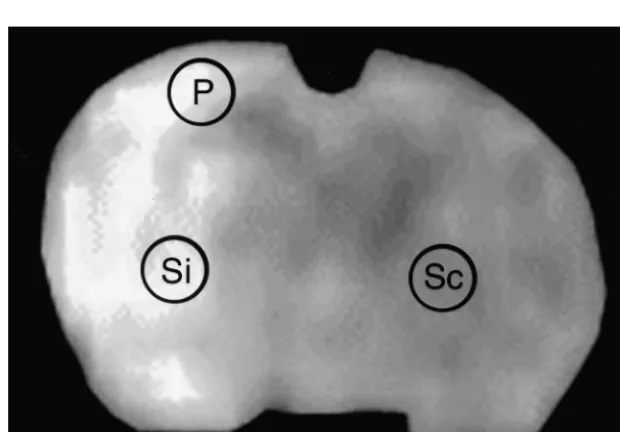
2.9. MRI analysis Because the scans could only be continued for a finite
period of time (20 min for each SD trial), the total number All analyses were performed by an investigator blinded of SDs could not be reliably determined; therefore, we to treatment groups, although information regarding the chose to only analyze parameters from the initial SD wave time point of the scan sets was provided. Data sets for all from each KCl application or ischemia experiment. From MRI scans from a specific time point were pooled, the KCl-induced SD experiments, ADC maps were gener-regardless of when the animal was euthanized. ROIs ated and ROIs were defined in the ipsilateral hemisphere delineating the DWI hyperintensity from four adjacent near the region of KCl application. ADC changes within slices were traced by hand and expressed as the percent the same ROI were computed from the sequential images area relative to the ipsilateral hemisphere. We have previ- and plotted as a function of time. The maximum ADC ously shown that these DWI hyperintensities at 24 h decrease, the time from initial ADC decline to maximal strongly correlate with infarction as seen by triphenyl ADC decrease (ADC decline time), and the time from tetrazolium chloride (TTC) staining [61]. ROIs were also maximal ADC decrease to ADC recovery (ADC recovery delineated within the striata (Fig. 1, Si) to determine the time) were measured from the first transient depolarization. percent change in ADC value between the ipsilateral For the SD experiments conducted in ischemic animals, ischemic (Si) and corresponding contralateral non-is- ROIs were identified within the cortex corresponding to a chemic (Sc) sides from the ADC maps. For comparison peri-infarct region (P, see Fig. 1). Another ROI within the between temperature groups, all ADCs from images ac- striatum was also defined (Si, Fig. 1) in order to measure quired under mild hypothermic conditions were corrected the time to terminal ADC decrease, defined as the time to to a brain temperature in a normothermic animal using the maximum ADC decline in regions where no ADC
re-previous correlative data. covery was observed during the 20-min scanning period.
Similar parameters from an ROI within the cortex (adja-cent to the area of KCl application) were measured from the KCl experiments.
2.10. Statistical analysis
Standard statistical methods were used to analyze data. Differences between groups and the various parameters was determined using one-way analysis of variance (ANOVA) and ANOVA with repeated measures to detect overall significance, followed by a post hoc multiple comparison procedure (Scheffe’s). Correlation was de-termined by Pearson’s correlation coefficient, followed by a linear regression. Statistical significance was determined at the P,0.05 level. All data are presented as mean6S.E.M.
Fig. 2. Striatal temperature in ischemic and non-ischemic brain under normothermic and mildly hypothermic conditions. Under normothermic 3. Results
conditions, rectal (d) and ischemic striatal (.) temperatures were similar, whereas non-ischemic striatal temperature (j) was
approximate-3.1. Correlative experiments ly 18C higher. Under mildly hypothermic conditions, rectal (s) and ischemic striatal (,) temperatures were similar, whereas non-ischemic striatal temperature (h) was approximately 28C higher. Cooling began
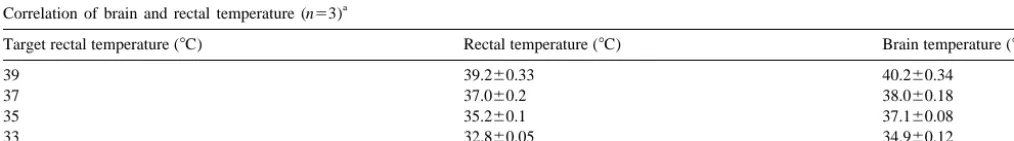
We found strong correlations between rectal and brain
immediately after occlusion of the middle cerebral artery (MCAO), and
temperature (r50.92, P,0.0001), with brain temperature
rewarming began immediately after reperfusion (arrow).
somewhat higher than rectal. The relationship was not entirely linear, as we found that when rectal temperature
was 378C, brain temperature was 388C, whereas when brain temperature under normothermic conditions (i.e. rectal temperature was 308C, brain temperature was 338C non-ischemic brain was corrected to 388C, and ischemic (Table 1). Furthermore, cortical and striatal brain tempera- brain was corrected to 378C).
tures were similar. Under ischemic conditions, striatal
temperature within the non-ischemic side followed patterns 3.2. Focal cerebral ischemia similar to those observed in non-ischemic animals.
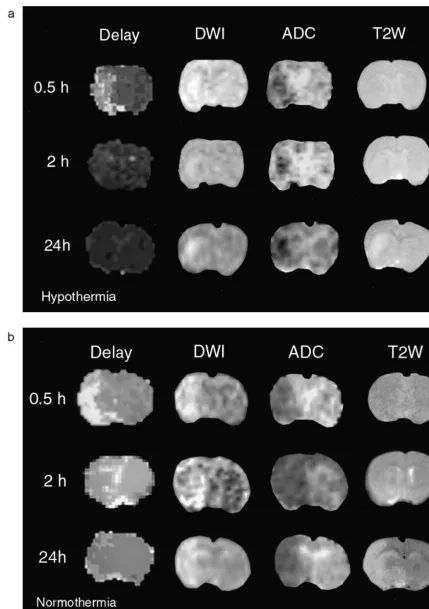
How-ever, the ischemic striatal temperature was lower than the There were no differences among any of the physiologi-non-ischemic striatum, and was no different from the rectal cal parameters (except temperature) between groups. For temperature (Fig. 2). Therefore, we found it possible to each temperature group, 22 DWI image sets (11 mildly reliably predict brain temperature from rectal temperature hypothermic and 11 normothermic sets) were available for measurements in this model. analysis at 0.5 h post-ischemia (intraischemic), 14 image ADC was found to vary linearly and directly with sets (seven mildly hypothermic and seven normothermic temperature. A 18C change in brain temperature corres- sets) were available for analysis 2.5 h post-ischemia (post-ponded to a 1.6% change in ADC. Our results are in close reperfusion) and 15 image sets (seven mildly hypothermic agreement with others’ who measured the temperature and eight normothermic sets) were available for analysis at dependence of the ADC in water and brain [18,23] as well 24 h. For the PWI scans, only six image sets were as in a mathematical model [32]. Although these changes available for analysis at the 24-h time point among are quite small in comparison to the ADC reduction normothermic animals, otherwise the numbers are the observed with ischemia, we used this relationship to same as for DWI.
correct all measured ADCs to the ADC corresponding to a Mild hypothermia inhibited DWI lesion growth early
Table 1
a
Correlation of brain and rectal temperature (n53)
Target rectal temperature (8C) Rectal temperature (8C) Brain temperature (8C)
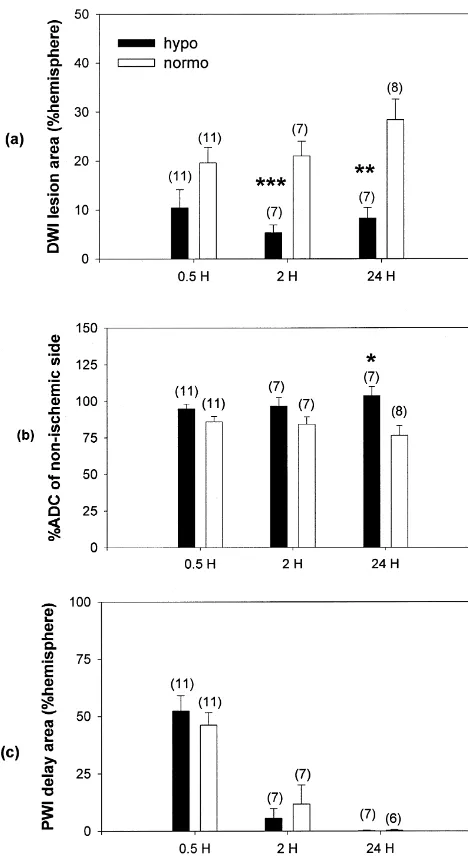
after the onset of ischemia and up to 24 h later. DWI lesion hypo 561.6%, normo 2163%, P,0.001; and 24 h: hypo areas (% of the ipsilateral hemisphere) were intraischemic: 862%, normo 2864%, P,0.01 (Figs. 3 and 4a). Striatal hypo 1064%, normo 1963.2%, P50.07; post-reperfusion: ADC recovery by 24 h was significantly improved among mildly hypothermic animals. ADC recovery, compared to the contralateral non-ischemic striata was 10466% among mild hypothermics versus 7766% ADC recovery among normothermics (P,0.05) (Fig. 4b). Areas of significant bolus peak delays were no different between groups at any time point. Cerebral perfusion was equally delayed during MCA occlusion, but returned to near normal in both groups shortly after reperfusion (Figs. 3 and 4c).
3.3. KCl- and ischemia-induced SDs
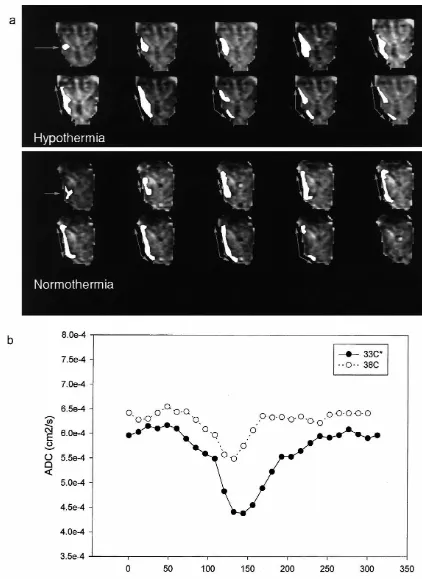
KCl application produced SD-like waves on DWI in non-ischemic animals which corresponded to the DC potential changes (data not shown). This correlation has been previously been described in detail by our group [12,48,49] and others [17,31]. Therefore, DC potential shifts were not measured for subsequent studies. Maximum ADC decreases were significantly lower with decreased temperature. Mild hypothermia prolonged ADC decline and ADC recovery times from the initial depolarization compared to normothermia (Table 2 and Fig. 5).
Among normothermic animals, ischemia resulted in SD-like ADC changes within peri-infarct zones. Mild hypo-thermia suppressed SD generation within cortex among two of three animals, and these animals also did not have any MRI-detected cortical injury. The third mildly hypo-thermic animal had a cortical lesion seen on DWI, and SD-like waves were observed in peri-infarct regions. ADC decline and recovery times within the cortical peri-infarct zone were slowed in this mildly hypothermic animal compared to the normothermics. SDs were detected within peri-infarct regions in the mildly hypothermic group with prolonged ADC decline and recovery (Table 3). ADC decline and recovery times were approximately three times longer among mildly hypothermic animals compared nor-mothermic ones (P,0.05). Time to terminal ADC de-crease was twice as long in mildly hypothermic animals compared to controls. Maximum ADC decreases were lower among the mildly hypothermic group even after
Fig. 4. Temperature-dependent, MRI-detected ischemic lesion changes. correction for temperature. (a) Mild hypothermia attenuates DWI lesion area. Significant reduction in
DWI lesion area (% of the ipsilateral hemisphere) among the hypothermic group is observed upon reperfusion (2 h) and at 24 h. Trends showing
reduced lesion size compared to the normothermic group are noted 4. Discussion intra-ischemically (30 min post-occlusion). (b) Mild hypothermia
im-proves recovery of the apparent diffusion coefficient (ADC) in focal Mild hypothermia has been shown to be an effective cerebral ischemia. Striatal ADC (% decrease relative to the contralateral,
treatment against cerebral ischemic injury by numerous
non-ischemic side) recovers to normal values in the hypothermia group by
groups [14,34]. Lowered temperatures are thought to
24 h. All ADCs are corrected to normothermic conditions. (c) Mild
hypothermia does not alter cerebral perfusion. Areas of bolus arrival preserve metabolic stores, but this has not been
con-delay from the PWI scans are no different between the hypothermic group sistently demonstrated in the setting of mild to moderate compared to the normothermic group. Upon reperfusion, these areas of hypothermia [8,15,25,28,53,57]. Several groups have also perfusion delay reverse and no delay is observed in either group by 24 h.
shown that mild hypothermia is associated with decreased
*P#0.05, **P#0.01 ***P#0.001 vs. normothermic group. Numbers in
accumulation of excitatory amino acids [4,7,21,33,42], and
parentheses indicate the number of images included in the analysis for
Table 2
a
Temperature dependence of SD-like ADC changes following KCl application (n54) 24
ADC, apparent diffusion coefficient; KCl, potassium chloride; ADCcor, ADC corrected to 388C; ADCcor Max, maximum ADCcor decrease.
b † ‡
*338C vs. 388C; 338C vs. 358C; 358C vs. 388C.
nitric oxide generation [24]. SDs have been thought to occlusion, we similarly show that mild hypothermia delays contribute to infarct growth due to increased calcium influx the time to maximum ADC decrease (the DWI ‘equiva-through glutamate activated calcium ion channels lent’ of terminal depolarization [48,49]). Our observations [5,13,20,43] and propagation through gap junctions [44]. are also in line with those by Katsura et al. [25] who DWI has gained considerable interest over the past few showed that mild hypothermia slowed the rate of extracel-years as a rapid, non-invasive way of detecting ischemic lular potassium ion accumulation, and delayed the fall in lesions by taking advantage of the early fluid shifts that ATP in a model of cardiac arrest. This is consistent with occur following stroke. DWI is also capable of detecting the notion that hypothermia preserves ATP hydrolases transient diffusion changes which have been correlated to needed to maintain ion gradients, and subsequently delays SDs [17,31,49]. Given that these ADC changes reflect or prevents cytotoxic edema formation.
alterations in ion and fluid homeostasis following ischemic There have been a few reports in the literature examin-insults [40], we show here that protection by mild hypo- ing SDs and the effects of hypothermia. To our knowledge, thermia appears to be related to enhanced recovery from this is the first report of temperature dependent SD/ SD-pathological fluid shifts and attenuated the DWI lesion like changes using DWI. However, other reports in the early after ischemia onset. Mild hypothermia also sup- literature have used electrophysiological methods to study pressed SD-like ADC changes. However, the ADC drop the temperature dependence of SDs. After topical applica-during the SD-like transients was lower among animals tion of KCl, Takaoka et al. [55] found that decreases in subjected to ischemia and KCl application at lower tem- temperature did not affect the brain’s ability to depolarize, peratures, and suggests that the source of the ADC is more but mild hypothermia did reduce the propagation speed of complex than previously believed. Using PWI, we show SDs. Following needle stab injury [59] and focal cerebral that the protective effects of mild hypothermia do not ischemia [9], the number of SDs were decreased in number appear to alter the area of cerebral perfusion deficit. and their onset was delayed with lower temperature. Jiang et al. [23] previously utilized a similar rodent Glutamate antagonists [22,45,56], calcium channel block-stroke model to examine the effects of mild hypothermia ers [52] and some anesthetics [50] have also been shown to within striatum using DWI and PWI. Consistent with our inhibit SDs. This led some to link SDs to glutamate results, they found that striatal ADC recovered more accumulation and subsequent infarct growth [5,20]. In fact, rapidly in animals subjected to mild hypothermia, with administration of various glutamate antagonists have been return of the ADC as early as 6 h post-ischemia in the shown to decrease the number of SDs as well as infarct mildly hypothermic group. We show that MRI-detected size [37,56], and mild hypothermia attenuates ischemia-lesion growth is inhibited soon after application of mild induced glutamate release [4,7,21,33,42]. Our data using hypothermia with a trend towards reduced DWI lesion size DWI are in agreement with this. We found that SD-like as early as 0.5 h after ischemia onset, and significant transient ADC changes are prolonged with mild hypo-reduction in lesion size by 2 h. We also observed improved thermia compared to normothermia, and following is-recovery of the ADC by 24 h with mild hypothermia. chemia, mild hypothermia completely inhibited these Since the decreased ADC is thought to reflect cytotoxic transient ADC changes in two of three cases. KCl-induced edema, mild hypothermia could protect ischemic brain by SDs have been shown to induce certain stress-associated restoring these fluid shifts. On the other hand, it is possible genes [19,46,52], and application of KCl during the that the early ADC recovery represents a ‘pseudonormali- ischemic period worsens ischemic injury [54]. Similarly, zation’, reflecting cell lysis and the development of mild hypothermia is associated with reduced expression of vasogenic edema. However, overall DWI lesion size at 24 heat shock protein 70 (HSP70), a stress-induced gene [10]. h was attenuated among the mild hypothermia group, Our finding that mild hypothermia inhibits or slows the making it more likely that the ADC recovery represented propagation of SD-like ADC transients is consistent with mild hypothermic rescue of ischemic tissue. Using electro- the notion that mild hypothermia reduces metabolic and physiological methods, others have shown that mild hypo- excitotoxic stresses to the brain.
Table 3
a
Temperature dependence of SD-like ADC changes following transient focal cerebral ischemia 24
Temperature group (8C) n ADCcorMax (310 s / cm) ADC decline (s) ADC recovery (s) Time to terminal ADC decrease (s)
Normo 3 5.660.3 126624 162643 162636
Hypo 3 4.460.1* 360690* 5286155* 366690*
a
ADC, apparent diffusion coefficient; KCl, potassium chloride; ADCcor, ADC corrected to 388C; ADCcorMax, maximum ADCcordecrease; n, number of animals studied; normo, normothermia; hypo, mild hypothermia. *P,0.01 (338C vs. 388C).
after temperature correction in both the KCl and ischemia gation and further lowers the ADC of SD-like transients, paradigms. The reasons for our observations are not clear, but is associated with better ADC recovery by 24 h. As but lower temperatures are known to alter membrane mild hypothermia is beginning to be studied at the clinical fluidity [1] and ion channel function [25,47]. Our results level for treatment of cerebral injury, DWI and PWI may could be explained in this light, particularly as temperature be useful in monitoring the response to such therapy as affects the function of proteins involved in regulating well as non-invasively studying ischemic pathophysiology cellular fluid and ion homeostasis. Furthermore, our data in human subjects.
suggest that these changes in ADC-detected fluid shifts are not necessarily damaging, since we observed robust
protection with mild hypothermia. Other reports of tem- Acknowledgements perature-dependent SD changes describe slowing and
reduced numbers of propagated waves [9]; however, This work was supported in part by funds from NIH amplitude was not altered [55] and ADC during SD has NINDS Grant RO1 NS 27292 (G.K.S.), NIH NINDS Grant not previously been measured. In our ischemia model, K08 NS01860 (M.A.Y.), Bernard and Ronni Lacroute early terminal ADC decreases within the ipsilateral mildly (G.K.S.) and the William Randolph Hearst Foundation hypothermic striata were also lower compared to nor- (G.K.S.). The authors would like to thank Dr Christian mothermic striata. Yet, there was better recovery of the Beaulieu and Mr David Kunis for expert technical assis-striatal ADC by 24 h, and reduction in DWI lesion size. tance, and Ms Beth Houle for preparation of the figures. Several groups have suggested that critically low ADCs
measured early after ischemia onset might predict irrevers-ible ischemic injury [11,16]. However, our data indicate
References
that the extent of early ADC decreases does not necessarily predict the fate of the tissue. Clearly, more investigation
[1] M.T. Almeida, J. Ramalho-Santos, C.R. Oliveira, M.C. de Lima,
into the source and significance of the ADC is needed.
Parameters affecting fusion between liposomes and synaptosomes.
PWI is also gaining interest as a rapid means of imaging Role of proteins, lipid peroxidation, pH and temperature, J. Membr. cerebral perfusion with the anatomic resolution of MRI Biol. 142 (1994) 217–222.
[2] T. Back, M. Hoehn-Berlage, K. Kohno, K.A. Hossmann, Diffusion
[30,58,62]. In our hands and others [41,62], the bolus
nuclear magnetic resonance imaging in experimental stroke.
Corre-arrival delay is the most sensitive PWI parameter
com-lation with cerebral metabolites, Stroke 25 (1994) 494–500.
pared to cerebral blood volume, mean transit time and
[3] A.E. Baird, A. Benfield, G. Schlaug, B. Siewert, K.O. Lovblad, R.R.
derived CBF, though not necessarily the most specific for Edelman, S. Warach, Enlargement of human cerebral ischemic lesion actual blood flow. Jiang et al. [23] also examined striatal volumes measured by diffusion-weighted magnetic resonance
imag-rCBF using an arterial spin labeling technique and found ing, Ann. Neurol. 41 (1997) 581–589.
[4] C.J. Baker, A.J. Fiore, V.I. Frazzini, T.F. Choudhri, G.P. Zubay, R.A.
that rCBF recovered in the mildly hypothermic group, but
Solomon, Intraischemic hypothermia decreases the release of
gluta-not in the normothermic group. Using other methods to
mate in the cores of permanent focal cerebral infarcts, Neurosurgery
measure blood flow, others have found that rCBF increases 36 (1995) 994–1001.
with reperfusion, but is blunted by mild hypothermia [5] E. Busch, M.L. Gyngell, M. Eis, M. Hoehn-Berlage, K.A.
Hos-[33,39]. Our results are somewhat in contrast to both of smann, Potassium-induced cortical spreading depressions during focal cerebral ischemia in rats: contribution to lesion growth
these findings in that we did not observe any changes in
assessed by diffusion-weighted NMR and biochemical imaging, J.
the areas of bolus arrival delay with mild hypothermia.
Cereb. Blood Flow Metab. 16 (1996) 1090–1099.
Therefore, our data suggest that protection from mild [6] E. Busch, M. Hoehn-Berlage, M. Eis, M.L. Gyngell, K.A. Hos-hypothermia is independent of changes in cerebral perfu- smann, Simultaneous recording of EEG, DC potential and
diffusion-sion. weighted NMR imaging during potassium induced cortical
spread-ing depression in rats, NMR Biomed. 8 (1995) 59–64.
In summary, we show using DWI and PWI that
intrais-´ [7] R. Busto, M.Y. Globus, W.D. Dietrich, E. Martinez, I. Valdes, M.D.
chemic mild hypothermia is neuroprotective against focal
Ginsberg, Effect of mild hypothermia on ischemia-induced release
cerebral ischemia, and that this protection is evident soon of neurotransmitters and free fatty acids in rat brain, Stroke 20 after ischemia onset, but is not associated with changes in (1989) 904–910.
1 K.M. Welch, The effects of post-ischemic hypothermia on the hypo- and hyperthermic rats: correlation to membrane fluxes of K ,
Brain Res 590 (1992) 6–12. neuronal injury and brain metabolism after forebrain ischemia in the
[26] N. Kawahara, S.D. Croll, S.J. Wiegand, I. Klatzo, Cortical spreading rat, J. Neurol. Sci. 107 (1992) 191–198.
depression induces long-term alterations of BDNF levels in cortex [9] Q. Chen, M. Chopp, G. Bodzin, H. Chen, Temperature modulation
and hippocampus distinct from lesion effects: implications for of cerebral depolarization during focal cerebral ischemia in rats:
ischemic tolerance, Neurosci. Res. 29 (1997) 37–47. correlation with ischemic injury, J. Cereb. Blood Flow Metab. 13
[27] S. Kobayashi, V.A. Harris, F.A. Welsh, Spreading depression induces (1993) 389–394.
tolerance of cortical neurons to ischemia in rat brain, J. Cereb. [10] M. Chopp, Y. Li, M.O. Dereski, S.R. Levine, Y. Yoshida, J.H.
Blood Flow Metab. 15 (1995) 721–727. Garcia, Hypothermia reduces 72-kDa heat-shock protein induction
[28] P. Kozlowski, A.M. Buchan, U.I. Tuor, D. Xue, Z.G. Huang, K.E. in rat brain after transient forebrain ischemia, Stroke 23 (1992)
Chaundy, J.K. Saunders, Effect of temperature in focal ischemia of
104–107. 31 1
rat brain studied by P and H spectroscopic imaging, Magn. [11] B.J. Dardzinski, C.H. Sotak, M. Fisher, Y. Hasegawa, L. Li, K.
Reson. Med. 37 (1997) 346–354. Minematsu, Apparent diffusion coefficient mapping of experimental
[29] J. Krivanek, V.I. Koroleva, Protein kinase C in the rat cerebral cortex focal cerebral ischemia using diffusion-weighted echo-planar
imag-during spreading depression, Neurosci. Lett. 210 (1996) 79–82. ing, Magn. Reson. Med. 30 (1993) 318–325.
[30] J. Kucharczyk, T. Roberts, M.E. Moseley, A. Watson, Contrast-[12] T. Els, J. Rother, C. Beaulieu, A. de Crespigny, M. Moseley,
enhanced perfusion-sensitive MR imaging in the diagnosis of Hyperglycemia delays terminal depolarization and enhances
repo-cerebrovascular disorders, J. Magn. Reson. Imaging 3 (1993) 241– larization after peri-infarct spreading depression as measured by
245. serial diffusion MR mapping, J. Cereb. Blood Flow Metab. 17
[31] L.L. Latour, Y. Hasegawa, J.E. Formato, M. Fisher, C.H. Sotak, (1997) 591–595. Spreading waves of decreased diffusion coefficient after cortical [13] G. Gido, T. Kristian, B.K. Siesjo, Induced spreading depressions in stimulation in the rat brain, Magn. Reson. Med. 32 (1994) 189–198. energy-compromised neocortical tissue: calcium transients and [32] D. Le Bihan, J. Delannoy, R.L. Levin, Temperature mapping with histopathological correlates, Neurobiol. Dis. 1 (1994) 31–41. MR imaging of molecular diffusion: application to hyperthermia, [14] M.D. Ginsberg, L.L. Sternau, M.Y.-T. Globus, W.D. Dietrich, R. Radiology 171 (1989) 853–857.
Busto, Therapeutic modulation of brain temperature: relevance to [33] E.H. Lo, G.K. Steinberg, Effects of hypothermia on evoked po-ischemic brain injury, Cerebrovasc. Brain Metab. Rev. 4 (1992) tentials, magnetic resonance imaging, and blood flow in focal
189–225. ischemia in rabbits, Stroke 23 (1992) 889–893.
[15] O. Haraldseth, T. Gronas, T. Southon, L. Thommessen, G. Borch- [34] J. Maher, V. Hachinski, Hypothermia as a potential treatment for grevink, P. Jynge, S.E. Gisvold, G. Unsgard, The effects of brain cerebral ischemia, Cerebrovasc. Brain Metab. Rev. 5 (1993) 277– temperature on temporary global ischaemia in rat brain. A 31- 300.
phosphorus NMR spectroscopy study, Acta Anaesthesiol. Scand. 36 [35] D.W. Marion, L.E. Penrod, S.F. Kelsey, W.D. Obrist, P.M. Kochanek,
(1992) 393–399. A.M. Palmer, S.R. Wisniewski, S.T. DeKosky, Treatment of
[16] Y. Hasegawa, M. Fisher, L.L. Latour, B.J. Dardzinski, C.H. Sotak, traumatic brain injury with moderate hypothermia, N. Engl. J. Med. MRI diffusion mapping of reversible and irreversible ischemic 336 (1997) 540–546.
injury in focal brain ischemia, Neurology 44 (1994) 1484–1490. [36] K. Matsushima, M.J. Hogan, A.M. Hakim, Cortical spreading [17] Y. Hasegawa, L.L. Latour, J.E. Formato, C.H. Sotak, M. Fisher, depression protects against subsequent focal cerebral ischemia in
Spreading waves of a reduced diffusion coefficient of water in rats, J. Cereb. Blood Flow Metab. 16 (1996) 221–226.
normal and ischemic rat brain, J. Cereb. Blood Flow Metab. 15 [37] S. Miettinen, F.R. Fusco, J. Yrjanheikki, R. Keinanen, T. Hirvonen, (1995) 179–187. R. Roivainen, M. Narhi, T. Hokfelt, J. Koistinaho, Spreading [18] Y. Hasegawa, L.L. Latour, C.H. Sotak, B.J. Dardzinski, M. Fisher, depression and focal brain ischemia induce cyclooxygenase-2 in Temperature dependent change of apparent diffusion coefficient of cortical neurons through N-methyl-D-aspartic acid receptors and water in normal and ischemic brain of rats, J. Cereb. Blood Flow phospholipase A2, Proc. Natl. Acad. Sci. USA 94 (1997) 6500–
Metab. 14 (1994) 383–390. 6505.
[19] J. Honkaniemi, B.A. States, P.R. Weinstein, J. Espinoza, F.R. Sharp, [38] J. Mintorovitch, G.Y. Yang, H. Shimizu, J. Kucharczyk, P.H. Chan, Expression of zinc finger immediate early genes in rat brain after P.R. Weinstein, Diffusion-weighted magnetic resonance imaging of permanent middle cerebral artery occlusion, J. Cereb. Blood Flow acute focal cerebral ischemia: comparison of signal intensity with
1 1
Metab. 17 (1997) 636–646. changes in brain water and Na ,K( )-ATPase activity, J. Cereb. [20] K.A. Hossmann, Periinfarct depolarizations, Cerebrovasc. Brain Blood Flow Metab. 14 (1994) 332–336.
Metab. Rev. 8 (1996) 195–208. [39] E. Morikawa, M.D. Ginsberg, W.D. Dietrich, R.C. Duncan, S. [21] F.P. Huang, L.F. Zhou, G.Y. Yang, Effects of mild hypothermia on Kraydieh, M.Y. Globus, R. Busto, The significance of brain tempera-the release of regional glutamate and glycine during extended ture in focal cerebral ischemia: histopathological consequences of transient focal cerebral ischemia in rats, Neurochem. Res. 23 (1998) middle cerebral artery occlusion in the rat, J. Cereb. Blood Flow
991–996. Metab. 12 (1992) 380–389.
[22] T. Iijima, G. Mies, K.A. Hossmann, Repeated negative DC deflec- [40] M.E. Moseley, Y. Cohen, J. Mintorovitch, L. Chileuitt, H. Shimizu, tions in rat cortex following middle cerebral artery occlusion are J. Kucharczyk, M.F. Wendland, P.R. Weinstein, Early detection of abolished by MK-801: effect on volume of ischemic injury, J. regional cerebral ischemia in cats: comparison of diffusion- and Cereb. Blood Flow Metab. 12 (1992) 727–733. T2-weighted MRI and spectroscopy, Magn. Reson. Med. 14 (1990) [23] Q. Jiang, M. Chopp, Z.G. Zhang, J.A. Helpern, R.J. Ordidge, J. 330–346.
Ewing, P. Jiang, B.A. Marchese, The effect of hypothermia on [41] T.B. Muller, O. Haraldseth, R.A. Jones, G. Sebastiani, F. Godtlieb-transient focal ischemia in rat brain evaluated by diffusion- and sen, C.F. Lindboe, G. Unsgard, Combined perfusion and diffusion-perfusion-weighted NMR imaging, J. Cereb. Blood Flow Metab. 14 weighted magnetic resonance imaging in a rat model of reversible (1994) 732–741. middle cerebral artery occlusion, Stroke 26 (1995) 451–457. [24] A. Kader, V.I. Frazzini, C.J. Baker, R.A. Solomon, R.R. Trifiletti, [42] K. Nakashima, M.M. Todd, Effects of hypothermia on the rate of
Effect of mild hypothermia on nitric oxide synthesis during focal excitatory amino acid release after ischemic depolarization, Stroke cerebral ischemia, Neurosurgery 35 (1994) 272–277. 27 (1996) 913–918.
[44] M. Nedergaard, A.J. Cooper, S.A. Goldman, Gap junctions are temperature on ischemic brain injury, Can. J. Neurol. Sci. 19 (1992) required for the propagation of spreading depression, J. Neurobiol. 317–325.
28 (1995) 433–444. [54] K. Takano, L.L. Latour, J.E. Formato, R.A. Carano, K.G. Helmer, Y. [45] T.P. Obrenovitch, E. Zilkha, Inhibition of cortical spreading depres- Hasegawa, C.H. Sotak, M. Fisher, The role of spreading depression sion by L-701,324, a novel antagonist at the glycine site of the in focal ischemia evaluated by diffusion mapping, Ann. Neurol. 39 N-methyl-D-aspartate receptor complex, Br. J. Pharmacol. 117 (1996) 308–318.
(1996) 931–937. [55] S. Takaoka, R.D. Pearlstein, D.S. Warner, Hypothermia reduces the [46] J.C. Plumier, J.C. David, H.A. Robertson, R.W. Currie, Cortical propensity of cortical tissue to propagate direct current
depolariza-application of potassium chloride induces the low-molecular weight tions in the rat, Neurosci. Lett. 218 (1996) 25–28.
heat shock protein (Hsp27) in astrocytes, J. Cereb. Blood Flow [56] T. Tatlisumak, K. Takano, M.R. Meiler, M. Fisher, A glycine site Metab. 17 (1997) 781–790. antagonist, ZD9379, reduces number of spreading depressions and
1
[47] B.M. Rodriguez, D. Sigg, F. Bezanilla, Voltage gating of Shaker K infarct size in rats with permanent middle cerebral artery occlusion, channels. The effect of temperature on ionic and gating currents, J. Stroke 29 (1998) 190–195.
Gen. Physiol. 112 (1998) 223–242. [57] Y. Tohyama, K. Sako, Y. Yonemasu, Hypothermia attenuates hy-[48] J. Rother, A.J. de Crespigny, D.A. H, K. Iwai, M.E. Moseley, perglycolysis in the periphery of ischemic core in rat brain, Exp.
Recovery of apparent diffusion coefficient after ischemia-induced Brain Res. 122 (1998) 333–338.
spreading depression relates to cerebral perfusion gradient, Stroke [58] D.C. Tong, M.A. Yenari, G.W. Albers, M. O’Brien, M.P. Marks, 27 (1996) 980–986. M.E. Moseley, Correlation of perfusion- and diffusion-weighted [49] J. Rother, A.J. de Crespigny, D.A. H, M.E. Moseley, MR detection MRI with NIHSS score in acute (,6.5 hour) ischemic stroke,
of cortical spreading depression immediately after focal ischemia in Neurology 50 (1998) 864–870.
the rat, J. Cereb. Blood Flow Metab. 16 (1996) 214–220. [59] M. Ueda, N. Watanabe, Y. Ushikubo, K. Kasai, T. Tsuzuki, K. Aoki, [50] R. Saito, R. Graf, K. Hubel, J. Taguchi, G. Rosner, T. Fujita, W.D. Y. Yamazaki, H. Samejima, The effect of hypothermia on CSD
Heiss, Halothane, but not alpha-chloralose, blocks potassium-evoked propagation in rats, No Shinkei Geka 25 (1997) 523–528. cortical spreading depression in cats, Brain Res. 699 (1995) 109– [60] S. Warach, J.F. Dashe, R.R. Edelman, Clinical outcome in ischemic
115. stroke predicted by early diffusion-weighted and perfusion magnetic
[51] S. Schwab, S. Schwarz, M. Spranger, E. Keller, M. Bertram, W. resonance imaging: a preliminary analysis, J. Cereb. Blood Flow Hacke, Moderate hypothermia in the treatment of patients with Metab. 16 (1996) 53–59.
severe middle cerebral artery infarction, Stroke 29 (1998) 2461– [61] M. Yenari, J. Palmer, G. Sun, A. de Crespigny, M. Moseley, G.
2466. Steinberg, Time-course and treatment response with SNX-111, an
21
[52] M. Shimazawa, H. Hara, T. Watano, T. Sukamoto, Effects of Ca N-type calcium channel blocker, in a rodent model of focal cerebral channel blockers on cortical hypoperfusion and expression of c-Fos- ischemia using diffusion-weighted MRI, Brain Res. 739 (1996) like immunoreactivity after cortical spreading depression in rats, Br. 36–45.
J. Pharmacol. 115 (1995) 1359–1368. [62] M.A. Yenari, A. de Crespigny, J.T. Palmer, S. Roberts, S.L. Schrier, [53] G.R. Sutherland, H. Lesiuk, P. Hazendonk, J. Peeling, R. Buist, P. G.W. Albers, M.E. Moseley, G.K. Steinberg, Improved perfusion Kozlowski, A. Jazinski, J.K. Saunders, Magnetic resonance imaging with rt-PA and hirulog in a rabbit model of embolic stroke, J. Cereb.
31