PAPER • OPEN ACCESS
Effect of sintering temperature on the
microstructure, electrical and magnetic properties
of Zn0.98 Mn0.02O material
To cite this article: K Sebayang et al 2018 IOP Conf. Ser.: Mater. Sci. Eng.309 012119
View the article online for updates and enhancements.
Related content
Effect of Sintering Temperature on the Microstructure and Mechanical Properties of Low-Cost Light-Weight Proppant Ceramics
K Y Wang, H J Wang, Y Zhou et al.
-Effect of sintering temperature on structural defects and superconducting properties in MgB2 + C4H6O5
M S A Hossain, J H Kim, X Xu et al.
-Effect of Sintering Temperature to Physical, Magnetic Properties and Crystal Structure on Permanent Magnet BaFe12O19 Prepared From Mill Scale
Ramlan, Muljadi, Priyo Sardjono et al.
Effect of sintering temperature on the microstructure,
electrical and magnetic properties of Zn0.98 Mn0.02O
material
K Sebayang2, D Aryanto1,3, S Simbolon1,3, C Kurniawan1, S F. Hulu2, T Sudiro1, M Ginting1, and P Sebayang1,3,*
1Research Center for Physics, Indonesian Institute of Sciences (LIPI), Puspiptek Bld.
440-442, Serpong 15314, Indonesia
2Department of Physics, Universitas Sumatera Utara, Medan 20155, Indonesia
3Department of Mechanical Engineering, Universitas Pamulang, Banten, Indonesia.
*E-mail: [email protected]
Abstract. Zn0.98Mn0.02O material was synthesized from ZnO and MnO2 powders using solid state reaction method. The microstructure, electrical and magnetic properties of Zn0.98Mn0.02O were studied as a function of sintering temperature. The X-ray diffraction analysis indicates that the main phase of synthesized sample is composed of hexagonal wurtzite ZnO phase. While the secondary phase of ZnMnO3 were found at the sintering temperature of 700°C and 900°C. The electrical properties measurement of Zn0.98Mn0.02O sample revealed that the resistivity and the dielectric constant of samples increase with the increase of sintering temperature. The ferromagnetic properties at room temperature were observed in the Zn0.98Mn0.02O samples sintered at 500°C and 700°C. It also found that the increase in sintering temperature leads to a tendency toward the changes in the magnetic properties into paramagnetic. The presence of ZnMnO3 secondary phases in Zn0.98Mn0.02O system is believed to be a factor that affects the decrease of the electrical and magnetic properties of the sample.
1. Introduction
Recently, the information technology devices have combined a magnetic (spin) and semiconductors (charge), such as the spintronics. The substitution of some transition metal elements in the compound of semiconductor crystal host can enhance the local magnetic moment at a low energy level system [1]. The electrons charge and spin in the spintronic can improve the device performance, such as high speed, better efficiency, and stability [2]. According to the theory, ZnO and GaN with Mn doping exhibit ferromagnetic properties above the room temperature [3]. These properties encourage many researchers doing an intensive work on the doping variation experiment of dilute magnetic semiconductor (DMS). The study on the DMS continues to grow because of the stable magnetic properties, especially in ZnO material after doped with Fe, Co, Ni, or Mn to achieve a high Curie temperature [4].
2
applications and magneto-optical devices [5,6]. The ZnO material also has magnetic properties when it was doped with the transition metal elements, such as Cr, Co Fe, Ni, and Mn [7–9]. Previous studies by Choudhury [10] showed that Zn2+ ions in the ZnO lattice could be replaced by a transition metal
ion. This produces a magnetic ion interaction with a carrier in the semiconductor band gap of ZnO and generates the exchange of spins magnetic interaction.
The Mn-doped ZnO is interesting to study since its Curie temperature (Tc) is above the room
temperature, so that it possess a ferromagnetic properties at room temperature[11]. The similarity of ionic radii of Mn and Zn atoms produces a high solubility of Mn into ZnO crystal lattice[12], which makes more carrier atoms available so that it can increase the ferromagnetic properties of ZnO at room temperature [13]. The Mn-doped ZnO were studied intensively using various methods, such as solid state reaction [14,15], ion implantation [16], solvothermal process [17,18], sol-gel [19] and coprecipitation method[11,20]. The results of previous studies show that the doping concentration, fabrication method and treatment temperature of the samples play an important role in generating Mn-doped ZnO sample characteristic. The good magnetic properties of the sample are obtained when the Mn-doped ZnO material does not contain a secondary phase[21]. Several studies have reported that the solubility limit of Mn doping on ZnO to avoid the formation of secondary phase is around 4 %at [15,22]. According to the report of Riyadi et al. [23], Banerjee et al. [17] and Chattopadhyay et al. [5] who doped ZnO with Mn up to 3 %at. obtained a single phase of Mn-doped ZnO with ferromagnetic properties. However, only a few studies reported the effect of sintering temperature on the fabrication of Mn-doped ZnO using solid state reaction method. The advantage of solid state reaction compared to the other methods is due to relatively simple, low cost and synthesized sample can be used further as a target in the ion implantation and thin film deposition [24].
In this study, the Mn-doped ZnO samples were synthesized using a solid state reaction method. The effect of sintering temperature on the microstructure, electrical and magnetic properties of Mn-doped ZnO was studied using X-ray diffractometer, I-V meter, C-V meter and VSM.
2. Experiment Method
The Zn0.98Mn0.02O bulk was prepared from ZnO and MnO2 powders by the solid state reaction method
using a high-speed shaker mill. The sample preparation process was done by mixing ZnO and MnO2
powders which contain 2 %at. of Mn dopant using stainless steel balls that were inserted into the cylindrical stainless steels vial with ball and powder ratio of 10:1. The powder mixing was carried out at the oscillation frequency of about 700/min. This process was done by wet milling for 3 hours using toluene as solvent. Afterward, the milled powders were dried in an oven at 100°C for 3 hours. The powders were then compacted by an automatic axial hydraulic press into a bulk with 15 mm in diameter and 2 mm in thickness using a load of 1500 kgf/cm2. Then the bulk samples were sintered at
elevated temperatures of 500°C, 700°C, and 900°C for 4 hours in air with a heating rate of 10°C/min. The X-ray diffraction data was collected by using a Smartlab Rigaku diffractometer with CuKα
radiation (λ = 1.5406 Å). The scanning range is 20°-60° (2θ) with a step of 0.02°. The lattice parameters such as lattice constant, d-spacing, crystalline size, strain and dislocation density were calculated based on the XRD data. The resistance measurement was carried out at room temperature using dual-probes of 8842A High Impedance Fluxe Multimeter. A 590 CV Keithley analyzer was used to obtain the capacitance at room temperature, which is then used to determine the dielectric constant of the sample. The magnetic properties were measured using Vibrating Sample Magnetometer 250, Dexing Magnet, ltd.
3. Results and Discussion
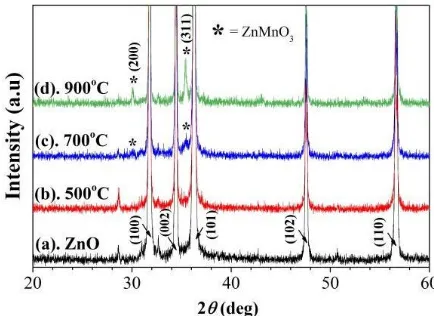
Figure 1 shows the XRD patterns of pure ZnO and Zn0.98Mn0.02O samples which prepared using a solid
by the presence of peaks that are identical to ZnO crystal planes of (100), (002), (101), (102) and (110) (JCPDS No. 00-005-0664). However, the Zn0.98Mn0.02O samples sintered at a temperature of 700°C
and 900°C show a secondary phase of ZnMnO3. The diffraction peaks of ZnMnO3 are confirmed at
the diffraction angle 2θ of around 30.09° and 35.44°, which show the crystal planes of (220) and (311). In general, the intensity of the main peak does not change significantly. But, the ZnMnO3 peak
intensity in the present study increases with the increase of the sintering temperature. This is the indication of the occurrence of ZnMnO3 crystal growth [25]. The formation of ZnMnO3 phase
probably due to Mn solubility in the ZnO crystal lattice is higher under non-equilibrium conditions [26]. The result of this work is also supported by previous studies using different methods [17,26,27], where in the secondary phases as MnO2, Mn2O3 or ZnMn2O4 appear at the sintering temperature above
500°C. Additionally, Fabbiyola et al. [11] with the co-precipitation method showed that the secondary phase of Mn3O4 appears when ZnO powder with 2 %at. Mn-dopant is dried at 200°C.
Table 1. Crystal parameters of Zn0.98Mn0.02O sample at (100) plane sintered at the elevated
temperature of 500°C, 700°C and 900°C 4 hours. Temperature of
The crystal size of the sample increases with the increase of the sintering temperature, where the crystal size is in the range of 46 to 52 nm. The increase of the sintering temperature from 500°C to 900°C accelerates the diffusion of grain Mn-doped ZnO at the grain boundaries, resulting from an agglomeration of a small grain into a larger grain. It affects the decrease of the lattice strain and dislocation density of the Mn-doped ZnO crystal system.
Figure 1. XRD patterns of (a) ZnO and Zn0.98Mn0.02O samples sintered at varying temperature of (b)
500°C, (c) 700°C, and (d) 900°C for 4 hours.
4
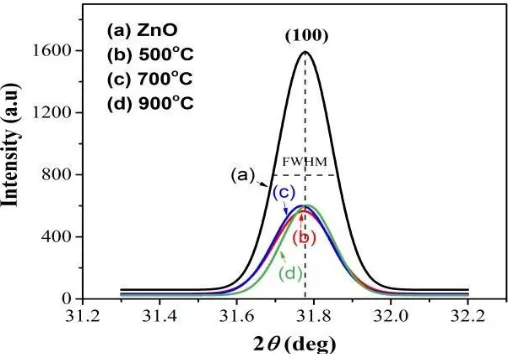
is shown in Figure 2 [28]. The diffraction peak intensity and the position are changes with the addition of 2 %at. Mn-dopant and the sintering temperature. This indicates that the presence of Mn-dopant and the increase of sintering temperature is likely to affect the quality of ZnO crystals. These results confirmed from the calculation of crystal parameters as presented in Table 1.
In Figure 2, it can be seen that the diffraction peak of the sample was shifted toward a smaller 2θ angle at the sintering temperature of 500°C and 700°C. This indicates the increase in the crystal lattice parameters (a and c), as shown by Neogiet al. [22]and Omri et al. [29]. The increase in lattice parameter is attributable to the substitution of Mn2+ ions replacing the position of Zn2+ ions in the ZnO
crystal lattice. The radius of Mn2+ ions (0.66 Å) is larger than Zn2+ ion (0.60 Å) which leads to an
increase in the lattice constants (a andc) of the ZnO crystal. While the diffraction peak of samples which is sintered at 900°C is shifted towards to a greater 2θ angle. This result is believed to be due to the presence of Mn3+ ions replacing the position of Zn2+ ions in the ZnO crystal lattice. The Mn3+ ion
has a smaller radius (0.58 Å) compared to that of Zn 2+ ions (0.60 Å) [30,31], which decrease the
lattice constant of ZnO crystal [32].
Figure 2. The intensity patterns of ZnO (a) and Zn0.98Mn0.02O samples on (100) plane sintered at the
elevated temperature of (b) 500°C, (c) 700°C and (d) 900°C for 4 hours.
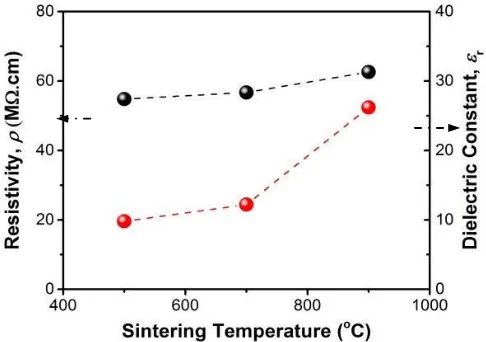
Figure 3 shows the resistivity and dielectric constant of Zn0.98Mn0.02O sample at varying sintering
temperature. From Figure 3 can be seen that the resistivity of Mn-doped ZnO increases with increasing sintering temperature, where the values are in the range of 54.8-62.6 MΩcm. The resistivity obtained in this work is smaller than the previous studies which were using a mechanical milling technique (resistivity of 84 MΩcm) [33]. However, the aforesaid resistivity is greater than the resistivity of the Mn-doped ZnO which was prepared by sol-gel method (29.42 MΩcm) [34] and sputtering [35]. The increase of the resistivity value in this work is related to the presence of ZnMnO3 phase, which leads
to the defects in the grain boundary area. Also, Mn doping builds a potential barrier at the grain boundaries [36,37] that causes the dopping of Mn2+ in ZnO is likely to decrease [38]. This case
strongly affects the decrease of the sample conductivity. The dielectric constant of Mn-doped ZnO samples, sintered at the elevated temperature of 500°C up to 900°C is in the range of 9.8 to 26.2 (see also Figure 3). The increase of the sintering temperature leads to an increase of the dielectric constant. This evidence is in accordance with the results of Das et al. [39], in which the impurity phase of ZnMnO3 at grain boundaries of the Zn0.98Mn0.02O acting as semi-blocking layers. In addition, the
presence of external electric field causes the carrier charge gather at the grain boundaries and leads to the polarization and an increase of the dielectric constant [40].
Figure 3. Resistivity and dielectric constant of Zn0.98Mn0.02O sample at varying sintering temperature.
Figure 4 shows the results of Vibrating Sample Magnetometer (VSM)-250 measurement of the pure ZnO and Zn0.98Mn0.02O samples sintered at elevated temperatures of 500°C, 700°C, and 900°C.
The magnetic hysteresis (M-H) curve indicates that the pure ZnO sample behaves as the diamagnetic. The presence of Mn dopant changes ZnO into ferromagnetic sample. In Figure 4, it is seen that the increase in sintering temperature on the synthesis of Mn-doped ZnO causes the decrease of the ferromagnetic properties. It is indicated by the decrease in the saturation (Ms) and the magnetic
remanence (Mr) of the samples. At 500°C, the magnetic parameters as magnetic saturation (Ms),
remanence (Mr) and coercivity (Hc) of the sample are 0.16 emu/g, 0.03 emu/g, and 277 Oe,
respectively. Whereas at 700°C, the Ms, Mr, and Hc of the sample are 0.04 emu/g, 0.01 emu/g, and 481
Oe, respectively. For Mn-doped ZnO sample sintered at 900°C, the sample exhibits paramagnetic properties indicated by the M-H curve which almost looks like a straight line. Compared to the results of previous studies, the present magnetic saturation (Ms) is relatively larger. Cahttopadhyay et al. [41]
reported that the Ms of Zn0.98Mn0.02O sample prepared by a solid state reaction method was 0.11
emu/g. While Hammad et al. [42], obtained the magnetic saturation of 0.194 emu/g for Zn0.98Mn0.02O
sample fabricated by sol-gel method.
Figure 4. Magnetic hysteresis curves (M-H) of pure ZnO (red line) and Zn0.98Mn0.02O samples sintered
at the elevated temperatures of 500°C (blue line), 700oC (magenta line) and 900°C (brown lines) for 4
6
The ferromagnetic properties of DMS are due to the exchange interaction between the carriers (holes or electrons from the valence band) and magnetic ions derived from a transition metal [10]. The substitution process causes the exchange interaction between the Mn2+ ion and Zn2+ion for a relatively
long time, so that almost all the Mn2+ ions aim towards the same spin. The polarized electrons in the
local spin and the conduction electrons will be easier to occur with the addition of Mn2+ concentration
[21]. Moreover, the ferromagnetic induction of ZnO-doped metal oxides sample is also possible to
appearance of paramagnetic properties. The presence of ZnMnO3 phase in the Zn1-xMnxO (x = 6, 8, 10
%at.) samples synthesized by the sol-gel method cause the sample becomes paramagnetic [22]. Based on the previous studies, the present results confirm that the change of the ferromagnetic into paramagnetic properties is due to the presence of a ZnMnO3 phase as the effect of the increase of the
sintering temperature.
4. Conclusion
The Zn0.98Mn0.02O was successfully synthesized by a solid state reaction method. The XRD data show
that all samples have a predominant crystal phase of hexagonal wurtzite. At high sintering temperatures, the secondary phase of ZnMnO3 was formed in the Zn0.98Mn0.02O system. The resistivity
and dielectric constant of Zn0.98Mn0.02O increase with the increase of the sintering temperature. The
Zn0.98Mn0.02O sample synthesized at low temperature exhibits ferromagnetic properties at room
temperature and close to paramagnetic properties at high sintering temperatures. The decrease of the magnetic properties of Zn0.98Mn0.02O synthesized at high temperatures is believed due to the presence
of a ZnMnO3 secondary phase.
5. Acknowledgements
This work was financially supported by Research Center for Physics, Indonesian Institute of Sciences.
6. References
[1] Pearton S J, Norton D P, Heo Y W, Tien L C, Ivill M P, Li Y, Kang B S, Ren F, Kelly J and Hebard A F 2006 Journal of Electronic Materials 35 863868
[2] Sharma P, Gupta A, Owens F J, Inoue A and Rao K V 2004 Journal of Magnetism and Magnetic Materials 282 115-121
[3] Dietl T, Ohno H, Matsukura F, Cibert J and Ferrand D 2000 Science287 1019-1022 [4] Sato K, Katayama-Yoshida H 2001 Jpn. J. Appl. Phys.40 L334–L336
[5] Chattopadhyay S, Dutta S, Banerjee A, Jana D, Bandyopadhyay S, Chattopadhyay S and Sarkar A 2009 Physica B 404 15091514
[6] Chaari M, Matoussi A and Fakhfakh Z 2011 Materials Sciences and Application 2 765-770 [7] Liu Y, Yang Y, Yang J, Guan Q, Liu H, Yang L, Zhang Y, Wang Y, Wei M, Liu X, Fei L, and
Cheng X 2011 Journal of Solid State Chemistry 184 1273-1278
[8] Saleh R, Djaja N F and Prakoso S P 2013 Journal of Alloys and Compounds546 48-56
[9] Ahmed F, Kumar S, Arshi N, Anwar M S, Koo B H and Lee C G 2012 Microelectronic Engineering 89 129-132
[11] Fabbiyola S, Kennedy L J, Dakhel A A, Bououdina M, Vijaya J J and Ratnaji T 2016 Journal of Molecular Structure1109 89-96
[12] Moontragoon P, Pinitsoontorn S and Thongbai P 2013 Microelectronic Engineering 108 158-162
[13] Yang J H, Zhao L Y, Zhang Y J, Wang Y X, Liu H L and Wei M B 2007 Solid State Communications 143 566-569
[14] Jayakumar O D, Gopalakrishnan I K and Kulshrestha S K 2006 Physica B 381 194-198
[15] Sanz R, Jensen J, Gonzalez-Diaz G, Martinez O, Vazquez M and Hernandez-Velez M 2009 Nanoscale Res. Lett.4 878-887
[16] Banerjee S, Rajendran K, Gayathri N, Sardar M, Senthilkumar S and Sengodan V 2008 Journal of Applied Physics104 043913(1-3)
[17] Ghoshal T, Kar S, Biswas S, De S K, Nambissan P M G 2009 J. Phys. Chem. C.113 3419-3425 [18] Karmakar R, Neogi SK, Midya N, Banerjee A and Bandyopadhyay S 2016 J. Mater Sci: Mater
Electron 27 6371-6381
[22] Neogi S K, Karmakar R, Misra A K, Banerjee A, Das D and Bandyopadhyay S 2013 Journal of Magnetism and Magnetic Materials 346 130-137
[23] Riyadi S, Muafif A, Nugroho A, Rusydi A and Tjia MO 2007 J. Phys.: Condens. Matter. 19 476214(1-8)
[24] Owens F J 2009 Journal of Magnetism and Magnetic Materials321 3734-3737
[25] Wang X L, Lai K H and Ruotolo A 2012 Journal of Alloys and Compounds542 147-150 [26] Zhang J, Skomski R and Sellmyer D J 2005 Journal of Applied Physics97 10D303(1-3) [27] Kolesnik S and Dabrowski B 2004 Journal of Applied Physics96 5379-5381
[28] Sebayang P, Hulu2. F, Nasruddin, Aryanto D, Kurniawan C, Subhan A, Sudiro T and Ginting M 2017 American Institute of Physics1862 030050(1-5)
[29] Omri K, Ghoul J E, Lemine O M, Bououdina M, Zhang B and Mir L E 2013 Superlattices and Microstructures60 139-147
[30] Abrishami M E, Hosseini S M and Kompany A 2011 Journal of Applied Sciences11 1411-1415 [31] Menon A S, Kalarikkal N and Thomas S 2013 Indian Journal of NanoScience1 16-24
[32] Lu Y, Lin Y, Xie T, Shi S, Fana H and Wang D 2012 Nanoscale 4 6393-6400 [37] Zhou Z, Kato K, Komaki T, Yoshino M, Yukawa H, Morinaga M and Morita K 2004 Journal of
the European Ceramic Society24 139-146
[38] Mote V D, Purushotham Y and Dole B N 2016 Materials and Design96 99-105
[39] Das J, Mishra D K, Srinivasu V V, Sahu D R and Roul B K 2015 Indian Journal of Physics89
1143-1151
[40] Arshad M, Ahmed A S, Azam A and Naqvi A H 2013 J. of Alloys and Compounds 77 469-474 [41] Chattopadhyay S, Neogi S K, Sarkar A, Mukadam M D, Yusuf S M, Banerjee A and
8
[42] Hammad T M, Griesing S, Wotocek M, Kuhn S, Hempelmann R, Hartmann U and Salem JK 2013 Appl. Nanosci. 3 153159