The activity of cyclin-dependent kinases (CDKs) on specific targets mediates the temporal regulation of plant cell cycle transitions. The sequential activity of CDKs and the spatial regulation of cell proliferation during plant development, however, are still poorly understood. Understanding these aspects depends on the identification of the downstream targets and upstream modulators of CDKs and their regulation in response to mitogenic and/or differentiation signals. Current efforts to elucidate the answers to these questions are very promising; in particular, recent works reveal the essential role that the retinoblastoma pathway plays in controlling cell cycle progression and, presumably, some developmental events.
Addresses
Centro de Biología Molecular ‘Severo Ochoa’, Consejo Superior de Investigaciones Científicas (CSIC) – Universidad Autónoma de Madrid (UAM), Cantoblanco, 28049 Madrid, Spain;
e-mail: [email protected]
Current Opinion in Plant Biology1998, 1:492–497 http://biomednet.com/elecref/1369526600100492 © Current Biology Ltd ISSN 1369-5266
Abbreviations
CAK CDK-activating kinase
CDK cyclin-dependent kinase
Rb retinoblastoma
RbIP Rb-interacting protein
STF S-phase-specific transcription factor
UTR untranslated region
Introduction
When cell responses are integrated at a higher organiza-tional level (organ, organism), the differences between plants and animals become very apparent (for example body organization and development). The lack of cell migration, the existence of a continuous morphogenetic process and the plasticity of the plant cell lead to a require-ment for continuous cell proliferation and the existence of highly regulated gene expression programs. In spite of these and other differences, the overall strategy which evolved in plant cells to regulate the events required to complete the cell division cycle seems to be similar to that in other eukaryotes, as these events are mediated by the action of cyclin-dependent kinases (CDKs) associated with cyclins [1,2,3•]. In mammalian cells, members of the retinoblastoma (Rb) family, also known as ‘pocket’ proteins, are crucial to control the passage of cells through G1 and G1/S, and the reactivation of G0 cells [4]. Rb operates as a tumor suppressor and as a negative regulator of cell prolif-eration. Three members comprise the Rb family, namely the Rb tumor suppressor, and the p107 and p130 proteins. Their function depends on the association with transcrip-tion factors of the E2F family [5]. These complexes are disrupted by sequential phosphorylation of the Rb moiety carried out by various cyclin-CDK complexes [6,7].
The existence of an analogous pathway in plants has been a matter of speculation until recently since none of the components had been identified. Advances in the study of G1/S regulators in plants, however, have indicated that a Rb-like pathway might operate in regulating plant cell growth. Surprisingly, to some extent, the components identified so far are similar to some of those functioning in mammalian cells and are, therefore, totally unrelated to those found in yeast. Recent efforts to understand G1/S regulation in plant cells, with particular emphasis on the Rb pathway, will be the subject of this review.
Plant Rb protein(s)
The hypothesis that a Rb-mediated pathway may exist in plants received initial support from two lines of evidence: first, the isolation of homologues of D-type cyclins from Arabidopsis [8] and alfalfa [9], which contain an LXCXE amino acid motif known to mediate Rb-binding in human cells; and second, the identification of proteins in gemi-niviruses (a group of plant DNA viruses with small genomes) that also contained the LXCXE motif required for efficient viral replication in cultured cells [10]. This, together with their absolute requirement for cell factors to complete the viral replication cycle, makes geminiviruses useful tools in plant cell cycle and DNA replication studies.
Early in the development of this field, a maize EST [11] was used to isolate cDNA clones encoding proteins with homol-ogy to human Rb family proteins from cDNA libraries made from maize endosperm (ZmRb, [12]) and two week old seedlings (ZmRb1, [13]). Several ZmRb1 cDNA clones, dif-fering in their 3′-untranslated region (3’UTR), were isolated (Q Xie and C Gutierrez, unpublished data). Analysis by Northern blot and rapid amplication of 5′cDNA ends poly-merase chain reaction in maize cells indicated the existence of mRNAs of a size consistent with that of the longest ZmRb1 cDNA isolated [13]. In due course, two classes of cDNAs encoding Rb-related proteins from two maize genes, RRB1 and RRB2, were isolated [14•]. The two longest RRB1cDNAs also differ in their 3′UTR and have the potential to encode a nuclear protein of 866 amino acids, whereas the RRB2cDNAs seem to represent partial clones [14•]. Interestingly, RRB transcripts are abundant in the shoot apex, but barely detected in 2-week seedlings. Thus, it is possible that expression of different members of the Rb protein family might be spatially and/or temporally regulat-ed. Data on the genomic structure of the maize Rb gene(s), on Rb from other plant species, and on the functional role of Rb should contribute significantly to the field.
Structural conservation between plant and
animal Rb proteins
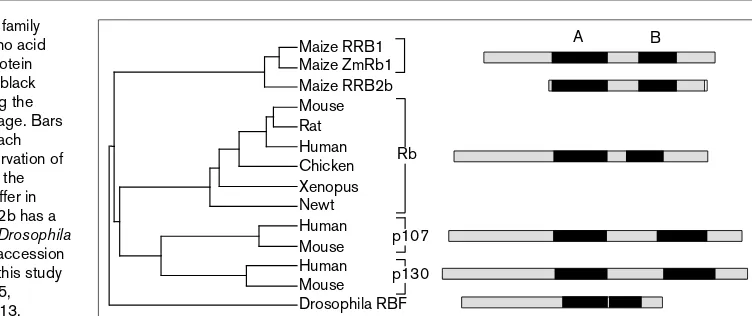
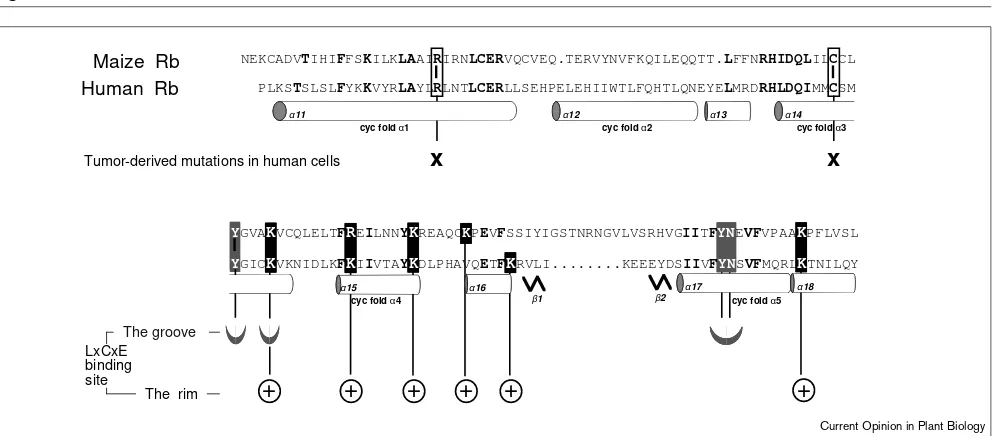
members, including plants. They contain the domains A and B (50–65% similarity), which together form the so-called A/B pocket, flanked by carboxy- and amino-terminal domains. All family members, apart from Drosophila, have a spacer of variable length between the A and B domains (Figure 1). Whether plant Rb is functionally equivalent to any of the human Rb family members or whether it shares some or all of their cell cycle regulatory properties remain to be determined. The conservation among pocket pro-teins does not occur merely at the level of their primary sequence. The crystal structure of the human A/B pocket domain [15] has revealed that the three-dimensional struc-ture might also be conserved, in particular, for two clusters of surface residues. One is the LXCXE-binding site, locat-ed within the B domain and consisting of a hydrophobic groove bordered by a positively charged rim (Figure 2). This explains the similarity in Rb-binding properties between animal virus oncoproteins, such as Simian Virus 40 large T antigen, and plant geminivirus Rb-bind-ing proteins [10,14•]. The other is located in the interface between the A and B domains. Interestingly, it is in these two clusters that mis-sense mutations frequently map in human tumors [16]. Thus, an exciting research topic for the future will be to identify the phenotypic consequences that mutations in these conserved clusters in Rb could have on plant cell cycle, growth and development. Furthermore, the D-type cyclins are crucial players in cell cycle regulation as high levels of cycD1 are frequently found in human tumors and cycD1 overexpression pro-motes hyperplasia and adenocarcinomas [17]. Although similar experiments are not available yet in plants, ectopic expression of the mitotic cyclin cyc1At stimulates growth without altering root development, and does not result in neoplasia [18].
Rb as a CDK substrate
Plants contain an abundant CDK gene family [3•]. The presence of consensus CDK phosphorylation sites (defined by the amino acid motif S/TP), strongly suggests
that regulation of Rb activity depends on its phosphoryla-tion level [13]. The cluster in the carboxy-terminal domain, known to be phosphorylated by different CDK/cyc complexes in human cells [19], could be of par-ticular relevance. Thus, it is conceivable, by analogy to animal cells, Rb phosphorylation by one or more CDK/cyc complexes allows the release of Rb-bound S-phase-specif-ic transcription factors (STFs).
The search for the CDK/cyc complex(es) acting on plant Rb is intense in a number of laboratories although, so far, their identification has been elusive. One could antici-pate that such activity should be present during the G1 and S phases. Some CDK activities have been character-ized in Arabidopsis [20] and in alfalfa cells [21••], although it remains to be determined whether they can functionally use Rb as a substrate. Complementary in vitrophosphorylation studies using maize Rb and human CDK/cyc complexes have shown that plant Rb can be readily phosphorylated by human CDK4/cycD, CDK2/cycA and CDK2/cycE complexes [22••]. Moreover, an intact pocket domain is required for effi-cient phosphorylation by CDK4/cycD, as in human cells, but not by the other complexes [22••]. Although the functional significance of these studies still needs to be addressed, it is conceivable that one (or more) plant CDK complexed to cycD and/or cycA, functionally equivalent to human CDK4/cycD and CDK2/cycA, reg-ulates plant Rb function by sequential phosphorylation. In this context, Rb has been also implicated in S phase regulation in the absence of an intervening mitosis, as an S-phase-associated kinase activity is increased in maize endosperm, coinciding with the initiation of endoredu-plication [12,23].
Future studies should focus on the identification of path-ways regulating the activity of CDK/cyc complexes. Steps towards this goal have been taken by the isolation of the Arabidopsis cak1Atgene encoding a CDK-activating
Figure 1
Comparison of plant and animal Rb family members. The tree is based on amino acid homologies of the region of each protein containing the A/B pocket domain (black boxes) and the spacer domain, using the PILEUP program of the GCG package. Bars at the right represent members of each subgroup to show the overall conservation of their domain organization. Note that the sequences of RRB1 and ZmRb1 differ in some residues, that the maize RRB2b has a different carboxy-terminus and that Drosophila RBF lacks the spacer domain. The accession numbers of the sequences used in this study are: AF007793, X98923, AF007795, M26391, P33568, M15400, U00113, A44879, Y09226, L14812, Q64701, S67171, U50850 and X96975.
Maize RRB1 Maize ZmRb1 Maize RRB2b Mouse Rat Human Chicken Xenopus Newt Human Mouse Human Mouse Drosophila RBF
p130 p107 Rb
A B
kinase (CAK) which is able to complement CAK muta-tions in yeasts and to specifically activate human CDK2 [24••]. Another plant gene with significant homology to CAKs is the rice R2gene whose expression is up-regulat-ed in meristems and part of the elongation zone in gibberellin-treated rice internodes, consistent with its potential role in G1/S progression [25•]. On the other side, the identification of other CDK-interacting proteins such as a homolog of the yeast p13suc1 gene from Arabidopsis [26] and an ArabidopsisCDK inhibitor (ICK1), related to human p27 protein, which interacts with both cdc2a and cycD3 and is induced by abscisic acid [27•] should contribute significantly to our understanding of the CDK regulatory network.
A broad spectrum of plant Rb-interacting
proteins
Since the isolation of cDNAs encoding plant Rb pro-teins, the identification of Rb-interacting proteins (RbIPs) has allowed us to take a glimpse at the potential roles of different Rb-containing complexes in plants. RbIPs belong to two groups according to the domain mediating binding to Rb.
LXCXE-containing RbIPs
First, as already mentioned, subgroup I geminiviruses (Mastreviruses) encode a RepA protein that binds plant Rb protein through its LXCXE motif. Examples are the wheat dwarf virus (WDF; [13]), the maize streak virus (MSV; [28]) or the bean yellow dwarf virus (BYDV; L Liu and J Stanley, personal communication) RepA protein. Some
changes of the motif, for example LXCXK, drastically reduce or abolish Rb-binding [13], whereas others, for example LXCXQ, do not have such strong effects (L Liu and J Stanley, personal communication). In Mastreviruses, the Rep protein, required for viral DNA replication, is translated from the same RepA-encoding mRNA after a splicing event [29]. This Rep protein contains an LXCXE motif but, interestingly, it does not bind to plant Rb ([28]; L Liu and J Stanley, personal communication; Q Xie and C Gutierrez, unpublished data). Presumably, the LXCXE motif is hidden since a carboxy-terminal deleted version of WDV Rep can bind to plant Rb (Q Xie and C Gutierrez, unpublished data). Second, the three Arabidopsis D-type cyclins bind to maize Rb [22••]. Interaction between RRB1 and cycD3 in yeast seems to be enhanced in a carboxy-ter-minal deleted cycD3 protein [14•].
LXCXE-lacking RbIPs
Geminiviruses from the other two subgroups (II or Curtoviruses and III or Begomoviruses), do not encode any protein homologous to Mastrevirus RepA but they all encode a Rep protein. Interestingly, Rep protein of tomato golden mosaic virus, a Begomovirus, lacks an LXCXE motif but still binds to maize RRB1 [14•]. Whether this represents a differ-ence in the strategy used by different geminiviruses to impinge on the Rb pathway is an open question. A second set of proteins, related to the S. cerevisiaeMSI1 protein [30] and the human RbAp46 and RbAp48 proteins [31], has been recently isolated from tomato (LeMSI1) and from Arabidopsis (AtMSI1-3) [32•]. Their function is unknown, but the human counterparts have been implicated in Figure 2
cyc fold α5
α18
β1 β2
+
α11
cyc fold α1 cyc fold α2
α12 α13 α14 cyc fold α3
Tumor-derived mutations in human cells Maize Rb
Human Rb
+
NEKCADVTIHIFFSKILKLAAIRIRNLCERVQCVEQ.TERVYNVFKQILEQQTT.LFFNRHIDQLILCCL
PLKSTSLSLFYKKVYRLAYLRLNTLCERLLSEHPELEHIIWTLFQHTLQNEYELMRDRHLDQIMMCSM
YGVAKVCQLELTFREILNNYKREAQCKPEVFSSIYIGSTNRNGVLVSRHVGIITFYNEVFVPAAKPFLVSL YGICKVKNIDLKFKIIVTAYKDLPHAVQETFKRVLI...KEEEYDSIIVFYNSVFMQRLKTNILQY
+
α17
LxCxE binding site
The rim The groove
x
x
α16
+
cyc fold α4
+
+
α15
Current Opinion in Plant Biology
Amino acid conservation and predicted structure of domain B of maize Rb derived from the crystal structure of the A/B pocket of human Rb [15]. Domain B contains eight α-helices (α11 through α18), five of which participate in the so-called ‘cyclin fold’ (α-helices cycfold α1 through cycfold α5). The two short βstrands (β1 and β2) are also shown. Note that the eight amino acid insertion present in maize Rb is
processes related to chromatin remodelling [33,34]. Assuming similar roles in plants, it is conceivable that they have implications in plant growth, morphogenesis and devel-opment. Third, a member of the E2F transcription factor family has recently been cloned in plants [35•] and, like the animal E2Fs, also lacks a LXCXE motif.
In addition, an increasing number of other proteins are known to interact with Rb in human cells [36]. It is expected, therefore, that more plant RBIPs will be identi-fied in the near future. Among them, some could belong to the family of human histone deacetylases which con-tribute to Rb-mediated transcriptional repression [37,38]. Others might be related to human oncogenes, differentia-tion and development factors. Still, it may occur that novel RBIPs, unique to plants, may have evolved as a conse-quence of the specific demands of the plant body organization and physiology.
Regulation of G1/S-specific gene expression
by Rb-bound transcription factors
The correct passage of cells throughout the cell cycle requires a strict temporal control of transcriptional activity ([3•] and references therein). For some genes, such as those encoding ribonucleotide reductase, histones, and cyclin D3, an up-regulation at G1/S and S phases has been found [39–41]. The molecular nature of S-phase-specific transcription factors, however, is not known. In human cells, expression of G1/S genes frequently depends on E2F transcription factors whose activity is modulated by binding to Rb family members [5].
Is G1/S specific gene expression in plants mediated by E2F-like proteins? The following evidence strongly sug-gests that this is the case: the ribonucleotide reductase and cyclin D3 promoters, but not those of histone genes, con-tain binding sites similar to those of human E2F (C Gigot, personal communication; J Murray, personal communica-tion); the structural conservation of the A/B pocket proteins [15]; maize Rb interacts, although weakly, with human E2F-1 in yeast [14•,22••,35•]; and finally, maize Rb represses E2F-dependent transcription in human cells [22••]. As the identification of plant E2F-like protein(s) and/or gene(s) has been so elusive to date, a significant step ahead has been the recent isolation of a cDNA encod-ing a plant E2F [35•] which has a domain organization similar that of the human E2F-1 subset of proteins. The identification of this novel component of the Rb pathway will allow further studies on its expression pattern and transcriptional activity related to Rb binding.
The Rb pathway in plant differentiation and
development
Components of the Rb pathway are involved not only in cell cycle control but also in mammalian [42,43] and Drosophila development [44,45]. Rb also seems to play a role in growth and development in plants. Thus, in maize leaves, a prolifer-ative gradient exists with a high amount of dividing cells in
the basal meristematic part and a progressive increase in dif-ferentiated cells along the leaf. In this difdif-ferentiated zone, Rb-immunoreactive ~110 kDa proteins accumulate, whereas they are barely detected (or absent) in the basal zone [22••]. Plant E2F mRNA can be detected in proliferating as well as in differentiated cells [35•]. These studies, together with the increasing evidence of the role of cell proliferation, and per-haps cell cycle regulators, in plant development [46] suggest a role for the components of the Rb pathway at some stages during plant morphogenesis and patterning.
Conclusions and prospects
A summary of the current knowledge of the Rb pathway in plants is shown in Figure 3. The picture is far from
Figure 3
A proposal for the regulation of the Rb pathway. The key component of the pathway is the complex constituted by Rb, E2F and DP proteins, the latter still to be identified. Plant Rb contains several potential CDK-phosphorylation sites (S/TP), in particular a carboxy-terminal cluster that may be phosphorylated by CDK/cyc complexes, for example,
CDK/cycD. Changes in the phosphorylation state of Rb, and presumably of its partners, could modulate their regulatory functions. The release of the Rb-bound E2F/DP factors after Rb phosphorylation by activated CDK/cycD would regulate genes required at the G1/S transition. It should be kept in mind that up-regulation at G1/S may also occur through other mechanisms. CDK is activated by specific phosphorylation by CAKs and its function inhibited by specific inhibitors (CKI). It is tempting to speculate that the expression of these G1/S regulators and/or their activities may be regulated by hormones, nutrients and/or negative growth signals. The normal Rb
phosphorylation pathway may be bypassed in geminivirus-infected cells by the action of virally encoded Rb-binding proteins. The presence of Rb and E2F messages in differentiated tissues suggests that, in addition to their role during the cell cycle, Rb and E2F/DP complexes may be crucial for the differentiation state of plant cells. These putative interactions have not been demonstrated yet to occur in vivo.
CDK Auxin
Hormones/Nutrients
Cytokinin
CycD3 CycD2 Sucrose
CKI
G1 / S Transition
P CycD - CDKP Geminiviruses
E2F
Rb
CycD - CDK ?
? Rb - binding
proteins
Rb DP ? Rb
E2F DP ?
Negative signals ? CAK
Differentiated state
?
complete, as many questions remain unanswered. On the basis of recent results, however, the field is moving fast and in extremely attractive directions. First, we need to identify, clone and characterize at the molecular level other components of the pathway. Second, since disrup-tion of G1/S regulators in mammals leads to transformation and tumor development, similar studies in plants will serve to explain why they are extremely refrac-tory to neoplastic transformation. Third, the normal G1/S events seem to be altered by geminivirus infection as up-regulation of genes, normally switched off in differentiated cells, occurs. One possibility is that this and/or other cellular effects of geminivirus proteins might be a consequence of the interference with the Rb path-way. If this is the case, an abnormal S-phase, or at least some S-phase functions and perhaps cellular DNA repli-cation, could be triggered. Fourth, if it is proven that Rb/E2F complexes participate in regulating differentia-tion decisions, a complete new window will be open to look at plant development. These are just a few of many aspects currently being investigated. Thus, we look for-ward to new and exciting results in the near future.
Acknowledgements
I thank D Dudits, C Gigot (deceased), H Hirt, D Inze, J Murray and J Stanley for sharing ideas and communicating unpublished results, E Martinez-Salas for comments on the manuscript, and past and present members of the lab for contributing with their work, discussions and stimulating environment. The work is partially supported by grants PB96-0919 (DGES), CI1*-CT94-0079 (European Union) and 06G/046/96 (Comunidad de Madrid) and by an institutional grant from Fundación Ramón Areces.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest ••of outstanding interest
1. Renaudin JP, Doonan J, Freeman D, Hashimoto J, Hirt H, Inze D, Jacobs T, Kouchi H, Rouze P, Sauter M et al.: Plant cyclins: a unified nomenclature for plant A-, B- and D-type cyclins based on sequence organization.Plant Mol Biol1996, 32:1003-1018. 2. Mironov V, van Montagu M, Inze D: Regulation of cell division in
plants: an Arabidopsis perspective. Prog Cell Cycle Res1997, 3:29-41.
3. Doonan J, Fobert P:Conserved and novel regulators of the plant
• cell cycle.Curr Opin Cell Biol1997, 9:824-830.
Updated discussion on the structure and function of the plant CDK family.
4. Weinberg RA: The retinoblastoma protein and cell cycle control.
Cell1995, 81:323-330.
5. Helin K: Regulation of cell proliferation by the E2F transcription factors. Curr Opin Genetics Dev 1998, 8:28-35.
6. Sherr CJ, Roberts JM: Inhibitors of mammalian G1 cyclin-dependent kinases.Genes Dev1995, 9:1149-1163.
7. Serrano M: The tumor suppressor protein p16INK4a.Exp Cell Res
1996, 237:7-13.
8. Soni R, Carmichael JP, Shah ZH, Murray JAH: A family of cyclin D homologs from plants differentially controlled by growth regulators and containing the conserved retinoblastoma protein interaction motif.Plant Cell 1995, 7:85-103.
9. Dahl M, Meskiene I, Bögre L, Ha DTC, Swoboda I, Hubmann R, Hirt H, Heberle-Bors E: The D-type alfalfa cyclin gene cycMs4
complements G1 cyclin-deficient yeast and is induced in the G1 phase of the cell cycle.Plant Cell 1995, 7:1847-1857.
10. Xie Q, Suarez-Lopez P, Gutierrez C: Identification and analysis of a retinoblastoma binding motif in the replication protein of a plant
DNA virus: requirement for efficient viral DNA replication.EMBO J
1995, 14:4073-4082.
11. Shen B, Carneiro N, Torres-Jerez I, Stevenson B, McCreery T, Helentjaris T, Baysdorfer C, Almira E, Ferl RJ, Habben JE, Larkins B: Partial sequencing and mapping of clones from two maize cDNA libraries.Plant Mol Biol1994, 26:1085-1101.
12. Grafi G, Burnett RJ, Helentjaris T, Larkins BA, DeCaprio JA, Sellers WR, Kaelin WG Jr: A maize cDNA encoding a member of the retinoblastoma protein family: involvement in endoreduplication.
Proc Natl Acad Sci USA 1996, 93:8962-8967.
13. Xie Q, Sanz-Burgos AP, Hannon GJ, Gutierrez C: Plant cells contain a novel member of the retinoblastoma family of growth regulatory proteins.EMBO J 1996, 15:4900-4908.
14. Ach RA, Durfee T, Miller AB, Zambryski PC, Hanley-Bowdoin L,
• Gruissem W: RRB1and RRB2encode maize retinoblastoma-related proteins that interact with a plant D-type cyclin and geminivirus replication protein.Mol Cell Biol 1997,17:5077-5086. This paper reports the cloning of cDNAs from two Zea maysgenes encod-ing Rb-related proteins, suggestencod-ing a complex pattern of messages. It also shows a weak interaction of maize RRB1 with human E2F-1.
15. Lee J-O, Russo AA, Pavletich NP: Structure of the retinoblastoma tumour-suppressor pocket domain bound to a peptide from HPV E7.Nature 1998, 391:859-865.
16. Kaye FJ, Kratzke RA, Gerster JL, Horowitz JM: A single amino acid substitution results in a retinoblastoma protein defective in phosphorylation and oncoprotein binding.Proc Natl Acad Sci USA
1990, 87:6922-6926.
17. Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV: Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice.Nature1994, 369:669-671.
18. Doerner P, Jorgensen J-E, You R, Steppuhn J, Lamb C: Control of root growth and development by cyclin expression.Nature1996, 380:520-523.
19. Mittnacht S: Control of pRB phosphorylation.Curr Opin Gen Dev
1998, 8:21-27.
20. Segers G, Gadisseur I, Bergounioux C, de Almeida Engler J, Jacqmard A, Van Montagu M, Inze D: The Arabidopsis cyclin-dependent kinase gene cdc2bAtis preferentially expressed during S and G2 phases of the cell cycle. Plant J 1996, 10:601-612. 21. Magyar Z, Meszaros T, Miskolczi P, Deak M, Feher A, Brown S,
•• Kondorosi E, Athanasiadis A, Pongor S, Bilgin M et al.: Cell cycle phase specificity of putative cyclin-dependent kinase variants in synchronized alfalfa cells.Plant Cell 1997, 9:223-235.
The cloning of multiple putative CDKs from alfalfa and measurement of in vitrokinase activity throughout the cell cycle is reported. It shows that pro-tein fractions bound to p13suc1-Sepharose or immunoprecipitated with Cdc2MsA/B antibodies contain elevated kinase activities at the G1/S and G2/M transitions, suggesting a multilevel regulatory system throughout the cell cycle.
22. Huntley R, Healy S, Freeman D, Lavender P, de Jager S, Greenwood J,
•• Makkerh J, Walker E, Jackman M, Xie Q et al.: The maize retinoblastoma protein homologue ZmRb1 is regulated during leaf development and displays conserved interactions with G1/S regulators and plant cyclin (D-type) proteins. Plant Mol Biol 1998, 37:155-169.
The first report of a temporal and spatial regulation of Rb level during maize leaf development. ZmRb1 protein binds in vitroto all three ArabidopsiscycD in a LXCXE-dependent manner and in yeast to human and DrosophilaE2F homologues. Interestingly, ZmRb1 is a good substrate for G1/S CDK/cyc complexes from human origin.
23. Grafi G, Larkins, BA: Endoreduplication in maize endosperm: involvement of M-phase promoting factor inhibition and induction of S-phase-related kinases.Science1995, 269:1262-1264. 24. Umeda M, Bhalerao RP, Schell, J, Uchimiya H, Koncz C: A distinct
•• cyclin-dependent kinase-activating kinase of Arabidopsis thaliana.Proc Natl Acad Sci USA1998, 95:5021-5026.
The first report of a functional CAK from Arabidopsis(Cak1At) able to complement CAK mutations in budding and fission yeast and to activate human CDK2 by specific phosphorylation at a threonine (T160) located in the T-loop. Cak1At does not phosphorylate the carboxy-terminal domain of RNA polymerase.
25. Sauter M: Differential expression of a CAK (cdc2-activating
and in the meristem and part of the elongation zone of gibberellin-treated rice internodes.
26. de Veylder L, Segers G, Glab N, Casteels P, van Montagu M, Inze D: The Arabidopsis Cks1At protein binds the cyclin-dependent kinases Cdc2aAt and Cdc2bAt.FEBS Lett 1997, 412:446-452. 27. Wang H, Qi Q, Schorr P, Cutler AJ, Crosby WL, Fowke LC: ICK1, a
• cyclin-dependent protein kinase inhibitor from Arabidopsis thalianainteracts with both Cdc2a and CycD3, and its expression is induced by abscisic acid. Plant J 1998, 15:501-510.
A study of ICK1, a CDK inhibitor related to human p27Kip1 protein, in terms of gene structure, interaction with both A. thalianaCdc2a and CycD3, and induction by the plant growth regulator abscisic acid.
28. Horvath GV, Pettko-Szandtner A, Nikovics K, Bilgin M, Boulton M, Davies JW, Gutierrez C, Dudits D: Prediction of functional regions of the maize streak virus replication-associated proteins by protein–protein interaction analysis.Plant Mol Biol1998, in press. 29. Schalk H-J, Matzeit V, Schiller B, Schell J, Gronenborn B: Wheat
dwarf virus, a geminivirus of graminaceous plants needs splicing for replication.EMBO J1989, 8: 359-364.
30. Rugieri R, Tanaka K, Nakafuku M, Kaziro Y, Toh-E A, Matsumoto K: MSI1, a negative regulator of the RAS-cAMP pathway in
Saccharomyces cerevisiae.Proc Natl Acad Sci USA1989, 86:8778-8782.
31. Qian Y-W, Wang Y-CJ, Hollingsworth RE Jr, Jones D, Ling N, Lee EY-HP: A retinoblastoma-binding protein related to a negative regulator of Ras in yeast.Nature1993, 364:648-652. 32. Ach RA, Taranto P, Gruissem W: A conserved family of WD-40
• proteins binds to the retinoblastoma protein in both plants and animals.Plant Cell1997, 9:1595-1606.
This paper reports the cloning by PCR using degenerate oligonucleotides of plant proteins related to yeast MSI1 and human RbAp46 and RbAp48 pro-teins. The plant proteins bind to maize RRB1 and to an ~65 kDa protein of unknown identity.
33. Taunton J, Hassig CA, Schreiber SL: A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p.
Science1996, 272:408-411.
34. Verreault A, Kaufman PD, Kobayashi R, Stillman B: Nucleosome assembly by a complex of CAF-1 and acetylated histones H3/H4.
Cell1996, 87:95-104.
35. Ramirez-Parra E, Xie Q, Boniotti MB, Gutierrez C: The cloning of
• plant E2F transcription factor reveals unique and conserved
features with animal G1/S regulators.Proc Natl Acad Sci USA
1998, in press.
This is the first report of the isolation of a cDNA encoding a plant E2F protein by yeast two-hybrid screening using ZmRb1 as a bait. It identifies a unique Rb-binding motif, evaluates its binding properties to different Rb family mem-bers and studies plant E2F expression in proliferating and differentiated cells.
36. Taya Y: RB kinases and RB-binding proteins: new points of view.
Trends Biochem Sci1997, 22:14-17.
37. Brehm A, Miska EA, McCance DJ, Reid JL, Bannister AJ, Kouzarides T: Retinoblastoma protein recruits histone deacetylase to repress transcription.Nature 1998, 391:597-601.
38. Magnaghi-Jaulin L, Groisman R, Naguibneva I, Robin P, Lorain S, Le Villain JP, Troalen F, Trouche D, Harel-Bellan A: Retinoblastoma protein represses transcription by recruiting a histone deacetylase.Nature 1998,391:601-605.
39. Atanassova R, Flénet M, Gigot C, Chaubet N:Functional analysis of the promoter region of maize (Zea mays) H3 histone gene in transgenic Arabidopsis thaliana.Plant Mol Biol1998,37:275-285. 40. Callard D, Mazzolini L: Identification of proliferation-induced genes in Arabidopsis thaliana. Characterization of a new member of the highly evolutionary conserved histone H2A.F/Z variant subfamily.
Plant Physiol 1997, 115:1385-1395.
41. Fuerst RA, Soni R, Murray JAH, Lindsey K: Modulation of cyclin transcript levels in cultured cells of Arabidopsis thaliana.Plant Physiol1996, 112:1023-1033.
42. Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA: Effects of an Rb mutation in the mouse.Nature1992, 359:295-300. 43. Yamasaki L, Jacks T, Bronson R, Goillot E, Harlow E, Dyson NJ:
Tumor induction and tissue atrophy in mice lacking E2F-1.Cell
1996, 85:537-548.
44. Du W, Vidal M, Xie J-E, Dyson N: RBF, a novel RB-related gene that regulates E2F activity and interacts with cyclin E in Drosophila.
Genes Dev1996, 10:1206-1218.
45. Brook A, Xie JE, Du W, Dyson N: Requirements for dE2F function in proliferating cells and in post-mitotic differentiating cells.EMBO J
1996, 15:3676-3683.