Phosphorus status affects postharvest respiration, membrane
permeability and lipid chemistry of European seedless
cucumber fruit (
Cucumis sati
6
us
L.)
L. Knowles, M. Rae Trimble
1, N. Richard Knowles *
Department of Horticulture and Landscape Architecture,Washington State Uni6ersity,Pullman,WA99164,USA Received 28 January 2000; accepted 30 June 2000
Abstract
Fruit of European seedless cucumber were grown in a greenhouse under low and high phosphorus (P) fertilization regimes. Tissue P concentration of fruit (number one grade) from low-P plants was, on average, 45% of that of fruit from high-P plants. Fruit P status affected membrane lipid chemistry and fruit respiration after harvest. Mesocarp tissue of low-P fruit had a lower concentration of phospholipids, lower level of unsaturation in various pools of fatty acids, and a greater rate of electrolyte leakage than that of high-P fruit. On average, respiration of low-P fruit was 21% higher than that of high-P fruit over a 16-day postharvest interval at 23°C. Moreover, low-P fruit experienced a climacteric in respiration that began about 40 h after harvest, reached a maximum at 72 h, and declined to preclimacteric levels by 90 h. The difference in respiration rate between low- and high-P fruit was as high as 57% during the climacteric. The respiratory climacteric was unique to the low-P fruit and was not associated with an increase in fruit ethylene concentration or ripening. Phosphorus nutrition can thus alter the postharvest physiology of cucumber fruit by affecting membrane lipid chemistry, membrane integrity and respiratory metabolism. © 2001 Elsevier Science B.V. All rights reserved.
Keywords:Cucumis sati6us; Phosphorus nutrition; Postharvest respiration; Membrane lipids; Electrolyte leakage
www.elsevier.com/locate/postharvbio
1. Introduction
The effects of phosphorus (P) nutrition on crop growth rate and fruit P content of greenhouse-grown, European seedless cucumber (Cucumis sa
-ti6us L.) are well established (Trimble and
Knowles, 1995a,b). However, studies on the ef-fects of P nutrition on the postharvest physiology of cucumber and other fruits are rare, with most of the research focussed on pome fruits. Phospho-rus nutrition has been shown to affect fruit firm-ness and the occurrence of low-temperature breakdown in apples (Sharples, 1980). Foliar ap-plications of P during the growing season in-creased tissue P concentration and reduced the * Corresponding author. Tel.: +1-509-3353451; fax: +
1-509-3356890.
E-mail address:[email protected] (N.R. Knowles).
1Present address: ICMS, Inc., P.O. Box 67, Highway 1A
East, Portage la Prairie, Manitoba, Canada R1N 3B2
incidence of storage disorders in apple (Yogarant-nam and Sharples, 1982). Lin and Ehret (1991) showed that the shelf-life of greenhouse cucumber (cv. Mustang) could be improved by increasing the concentrations of N, P, K, Ca, S and Mg in the nutrient solution, but it was not possible to isolate a specific effect due to P from their data.
The effect of P on the postharvest physiology of fruit may be attributed to its role as a component of phospholipids, a major constituent of cell membranes. Alterations in membrane composi-tion occur naturally during fruit ripening and senescence and are also a mechanism by which cells adapt to changing environmental conditions. For example, phospholipid content increased in apple during storage at 0°C, purportedly as an adaptation to maintain membrane function at this low temperature (Lurie et al., 1987). Phosphorus deficient plant organs may be unable to make this adaptation. It is commonly observed in fruit tis-sue that membrane function declines with ripen-ing and senescence, and this occurs concomitant with a loss of phospholipids and/or changes in their constituent fatty acids. The most frequently reported change in fatty acid chemistry is an increase in saturation at the expense of unsatura-tion (Lurie and Ben-Arie, 1983; Lester and Stein, 1993; Palma et al., 1995; Rogiers et al., 1998a).
A few studies have indicated that P content can potentially affect the postharvest respiration rate of fruit, which is often inversely related to storage and shelf-life (Kader, 1987). Letham (1969) found that higher levels of P fertilization resulted in higher P concentration in apple and that fruit P content was negatively correlated with respiration rate. Another study of respiration in apple found no relation between tissue P concentration and respiration rate (Davenport and Peryea, 1989). In this latter study, differences in tissue P were due to natural variation among fruits and the applica-tion of CaCl2 sprays, and were not as great as those induced by varied P nutrition.
Despite inconsistencies in the literature, it is evident that plant P nutrition affects fruit P status and this in turn may have a significant influence on postharvest physiology and thus storability and shelf-life of fruit. The experiments described herein were designed to induce differences in P
concentration of European seedless cucumber fruit so that the effects of fruit P-status on respi-ration rate, membrane lipid chemistry and mem-brane function could be assessed after harvest.
2. Materials and methods
2.1. Plant culture
European seedless cucumber (Cucumis sati6us
L., cvs. Carmen and Corona) fruit were grown from seed in a mixture of coarse sand, fine sand and loam (2:2:1, v/v/v) in a research greenhouse at the University of Alberta, Edmonton under natural light supplemented with high pressure sodium lamps (Sylvania 400 W; 450 mmol m−2 s−1, 1 m above bench; 16 h photoperiod), as described previously (Trimble and Knowles, 1995b). To produce fruit containing relatively low and high P levels, plants were fertilized with 250 mL of 120 or 480 mg l−1P (KH
2PO4) three times per week, starting 26 days after planting. Phos-phorus treatments thus consisted of 90 and 360 mg of elemental P per plant per week. Potassium levels were held constant by adding KCl to the 90 mg P per week treatments. All other nutrients were applied uniformly (pH 6) to each pot by an automated fertigation system (Harrow Fertigation Manager, Labbate Control Systems, Leamington, ON) according to nutrient levels recommended for cucumber fruit production (Adamson and Maas 1981; Mirza, 1990). Fertigation began 26 days after planting. Each plant initially received 1 l d−1 of nutrient solution containing 50 mg l−1 NO3– N, 5 mg l−1 NH4– N, 75 mg l−1K, 40 mg l−1 Ca, 40 mg l−1 Mg, and 400 mg l−1(max) S. The volume of fertilizer and concentration of nutrients were gradually increased with plant growth, so that by 64 days after planting each plant received 5.4 l per day of solution containing 200 mg l−1NO
3– N, 20 mg l −1 NH
4– N, 275 mg l−1K, 120 mg l−1Ca, 40 mg l−1Mg and 400 mg l−1(max) S, as previously described (Trimble and Knowles, 1995b). Micronutrients in the fertiga-tion solufertiga-tion were maintained at constant levels (0.840 mg l−1Fe, 0.547 mg l−1Mn, 0.164 mg l−1
0.102 mg l−1Cu, and 0.072 mg l−1Mo) through-out the study.
The plants were trained to maintain a single leader, according to guidelines for a sequence cropping production system (Adamson and Maas, 1981; Mirza, 1990). Fruit from plants grown un-der the two phosphorus regimes were harvested after reaching a minimum of 30 cm length and 42 mm diameter (Canada No. 1 grade), which corre-sponded to a fresh weight of between 300 and 400 g. These fruit were then used to characterize the effects of P nutrition on fruit P content, mem-brane lipid chemistry, memmem-brane permeability, and whole-fruit respiration during storage at 23°C.
2.2. Respiration
Respiration of low- and high-P fruit was ini-tially compared over a 16-day period at 23°C starting 1 day after harvest. Fruit (cv. Carmen), harvested 77 days after planting, were blocked for fresh weight and enclosed in 5.7 L plastic cham-bers (one fruit per chamber, six replicates) con-taining inlet and outlet air-flow ports. The chambers were attached to a flow-board which distributed a constant flow of humidified air (120 mL min−1) to each chamber. Fruit respiration was determined daily by quantifying CO2in 1 mL samples of chamber effluent. The CO2 was ana-lyzed with a Hewlett Packard 5890A gas chro-matograph (GC) equipped with a thermal conductivity detector and a 2.4 m stainless steel column (3.2 mm o.d.) packed with HaySep T. The carrier gas (He) flow rate was 30 ml min−1 and the column was 100°C. Injector and detector port temperatures were 140°C.
A follow-up study was conducted to more clearly characterize the differences in fruit respira-tion between low- and high-P fruits that were identified within 4 days of harvest in the longer-term study (above). Canada No. 1 grade fruit for this latter study were harvested from 82-day-old plants (cv. Corona), blocked for fresh weight and immediately placed into chambers (one fruit per chamber, 10 replicates) in an open airflow system at 23°C as above. The outflows from each cham-ber were connected via a manifold to an infra-red
gas analyzer (LI-COR model LI 6262, Lincoln, Nebraska) that was programmed to read CO2 concentration from each chamber at 4 h intervals over the 4-day postharvest period. Respiration rates of fruit from low- and high-P plants were calculated as the rate of CO2 produced (m mol min−1kg−1) on a fresh weight basis (9SE) and plotted against time after harvest.
2.3. Internal ethylene
The concentration of ethylene in Canada No. 1 grade fruit from high- and low-P-grown plants (cv. Corona) was quantified at 70 h after harvest. The fruit had been stored at 23°C (95% RH) prior to ethylene extraction and analysis. Ethylene was extracted from the fruit under vacuum, as de-scribed in Beyer and Morgan (1970). Gas chro-matographic separation and quantification of ethylene was according to Rogiers et al. (1998b).
2.4. Electrolyte leakage
2.5. Tissue phosphorus
Whole fruit from the respiration studies and mesocarp tissue from the electrolyte leakage stud-ies were lyophilized in preparation for P analysis. The dried tissue was ground with a Wiley mill through a 40 mesh screen and 100 mg of tissue was ashed overnight in a muffle furnace (550°C). The ash was digested in 1 mL HCl for 20 min, diluted with 9 mL 0.72 N H2SO4 and centrifuged (1640× g, 30 min) to settle any undigested mat-ter. Total P of a 200 mL aliquot was determined using the methods of Serrano et al. (1976).
2.6. Lipid extraction and analysis
Freeze-dried, ground tissue of fruit from the 16-day respiration study was extracted for 30 s in 10 vol boiling isopropanol, and 60 s further after the addition of 20 vol of CHCl3. The extract was filtered and re-extracted in 20 vol CHCl3:MeOH (2:1 v/v). The combined filtrates were washed with 0.2 vol of 0.15% KCl and evaporated to dryness. An aliquot of lipid extract was concentrated and spiked with phosphatidylcholine dipentade-canoyl and phosphatidylethanolamine diheptade-canoyl as internal standards. The samples, along with an external standard of nonadecanoic acid, were applied to thin layer chromatography plates of 0.5 mm silica gel G and developed in hex-ane:Et2O:HOAc (70:30:1, v/v/v). The separated compounds were visualized with 0.05% 2%,7% -dichlorofluorescein in EtOH and the band corre-sponding to nonadecanoic acid was collected and reserved for analysis as the unesterified fatty acid fraction. The phospholipids remaining at the origin on the plates were eluted from the silica gel in CHCl3:MeOH:H2O (10:5:1, v/v/v), reapplied to another plate (0.5 mm silica gel H) and developed in CHCl3:MeOH:HOAc:H2O (50:25:7:3, v/v/v/v). Narrow vertical lanes of the plates were sprayed with molybdenum blue reagent for the identifica-tion of phospholipids (Dittmer and Lester, 1964). Ninhydrin and Dragendorf’s reagents (Zweig and Sherma, 1972), in combination with authentic ex-ternal standards, were used to verify the locations of PE and PC, respectively.
Unesterified fatty acids, PC, PE and an aliquot of crude extract were transesterified for gas chro-matography by refluxing with 2% (v/v) H2SO4 in MeOH for 2 h. Fatty acid methyl esters were recovered in hexane according to Christie (1989). These were analyzed on a Hewlett Packard 5890A gas chromatograph using a flame ionization detec-tor and a 1.8 m×3 mm stainless steel column of 10% SP2330 on 100/120 mesh Chromosorb WAW. The operating temperature was 185°C with a N2 flow rate of 30 mL min−
1
. The instru-ment was calibrated with authentic standards of methylpalmitate (16:0), methylstearate (18:0), methyloleate (18:1), methylinoleate (18:2) and methylinolenate (18:3). Double bond indices (DBI) were then calculated for fatty acids in the total, unesterified, PC and PE fractions according to the following equation (Liljenberg and Kates, 1985):
[%18:1+2(%18:2)+3(%18:3)] [%16:0+%18:0] ,
where% indicates mol percent.
To quantify lipid P, an aliquot of lipid extract was subjected to thin layer chromatography as described above for the separation of lipid classes, but no internal standards were included. PC and PE were located as before and transferred to a 30 mL digestion flask. A sample of crude extract was applied to silica gel and removed without develop-ment for analysis of total lipid P. The fractions were digested in 0.65 mL perchloric acid for 20 min. Phosphorus was analyzed according to Rouser et al. (1969).
3. Results and discussion
3.1. Tissue P le6els, total lipid and phospholipids
Table 1
Phosphorus (P) levels in fruit tissue, total lipid, phosphatidylcholine (PC), and phosphatidylethanolamine (PE) fractions of European seedless cucumber fruit (cv. Carmen), as affected by P nutrition. Canada No. 1 grade fruit (see Section 2) were harvested from both treatments and P-levels were quantified (dry weight basis) after profiling whole-fruit respiration rates over a 16-day postharvest interval at 23°C (see Fig. 2)a
Phosphorus content (mmol kg−1)
Fruit Tissue
P Nutrition (mg per plant per week) Total lipid PC PE
11.5
90 63 1.90 1.29
360 198d, *** 13.7c, ** 2.40b, * 2.01c, **
aData are average of 6 fruit. Differences betweenPnutrition levels were significant at: b, *P50.07;
c, **P50.05; d, ***P50.01 levels.
week) is consistent with that recommended for greenhouse cucumber production in soilless cul-ture systems in Alberta (Mirza, 1990). Previous studies showed insignificant increases in plant growth and yield with P applications above this rate (Trimble, 1993; Trimble and Knowles, 1995b). Typical P deficiency symptoms, consisting of delayed fruit set and localized interveinal le-sions of desiccated leaf tissue, were noted on the low-P plants late in development; however, no visible symptoms of P deficiency were evident on the fruit. In fact, while the low-P plants had produced 26% fewer marketable fruit than the high-P plants by 92 days after planting, the Canada No. 1 grade fruit from both treatments were outwardly indistinguishable at harvest. This is relevant from a practical standpoint in that an effect of P-nutrition on fruit physiology after har-vest would likely impact relative storability and quality and, in the absence of visible symptoms, fruit could not be sorted accordingly.
The concentration of P in the lipid fraction of fruit at the end of the postharvest period de-pended on P fertilization level (Table 1). Total lipid P in fruit from low-P plants was only 16% less than that in fruit from high-P plants, which contained three times the total P of low-P fruit on a dry weight basis. Lipid P in the low-P grown fruit accounted for 18.3% of the total tissue P, compared with only 6.9% in the high-P grown fruit. These results indicate that, when fruit are produced under low levels of P nutrition, lipids
receive priority for P over other P pools, and thus P content of the lipid fraction is not reduced to the same extent as total P levels would indicate.
Concentrations of the two membrane phospho-lipids, PC and PE, were quantified via P analysis after separation from the crude extract, and were dependent on the level of P nutrition. These two phospholipids accounted for 28% and 32% of total lipid P in fruit from low- and high-P plants, respectively. Fruit from high-P plants contained
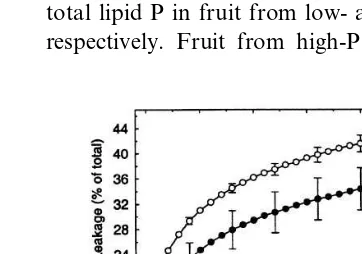
Fig. 1. Time course of electrolyte leakage from mesocarp tissue taken from Canada No. 1 grade European seedless cucumber fruit (cv. Corona) grown under low and high levels of P nutrition. Following harvest, fruit were held at 23°C for 3 days prior to leakage measurements. Leakage is expressed as a percentage of total electrolytes within the tissue.F-value for the interaction of P nutrition and time was significant at the 0.01 level. Data are the average of 4 fruit 9standard error which, for clarity, is only shown every 4 min. Inset shows total P concentrations in fruit (dry weight basis). ***Difference
Fig. 2. Postharvest changes in respiration rates (CO2
evolu-tion) of Canada No. 1 grade European seedless cucumber fruit (cv. Carmen) grown under low and high levels of P nutrition. Fruit respiration was monitored in an open air-flow system at 23°C (95% RH).F-value for the interaction of P nutrition and time was significant at the 0.01 level. Data are the average of 6 fruit9standard error. Inset shows total P concentrations in fruit (dry weight basis). ***Difference between P treatments
was significant at the 0.01 level.
3.2. Fatty acid profiles
In light of the effects of P nutrition on the concentrations of total lipid and phospholipids in fruit, it was of interest to determine whether fatty acid saturation indices were also affected. Five major fatty acids in different lipid pools in low-and high-P fruit were quantified low-and the DBI reported (Table 2). The DBI of total fatty acid and unesterified fatty acid fractions in low-P fruit were 21 and 30% lower than those in high-P fruit, respectively. As fatty acids are integral compo-nents of phospholipids, changes in their chemistry can alter the physical attributes of membranes. In particular, high levels of acyl-group unsaturation are important in conferring increased membrane fluidity and function (Yoshida and Uemura, 1984), especially during chilling (Lynch and Steponkus, 1987). In addition, unesterified fatty acids are substrates for peroxidation, a process that can hasten postharvest deterioration. It is typical for ripening and senescing fruit tissues to undergo qualitative changes in the acyl composi-tion of membrane lipids. Lester and Stein (1993) determined that the ratio of saturated:unsaturated fatty acids in muskmelon plasma membrane in-creased with maturation of the fruit. A similar change in fatty acids occurred in the microsomal membrane fraction of apple during ripening (Lurie and Ben-Arie, 1983). While DBI of the PC fraction in our cucumber fruit was not affected by P nutrition, DBI of the PE fraction of low-P fruit was 18% lower than that of high-P fruit (Table 2). Phosphorus nutrient stress thus affected increased saturation of fatty acids in a major phospholipid pool, reflecting a change in the molecular species of fatty acids in membranes of the harvested cucumber fruit.
Though speculative, it is possible that the higher level of unsaturation in the unesterified fatty acid pool (Table 2), relative to the phospho-lipids, is a result of the positional specificity of a particular phospholipase. The lysophospholipid thus formed would not be included in the pool of native PC or PE (Table 1) resolved by thin layer chromatography. This hypothesis could be tested by a more detailed examination of the phospho-lipid and lysophosphophospho-lipid pools of low- and high-P fruit.
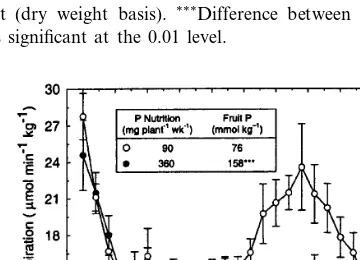
Fig. 3. Postharvest changes in respiration rates (CO2
evolu-tion) of Canada No. 1 grade European seedless cucumber fruit (cv. Corona) grown under low and high levels of P nutrition. Fruit respiration was monitored in an open air-flow system at 23°C (95% RH).F-value for the interaction of P nutrition and time was significant at the 0.01 level. Data are the average of 10 fruit 9standard error. Inset shows total P concentrations in fruit (dry weight basis).***Difference between P treatments
was significant at the 0.01 level.
3.3. Electrolyte leakage
Since phospholipids and their constituent fatty acids are important components of membranes, we tested the possibility that the low-P-induced changes in concentration and saturation of phos-pholipids affected membrane integrity in the har-vested fruit. Changes in membrane integrity that effect increased permeability can readily be demonstrated by measuring leakage of electrolytes from tissue (Ross, 1974; Parrish and Leopold, 1978; Barber and Thompson, 1980). Tissue from low-P fruit leaked electrolytes at a faster rate initially, and lost a greater percentage of total electrolytes over a 30 min interval, than that from high-P fruit (Fig. 1). After 30 min, tissue from low-P fruit had leaked 19% more total electrolytes than tissue from high-P fruit. Since the concentra-tion of electrolytes (i.e. maximum conductivity) on a fresh weight basis was equal in tissue from the low- and high-P fruit, the greater efflux of electrolytes from low-P tissue is indicative of re-duced membrane integrity. Moreover, the higher membrane permeability was in close agreement with the 18% lower DBI in the PE fraction of low-P fruit (Table 2). Negative correlations be-tween permeability and DBI of membrane lipids have been shown during aging of vegetative tis-sues and with changes in fruit development. For example, aging of potato tubers during cold stor-age was accompanied by a progressive increase in the degree of saturation of membrane lipids and this correlated with a significant decline in mem-brane integrity (Knowles and Knowles, 1989).
Leakage of electrolytes from muskmelon fruit tis-sue increased with ripening, correlating with an increase in the degree of saturation of plasma membrane phospholipid fatty acids (Lester and Stein, 1993). Our study is the first to demonstrate that P nutrition can affect membrane function (permeability) of harvested cucumber fruit through an effect on membrane phospholipid con-centration and saturation level. If the basal metabolic rates of fruit are altered by P content, then P nutrition could potentially affect the rate of deterioration of fruit after harvest.
3.4. Fruit respiration
Respiration rates of low- and high-P fruit (cv. Carmen) were compared as a general indication of overall metabolic rate. Fruit harvested at 77 days after planting from low-P plants had a 22% higher (PB0.01) respiration rate (6.0mmol min−1kg−1) than fruit from high-P plants (4.9 mmol min−1 kg−1) when averaged over the 16-day postharvest interval (Fig. 2). Respiration rates declined over the 16-day period, such that rates at day 1 were 153% higher than those at day 16, regardless of the level of P nutrition (PB0.01). However, low-P plants produced fruit that experienced a climac-teric in respiration within 5 days of harvest. At its maximum (72 h postharvest), respiration of low-P fruit was 35% greater than that of high-P fruit. The increase in respiration was unique to the low-P fruit.
To further document and characterize the effect of P nutrition on fruit respiration rates, a different
Table 2
Double bond indices (DBI) of fatty acids in various lipid fractions of Canada No. 1 grade European seedless cucumber fruit (cv. Carmen), as affected by P nutritiona
Lipid fraction (DBI of fatty acids) P Nutrition (mg per plant per week)
PC PE
Total Unesterified
2.60
90 5.56 5.07 2.74
3.35b
2.34 nsd
7.21c
360 7.03c
aData are from the same fruit described in Table 1.
bDifferences betweenPnutrition levels were significant atP50.07.
cultivar (Corona) was produced under similar cul-tural conditions and respiration was monitored at 4 h intervals for 100 h after harvest (Fig. 3). Fruit with the lower P concentration from low-P plants again showed a respiratory climacteric that lasted from 50 – 90 h after harvest. Low-P fruit had a 57% greater respiration rate than high-P fruit at the climacteric maximum (about 70 h after har-vest). The fruit grown with high P fertilization had higher tissue P (Fig. 3, inset) and did not show a rise in respiration during this period. Phosphorus nutrition thus affected respiratory metabolism of cucumber fruit after harvest. Since respiration and shelf-life of fruits are often in-versely related (Salunkhe and Desai, 1984; Kader, 1987), further research on the effects of P nutri-tion on postharvest deterioranutri-tion of cucumber fruit is warranted.
In addition to P-induced respiratory differ-ences, respiration rates of cucumber at harvest can depend on fruit and/or plant maturity. Fruit (cv. Carmen) harvested at 77 days after planting respired at a greater rate than those harvested at 54 days after planting (Trimble, 1993). When fruit maturity was defined as days after anthesis, postharvest respiration rates of cv. Deliva fruit increased with maturity, while shelf-life declined (Kanellis et al., 1986). Similar to our results, ‘Deliva’ fruit harvested 25 or 30 days after anthe-sis underwent a peak in respiration a few days after harvest, with the most pronounced peak in the more mature fruit (Kanellis et al., 1986). Although shelf-life was not determined in our study, it is reasonable to speculate that fruit with higher respiration rates due to low tissue P would also exhibit a more rapid loss in postharvest quality.
3.5. Internal ethylene
Like field cucumbers, European seedless green-house cucumbers are harvested at a physiologi-cally immature stage and thus lack the ability to complete many of the changes associated with full ripening. As such, cucumbers of commercial ma-turity behave as nonclimacteric fruit (Kanellis et al., 1986). If left to ripen on the plant, or if harvested after attaining physiological maturity,
cucumber fruit may indeed exhibit climacteric be-havior (Abeles et al., 1992). Nevertheless, to confirm that the low-P-related respiratory climac-teric (Figs. 2 and 3) was not induced by ethylene and associated with ripening, ethylene concentra-tions were compared at 72 h after harvest (coin-ciding with the respiratory peak in the low-P fruit). The low- and high-P fruit contained 2.7 and 2.5 nmol L−1 ethylene, respectively, and the difference was not significant. Saltveit and McFeeters (1980) noted that a burst of ethylene could occur from processing cucumber anywhere from 5 – 25 days after harvest, but this increase in ethylene production was not associated with a concomitant increase in respiration. The increase in respiration rate of our low-P fruit was not associated with ripening and was likely a P stress response. Phosphorus stress during growth either indirectly, through influencing the rate at which fruits mature, or directly, through an effect on membrane composition and function, affects the postharvest respiration of cucumber fruit.
In apple, respiration of harvested fruit did not correlate with fruit P over a wide range of tissue P concentrations (164 – 618 mg kg−1
, dry weight basis) (Davenport and Peryea, 1989). However, in a long-term study of the effects of N, P and K fertilization levels on postharvest respiration of apple, Letham (1969) found that fruit with the highest P concentration had the lowest respiration rate while those with the lowest P concentration had the highest respiration rate. Low-P apple fruit initiated the climacteric sooner than high-P fruit, indicating that lower levels of tissue P were suffi-cient to alter fruit developmental physiology. Fruit P concentrations in Letham’s study were within the range of those reported in Davenport and Peryea’s work, but were induced as a result of manipulation of P fertilization levels during production.
4. Summary and conclusions
mem-brane permeability and thus greater leakage of electrolytes than high-P fruit. This increased ion leakage was associated with qualitative changes in the fatty acyl composition of membranes that resulted in increased saturation, and decreased concentrations of the two most abundant phos-pholipids, PC and PE. The respiratory burst within days after harvest is indicative of stress caused by P deficiency. It is worth noting that over the postharvest intervals used in these stud-ies, no differences were apparent in the outward appearance of the Canada No. 1 grade fruit from low- and high-P plants. Based on studies with other plant commodities however, it is reasonable to speculate that low-P cucumber fruit will lose quality and deteriorate faster after harvest than high-P fruit, due to their higher metabolic rates and increased membrane leakiness, although this remains to be established.
References
Abeles, F.B., Morgan, P.W., Saltveit, M.E., 1992. Fruit ripen-ing, abscission, and postharvest disorders. In: Ethylene in Plant Biology. Academic Press, Inc, San Diego, CA, pp. 182 – 220.
Adamson, R.M., Maas, E.F., 1981. Soilless culture of seedless greenhouse cucumbers and sequence cropping. Agriculture Canada Publication 1725E.
Barber, R.F., Thompson, J.E., 1980. Senescent-dependent in-crease in the permeability of liposomes prepared from bean cotyledon membranes. J. Exp. Bot. 31, 1305 – 1313. Beyer, E.M., Morgan, P.W., 1970. A method for determining
the concentration of ethylene in the gas phase of vegetative plant tissue. Plant Physiol. 46, 352 – 354.
Christie, W.W., 1989. Gas Chromatography and Lipids. The Oily Press, Ayr, p. 307.
Davenport, J.R., Peryea, F.J., 1989. Whole fruit mineral ele-ment content and respiration rates of harvested ‘Delicious’ apples. J. Plant Nutr. 12, 701 – 713.
Dittmer, J.C., Lester, R.L., 1964. A simple, specific spray for the detection of phospholipids on thin-layer chro-matograms. J. Lipid Res. 5, 126 – 127.
Kader, A.A., 1987. Respiration and gas exchange of vegeta-bles. In: Weichmann, J. (Ed.), Postharvest Physiology of Vegetables. Marcel Dekker Inc, New York, pp. 25 – 44. Kanellis, A.K., Morris, L.L., Saltveit, M.E., Jr., 1986. Effect
of stage of development on postharvest behavior of cucum-ber fruit. HortScience 21, 1165 – 1167.
Knowles, L.O., Knowles, N.R., 1989. Correlations between electrolyte leakage and degree of saturation of polar lipids from aged potato (Solanum tuberosum L.) tuber tissue. Ann. Bot. 63, 331 – 338.
Lester, G., Stein, E., 1993. Plasma membrane physiochemical changes during maturation and postharvest storage of muskmelon fruit. J. Am. Soc. Hort. Sci. 118, 223 – 227. Letham, D.S., 1969. Influence of fertilizer treatment on apple
fruit composition and physiology. II. Influence on respira-tion rate and contents of nitrogen, phosphorus and titrat-able acidity. Aust. J. Agric. Res. 20, 1073 – 1085. Liljenberg, C., Kates, M., 1985. Changes in lipid composition
of oat root membranes as a function of water deficit stress. Can. J. Biochem. and Cell Biol. 63, 77 – 84.
Lin, W.C., Ehret, D.L., 1991. Nutrient concentration and fruit thinning affect shelf life of long English cucumber. HortScience 26, 1299 – 1300.
Lurie, S., Ben-Arie, R., 1983. Microsomal membrane changes during the ripening of apple fruit. Plant Physiol. 73, 636 – 638.
Lurie, S., Sonego, L, Ben-Arie, R., 1987. Permeability, micro-viscosity and chemical changes in the plasma membrane during storage of apple fruit. Sci. Hort. 32, 73 – 83. Lynch, D.V., Steponkus, P.L., 1987. Plasma membrane lipid
alterations associated with cold acclimation of winter rye seedlings (Secale cerealeL. cv Puma). Plant Physiol. 83, 761 – 767.
Mirza, M., 1990. Greenhouse Vegetable Production Guide, 1990-1991 for Commercial Growers. Alberta Agriculture Agdex 250/15-1.
Palma, T., Maragoni, A.G., Stanley, D.W., 1995. Environ-mental stresses affect tomato microsomal membrane func-tion differently than natural ripening and senescence. Postharvest Biol. and Tech. 6, 257 – 273.
Parrish, D.J., Leopold, C.A., 1978. On the mechanism of aging in soybean seeds. Plant Physiol. 61, 365 – 368. Rogiers, S.Y., Mohan Kumar, G.N., Knowles, N.R., 1998a.
Maturation and ripening of fruit of Amelanchier alnifolia Nutt. are accompanied by increasing oxidative stress. Ann. Bot. 81, 203 – 211.
Rogiers, S., Kumar, G.N.M., Knowles, N.R., 1998b. Regula-tion of ethylene producRegula-tion and ripening by saskatoon (Amelanchier alnifolniaNutt.) fruit. Can. J. Bot. 76, 1743 – 1754.
Ross, C.W., 1974. Membranes, permeability, and nutrient relations. In: Yessne, S. (Ed.), Plant Physiology Labora-tory manual. Wadsworth Publishing Co., Inc, Belmont, CA, pp. 52 – 67.
Rouser, G., Fleischer, S., Yamamoto, A., 1969. Two-dimen-sional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 5, 595 – 596.
Saltveit, M.E., Jr., McFeeters, R.F., 1980. Polygalacturonase activity and ethylene synthesis during cucumber fruit devel-opment and maturation. Plant Physiol. 66, 1019 – 1023. Salunkhe, D.K., Desai, B.B., 1984. Postharvest Biotechnology
of Fruits, vol. 1. CRC Press, Boca Raton, Florida, pp. 23 – 42.
Sharples, R.O., 1980. The influence of orchard nutrition on the storage quality of apples and pears grown in the United Kingdom. In: Atkinson, D., Jackson, J.E., Sharples, R.O., Waller, W.M. (Eds.), Mineral Nutrition of Fruit Trees. Butterworths, Sevenoaks, pp. 17 – 28.
Suttle, J.C., Kende, H., 1980. Ethylene action and loss of membrane integrity during petal senescence in Trades -cantia. Plant Physiol 65, 1067 – 1072.
Trimble, M.R., Knowles, N.R., 1995a. Influence of vesicular-arbuscular mycorrhizal fungi and phosphorus on growth, carbohydrate partitioning and mineral nutrition of green-house cucumber (Cucumis sati6usL.) plants during estab-lishment. Can. J. Plant Sci. 75, 239 – 250.
Trimble, M.R., Knowles, N.R., 1995b. Influence of phospho-rus nutrition and vesicular-arbuscular mycorrhizal fungi on growth and yield of greenhouse cucumber (Cucumis sati6us L.). Can. J. Plant Sci. 75, 251 – 259.
Trimble, M.R. 1993. Growth, development and postharvest physiology of greenhouse cucumber (Cucumis sati6usL.) as affected by phosphorus nutrition and vesicular-arbuscular mycorrhizae. MSc Thesis, Dept. of Plant Science, Univer-sity of Alberta, Edmonton, Alberta, Canada. 145 pages. Yogarantnam, N., Sharples, R.O., 1982. Supplementing the
nutrition of Bramley’s Seedling apple with phosphorus sprays: Effects on fruit composition and storage quality. J. Hort. Sci. 57, 53 – 59.
Yoshida, S., Uemura, M., 1984. Protein and lipid composi-tions of isolated plasma membranes from orchard grass (Dactylis glomerataL.) and changes during cold acclima-tion. Plant Physiol. 75, 31 – 37.
Zweig, G., Sherma, J., 1972. Detection reagents for paper-and/
or thin-layer chromatography. In: Handbook of Chro-matography, vol. II. CRC Press, Cleveland, Ohio, pp. 103 – 189.