Inhibition of cytoplasmic DNA synthesis may be an initial signal
for the induction of sexual reproduction in
Closterium ehrenbergii
Machiko Imaizumi
a,*, Jin Hamada
baDepartment of Biology,Shiga Uni6ersity of Medical Science,Ohtsu520-2192,Japan
bDepartment of Community Medicine,Faculty of Medicine,Toyama Medical and Pharmaceutical Uni6ersity,Toyama930-0152,Japan
Received 9 November 1999; received in revised form 8 August 2000; accepted 8 August 2000
Abstract
We investigated the effect that inhibitors of cytoplasmic DNA (cytDNA) and/or nuclear DNA (nDNA) synthesis have on the frequency of zygote formation inClosterium ehrenbergiiand whether these inhibitors correlate with arrest of pyrenoid production and enlargement of pyrenoidal size accompanying pregametogenesis in the alga. CytDNA inhibitors, ethidium bromide (EB), acridine orange and novobiocin (NB), enhanced zygote formation in the presence of nitrate, and these chemicals correlated with both a decrease in pyrenoid production and an increase in pyrenoidal size. By contrast, a nDNA inhibitor, aphidicolin (APC), enhanced zygote formation in the presence and absence of nitrate, and correlated with only an increase in pyrenoidal size. Meanwhile, inhibitors of both nDNA and cytDNA synthesis, mitomycin C (MC), hydroxyurea (HU) and arabinosyl cytosine (Ara-C), enhanced zygote formation in the presence and/or absence of nitrate. In addition, MC and HU correlated with only an increase in pyrenoidal size and Ara-C only a decrease in pyrenoid production. This result seemed to indicate that MC and HU functioned as selective inhibitors of nDNA synthesis, and Ara-C as a selective inhibitor of cytDNA synthesis. It is suggested that differentiation into pregametes correlates with the inhibition of both nDNA and cytDNA synthesis, levels of which are probably low, the inhibition of cytDNA synthesis being the initial signal for pregametogenesis. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Closterium ehrenbergii; DNA synthesis inhibitor; Pyrenoid; Sexual reproduction; Zygote formation
www.elsevier.com/locate/plantsci
1. Introduction
Cells of Closterium ehrenbergii form zygotes ef-fectively when two heterothallic strains are mixed under nitrogen source (KNO3, Ca(NO3)2 · 4H2
O)-depleted conditions [1,2]. The presence of a nitro-gen source lowers the frequency of zygote formation. This suggests that some pathway in nitrogen metabolism regulates the induction of zygote formation (sexual reproduction) in this alga. However, the regulatory mechanism remains largely unknown. In a preliminary study, we found
that the frequency of zygote formation in this alga is greatly increased when ethidium bromide (EB), which is an inhibitor of cytoplasmic DNA (cytDNA) synthesis, is added to the alga under conditions in which nitrate is present. This result indicates that the inhibition of cytDNA synthesis is a signal for the induction of zygote formation in the alga. Hamada [3] suggested, on the other hand, that nuclear DNA (nDNA) duplication did not occur during gametogenesis in this alga and that inhibition of nDNA synthesis was involved in the induction of zygote formation. It was reported that nuclear and organellar DNAs replicated at different times in the cell cycles [4 – 7] and their replication cycles were tightly coupled with each other [8]. All this evidence suggests that the
inhibi-* Corresponding author. Tel.: +81-77-5482123; fax: + 81-77-5482048.
E-mail address:[email protected] (M. Imaizumi).
tion of nDNA and/or cytDNA synthesis is closely involved in the induction of zygote formation.
We noticed that the pyrenoids in C. ehrenbergii were greatly enlarged during sexual reproduction compared with those during asexual reproduction. This would suggest that regulation of pyrenoid function occurs during the conversion from asex-ual to sexasex-ual reproduction in this alga. In the present study, we investigated the ratio of pyrenoid/cell number, and the pyrenoidal sizes of the alga throughout the asexual and sexual stages of reproduction, and found that the modulation of pyrenoid production and function accompanied the formation of pregametes (pregametogenesis). The present study investigated whether the inhi-bition of nDNA and/or cytDNA synthesis is in-volved in the induction of zygote formation in C. ehrenbergiiand if so, which form of inhibition is an initial signal for the induction. To this end, the effect of selective inhibitors of cytDNA or nDNA synthesis on the frequency of zygote formation in the alga, as well as on the modulation of pyrenoid production and pyrenoidal size during pregameto-genesis, was examined.
2. Materials and methods
2.1. Strains and culture conditions
Previously described [3] heterothallic strains of C. ehrenbergii, M-16-4a (mating-type plus: m+)
and M-16-4b (mating-type minus: m−) were used
in the present study. They were cultured in C medium [9] at 23°C, and illuminated with fluores-cent lamps of 2.25×10−1 W/m2 (1500 lx) with a
16-h per day photoperiod.
2.2. Chemicals
For staining pyrenoids, carmine (Merck, C.I. No. 75470) was purchased from Nacalai Tesque (Kyoto, Japan). As DNA synthesis inhibitors, the following chemicals were used. EB and Mitomycin C (MC) were purchased from Aldrich Chemical Co. Inc. (Milwaukee, USA) and Kyowa Hakko Co. (Tokyo, Japan), respectively. Acridine orange (AO), novobiocin (NB, sodium salt) and ara-binosyl cytosine (Ara-C) were from Nacalai Tesque. Aphidicolin (APC) and hydroxyurea (HU) were from Sigma Chemical Co. (St. Louis, USA).
2.3. Experimental procedures
To investigate the frequency of zygote forma-tion, vegetative cells of each strain were grown for 17 days in C medium and collected, and approxi-mately 104cells of each strain were mixed together.
The mixed cells were centrifuged (at 72×g for 1 min), washed twice with a nitrogen-deficient conju-gation medium (MIH medium [10]), and suspended in the same medium (2×103 cells per ml).
Subse-quently, a 0.1-ml aliquot of the suspension was inoculated per well of a Linbro tissue culture multi-well plate (ICN Biomedicals, Inc.) contain-ing 0.7 ml of MIH medium with or without a nitrate source (final concentrations of 16.9 mg/l of Ca(NO3)2 · 4H2O and 13.1 mg/l of KNO3). To
examine the effect of DNA synthesis inhibitors on zygote formation, the DNA inhibitors above were added to the MIH medium at various concentra-tions. The cells were incubated for 5 days under the conditions described above, except that the light intensity was elevated to 3000 lx to induce zygote formation. Counting of zygotes, gametes and vege-tative cells was achieved under a stereomicroscope at 10× magnification. A visual field was selected for each test well, and a minimum of 100 cells were counted. The frequency of zygote formation (%) was calculated from the equation: 2Nz×100/
(2Nz+Ng+2Nv), where Nz is the number of zygotes (normal plus abnormal zygotes), Ng is the number of gametes and Nv is the number of vegetative cells.
To test the effect of DNA synthesis inhibitors on growth (asexual reproduction), vegetative cells of the m+ strain were grown for 17 days in C
medium, collected by centrifugation and suspended in fresh C medium (103cells per ml). Subsequently,
10-ml aliquots of the suspension were inoculated
into wells containing 790 ml of C medium together
with the test chemicals at various concentrations. The culture was maintained for 5 days under illumination of 2000 lx, and then the growth rate per day was calculated.
in fresh C medium (2.5×103 cells per ml). Four
0.1-ml aliquots (containing about 250 cells each) of each cell suspension were separately inoculated into respective petri dishes which contained 25 ml of C medium. Subsequently, the inocula were in-cubated for 4, 11, 17 or 22 days under illumination of 2000 lx, and at the end of each culture period, cells of each strain were harvested. To obtain cells at pregamete and gamete stages, vegetative cells of each strain grown for 17 days in C medium were collected and mixed together in the cell number noted above. The mixed cells were washed twice with MIH medium, and then inoculated into a petri dish containing 25 ml of MIH medium (300 cells per ml). Subsequently, the inoculum was in-cubated for 4 days with illumination of 3000 lx and then harvested. To count the number of pyrenoids/cell, pyrenoids were stained according to Rosowski and Hoshaw [11] (Fig. 1L and M), with the exception that 50% propionic acid which contained 4% ferric ammonium sulfate was used as the mordant, followed by 3-D counting under an Olympus BX50 microscope. To determine pyrenoidal size, ten or more cells were selected for each stage and photographed under the micro-scope at 100×. For each stage, the diameters of 500 pyrenoids were measured on microphoto-graphs enlarged at a final magnification of 400×. To investigate the effects of DNA synthesis inhibitors on pyrenoid production and pyrenoidal size, vegetative cells of each strain were grown for 17 days, collected and inoculated separately into petri dishes which contained 25 ml of C medium (300 cells per ml) with or without the test chemi-cals. The cells were incubated for 5 days with illumination of 3000 lx and then harvested. This was followed by measurement of pyrenoids/cell number and pyrenoid diameter.
2.4. Statistical analysis
Experiments to investigate the frequency of zygote formation and growth rate per day were performed in triplicate. Experiments on pyrenoid number and size were repeated more than twice, and the same tendencies were observed. To com-pare treatment means with a control mean, statis-tical analyses were carried out using Student’s t-test, and the degree of significance was expressed in terms of P-values.
3. Results
3.1. Induction of zygote formation by DNA synthesis inhibitors
When two heterothallic strains ofC. ehrenbergii are mixed, zygotes are induced (Fig. 1K). In the present study, the effect that inhibitors of DNA synthesis have on the frequency of zygote forma-tion in the alga was investigated (Table 1). In-hibitors of prokaryotic-type DNA synthesis, EB, AO and NB, which are considered to prevent cytDNA synthesis, increased the frequency of
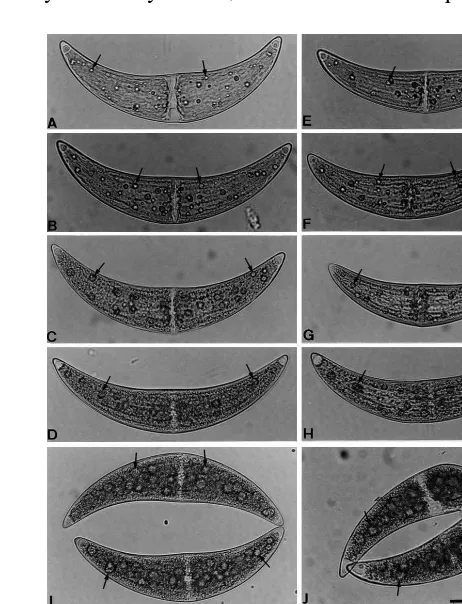
Fig. 1.C.ehrenbergiicells. Photomicrographs shown in (A – J) and (K – M) are enlarged at the same magnification, respec-tively. Scale bars, 100mm. Arrows indicate pyrenoids. (A – D)
Vegetative cells of an m+strain during asexual reproduction.
(E – H) Vegetative cells of an m−strain during asexual
repro-duction. Vegetative cells were grown for 4 days (A and E), 11 days (B and F), 17 days (C and G) and 22 days (D and H) after inoculation in C medium. Each cell comprised two identical semicells and the nucleus was localized in the central region of the cell. (I) A pair of pregametes. (J) A pair of gametes. (K) A pair of normal zygotes. They were almost spherical and had thick walls. (L – M) M+strain cells treated
according to Rosowski and Hoshaw [11] to stain pyrenoids. Before treatment, the cells were grown for 5 days in C medium in the absence (L) or presence (M) of 2.5×10−6M
Table 1
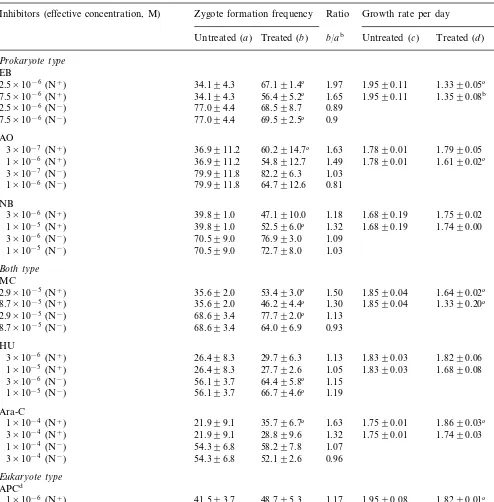
Induction of zygote formation inC.ehrenbergiiby the administration of DNA synthesis inhibitorsa
Ratio
Inhibitors (effective concentration, M) Zygote formation frequency Growth rate per day Ratio
Treated (b) b/ab Untreated (c) Treated (d) d/cb
Untreated (a)
Prokaryote type
EB
2.5×10−6(N+) 34.194.3 67.191.4c 1.97 1.9590.11 1.3390.05c 0.68
56.495.2c 1.65 1.9590.11
34.194.3 1.3590.08b
7.5×10−6(N+) 0.69
68.598.7 0.89 2.5×10−6(N−) 77.094.4
69.592.5c 0.9
77.094.4 7.5×10−6(N−)
AO
60.2914.7c 1.63 1.7890.01
3×10−7(N+) 36.9911.2 1.7990.05 1.01
54.8912.7 1.49 1.7890.01
36.9911.2 1.6190.02c
1×10−6(N+) 0.9
79.9911.8
3×10−7(N−) 82.296.3 1.03
1×10−6(N−) 79.9911.8 64.7912.6 0.81
NB
47.1910.0
3×10−6(N+) 39.891.0 1.18 1.6890.19 1.7590.02 1.04
1×10−5(N+) 39.891.0 52.596.0c 1.32 1.6890.19 1.7490.00 1.04
76.993.0 1.09 70.599.0
3×10−6(N−)
72.798.0 1.03 1×10−5(N−) 70.599.0
Both type
MC
2.9×10−5(N+) 35.692.0 53.493.0c 1.50 1.8590.04 1.6490.02c 0.89
8.7×10−5(N+) 35.692.0 46.294.4c 1.30 1.8590.04 1.3390.20c 0.72
77.792.0c 1.13
68.693.4 2.9×10−5(N−)
68.693.4
8.7×10−5(N−) 64.096.9 0.93
HU
29.796.3 1.13 1.8390.03
26.498.3 1.8290.06
3×10−6(N+) 0.99
26.498.3
1×10−5(N+) 27.792.6 1.05 1.8390.03 1.6890.08 0.92
56.193.7
3×10−6(N−) 64.495.8c 1.15
66.794.6c 1.19
56.193.7 1×10−5(N−)
Ara-C
35.796.7c
1×10−4(N+) 21.999.1 1.63 1.7590.01 1.8690.03c 1.06
28.899.6 1.32 1.7590.01
21.999.1 1.7490.03
3×10−4(N+) 0.99
1×10−4(N−) 54.396.8 58.297.8 1.07
52.192.6 0.96 54.396.8
3×10−4(N−)
Eukaryote type
APCd
1×10−6(N+) 41.593.7 48.795.3 1.17 1.9590.08 1.8290.01c 0.93
50.593.9c 1.22 1.9590.08
41.593.7 1.5890.04c
3×10−6(N+) 0.81
81.094.5c 1.10
1×10−6(N−) 73.992.6
52.6912.3c 0.71
73.992.6 3×10−6(N−)
aAll measurements were made at the end of 5 days of culture. Values represent the mean9S.D. of triplicates. N+, nitrate
present. N−, nitrate absent.
bTreatment mean/non-treatment mean. cPB0.05 vs. untreated cultures.
dSince APC was dissolved in dimethyl sulfoxide (DMSO), DMSO was added to the medium at a final concentration of 0.03%
in the experiments with APC.
zygote formation in the presence of nitrate. The increases were 97, 63 and 32% of the values for non-treated cultures at the most effective concen-trations, 2.5×10−6, 3×10−7 and 10−5 M,
consid-ered to block nDNA synthesis, increased the zygote formation frequency in both the presence and absence of nitrate. The frequency increases at the most effective concentrations were 22 and 10% of non-treated values in the presence and absence of nitrate, respectively. On the other hand, in-hibitors of both prokaryotic and eukaryotic DNA synthesis, MC, HU and Ara-C, increased the fre-quencies in the presence and/or absence of nitrate. Namely, at the most effective concentration, 2.9×
10−5 M, MC elevated the frequencies by 50 and
13% in the presence and absence of nitrate, respec-tively. HU at 3×10−6M increased the frequency
by 15% in the absence of nitrate, and Ara-C at 10-4 M by 63% in its presence.
The effects of these inhibitors on the growth rate per day in the alga were tested (Table 1). NB, HU and Ara-C little affected the growth rate at concentrations effective for zygote formation. By contrast, EB, AO, APC and MC decreased it by 10 – 30% at these concentrations.
3.2. Numbers of pyrenoids/cell and pyrenoid diameters at asexual and sexual reproduction stages
To investigate the numbers of pyrenoids/cell and pyrenoid diameters during asexual reproduc-tion, vegetative m+ and m− strain cells of C.
ehrenbergiiwhich were grown for 4 days (early-log phase, Fig. 1A and E), 11 days (mid-log phase, Fig. 1B and F), 17 days (late-log phase, Fig. 1C and G) and 22 days (stationary phase, Fig. 1D and H) in C medium were observed. In these cells, pyrenoids were almost spherical and distributed randomly.
In early-log phase, the average number of pyrenoids/cell was 57.5912.8 (n=57) and 42.39 9.5 (n=63) for m+ and m− vegetative cells,
re-spectively. This number decreased gradually with the advance of stage in both strains; the averages were 58.6913.9 (n=76) and 45.7910.4 (n=70) in m+ cells at mid-log and late-log phases,
respec-tively, and 38.198.8 (n=63) and 35.698.1 (n=
50) in m− cells at mid-log and late-log phases,
respectively. And, they reached 39.5911.0 (n=
47) and 34.596.9 (n=48) in the respective cells at the stationary phase. On the other hand, in early-log phase, the averages of pyrenoid diameters were 5.391.2 mm (n=500) and 5.291.4 mm (n=500)
in m+ and m−vegetative cells, respectively. These
diameters increased gradually as the stage pro-gressed in both strains of cells; the averages were 5.891.4 mm (n=500) and 6.391.8 mm (n=500)
in m+ cells at mid-log and late-log phases,
respec-tively, and 5.691.4 mm (n=500) and 6.591.8 mm (n=500) in m− cells at mid-log and late-log
phases, respectively. They reached 6.991.8 mm
(n=500) and 7.091.8 mm (n=500) in the
respec-tive cells at stationary phase.
During sexual reproduction, the number of pyrenoids/cell and pyrenoid diameters were deter-mined at pregamete (Fig. 1I) and gamete (Fig. 1J) stages. At these stages, pyrenoids were also almost spherical and distributed randomly within cells. The average number of pyrenoids/cell was 38.6910.8 (n=47) and 20.196.7 (n=48) at the pregamete and gamete stages, respectively. There were no significant differences between the average number of pyrenoids per cell at the pregamete stage and vegetative stage in either strain of cells grown for 17 days in C medium (late-log phase), which were mixed to induce zygote formation. Moreover, the average number per cell at the gamete stage was half of that at the pregamete stage. These results imply that pyrenoid produc-tion occurs neither during pregametogenesis nor during gametogenesis. On the other hand, at the pregamete and gamete stages, the pyrenoid diame-ters were greatly increased compared to those during asexual reproduction; the average value being 10.692.4 mm (n=500) at pregamete and
gamete stages. The result that the averages of pyrenoid diameters at the pregamete and gamete stages were the same indicates an increase of pyrenoidal size during pregametogenesis.
3.3. Modulation of pyrenoid production and pyrenoidal size by DNA synthesis inhibitors
To investigate whether inhibition of DNA syn-thesis is involved in the modulation of pyrenoid production and pyrenoidal size during pregameto-genesis, vegetative m+ and m− strain cells of C.
ehrenbergii were incubated separately with DNA inhibitors, and the number of pyrenoids/cell and pyrenoid diameters were determined (Table 2 and Figs. 2 and 3). Comparison of the average number of pyrenoids/cell and pyrenoid diameters between untreated cultures and cultures treated with EB (2.5×10−6M) indicated that EB caused arrest of
pyrenoidal size. Treatment with AO and NB also caused both a decrease in pyrenoid numbers and an increase in diameter in both strains at concen-trations effective for zygote formation. By con-trast, APC caused only the pyrenoid diameter to increase: it actually evoked an increase in pyrenoid numbers at concentrations sufficient to decrease the growth rate. MC, HU and Ara-C at the effec-tive concentrations caused either the number of pyrenoids/cell to decrease or the pyrenoid diame-ter to increase in both strains of cell. That is, MC and HU increased the diameter, and Ara-C de-creased the number. In addition, MC and HU caused the pyrenoid number to increase at higher
concentrations, 8.7×10−5 and 10−5 M,
respec-tively, like APC. These results indicate that the inhibition of nDNA and/or cytDNA synthesis is closely involved in the modulation of pyrenoid production and pyrenoidal size.
4. Discussion
EB and acridines, and NB were reported to inhibit the synthesis of circular DNA by interca-lating with and inhibiting the gyrase activity of, respectively, the DNA [12,13]. Since cytDNA was shown to be a closed circular duplex molecule in
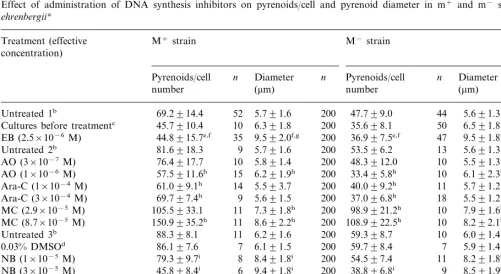
Table 2
Effect of administration of DNA synthesis inhibitors on pyrenoids/cell and pyrenoid diameter in m+ and m− strains of C.
ehrenbergiia
M−strain
M+strain
Treatment (effective concentration)
Pyrenoids/cell n Diameter n Pyrenoids/cell n Diameter n
(mm)
Cultures before treatmentc 45.7910.4 35.698.1
EB (2.5×10−6M) 44.8915.7e,f 35 9.592.0f,g 200 36.997.5e,f 47 9.591.8f,g 200
aM+ and M− vegetative cells grown for 17 days in C medium (late-log phase) were incubated separately for 5 days in C
medium in the presence or absence of DNA inhibitors with illumination of 3000 lx, and then the pyrenoids/cell number and pyrenoid diameters were examined. Values represent the mean9S.D. of samples.n, the number of samples.
bCultures in the absence of DNA inhibitors. In the experiments with EB, Untreated 1 was used for the control. Similarly,
Untreated 2 and Untreated 3 were used in those with AO, Ara-C and MC and those with NB, HU and APC, respectively. To dissolve APC, 0.03% DMSO was added to the medium.
cVegetative cells grown for 17 days in C medium (late-log phase). dCultures treated with 0.03% DMSO.
eP\0.2 vs. cultures before treatment. fPB0.05 vs. untreated 1.
gPB0.05 vs. cultures before treatment. hPB0.05 vs. untreated 2.
iPB0.05 vs. untreated 3.
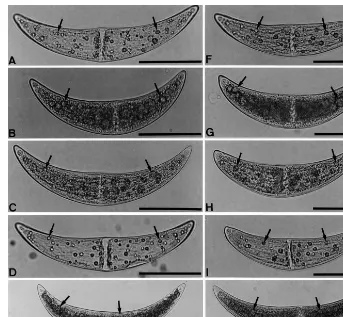
Fig. 2. Separate treatment of vegetative cells of m+and m−strains ofC.ehrenbergiiwith EB, AO, Ara-C or MC. M+and M−
strain cells were grown separately for 5 days in C medium under illumination of 3000 lx in the presence or absence of inhibitors of DNA synthesis. (A – E) M+strain cells. (F – J) M−strain cells. Arrows indicate pyrenoids. Scale bars, 100
mm. (A and F) Cells
grown in the absence of any inhibitors. (B and G) Cells grown in the presence of 2.5×10−6M EB. Pyrenoids are enlarged and
pyrenoid numbers are decreased. (C and H) Cells grown in the presence of 10−6M AO. Pyrenoids are somewhat enlarged and
pyrenoid numbers are decreased. (D and I) Cells grown in the presence of 3×10−4M Ara-C. Pyrenoid numbers are decreased.
(E and J) Cells grown in the presence of 8.7×10−5M of MC. Pyrenoids are enlarged and pyrenoid numbers are increased.
many eukaryotes including eukaryotic algae [14 – 18], these chemicals are considered to be selective inhibitors of cytDNA synthesis. Perlman and Mahler [19] reported that EB inhibited mitochon-drial DNA (mtDNA) replication by over 95% in Saccharomyces at 20 mg/l (equal to 5×10−5 M),
while nDNA replication was only slightly affected. Flechtner and Sager [20] also showed that EB inhibited chloroplast DNA (chlDNA) replication by 7.1 – 42.9% in Chlamydomonas at 5 – 10 mg/l, while permitting nDNA replication. An acridine, acriflavin, selectively inhibited kinetoplast DNA in Leishmania at the concentration of 5×10−5– 3×
10−4 M [21] and NB inhibited DNA synthesis in
Escherichia coli by 80% at 1.8×10−4 M [22]. In
our experiments, administration of EB, AO and NB enhanced zygote formation inC.ehrenbergiiat concentrations of 2.5 – 7.5×10−6, 3×10−7– 10−6
and 10−5 M, respectively, in the presence of
ni-trate (Table 1). The concentrations at which AO and NB enhanced zygote formation were one to two orders of magnitude lower than those re-ported to inhibit DNA synthesis. This result sug-gests that inhibition of cytDNA synthesis, levels of which are probably low, are involved in the induc-tion of zygote formainduc-tion in the alga. On the other hand, APC was reported to inhibit eukaryotic DNA polymerase aactivity in HeLa cells [23] and
sea urchin embryo [24]. The chemical inhibited DNA synthesis in HeLa cells by 25 – 95% at 2×
10−5– 2×10−3 M [23], and inhibited nucleic acid
synthesis in isolated mesophyll cells of Zinnia ele-gans by 50 – 80% at 10−5– 10−4 M [25]. In our
experiments, APC enhanced zygote formation in C. ehrenbergii at 10−6– 3×10−6 M in the
inhibited the synthesis of DNA or nucleic acid in HeLa cells andZ. elegans. This result suggests that inhibition of nDNA synthesis, levels of which seem to be low, is involved in the induction of zygote formation in the alga.
MC and HU were reported to inhibit both prokaryotic and eukaryotic DNA synthesis by in-ducing cross-linking between DNA double strands [12] and by inhibiting the activity of ribonucleotide reductase [26], respectively. Ara-C, also an in-hibitor of both DNA, was metabolized in vitro to Ara-CTP, and inhibited DNA polymerase activity [27] and DNA synthesis at the level of nucleotide metabolism [12]. 10−5 M MC and 10−5– 10−4 M
Ara-C inhibited nucleic acid synthesis in Z. ele-gans mesophyll cells by 80 and 80 – 90%, respec-tively, [25]. Ara-C (4 mM) inhibited DNA synthesis in E. coli by 95% [12]. HU inhibited DNA synthesis in Chlamydomonas reinhardi by 71% at 5×10−3 M and in Cymbidium almost
completely at 10−4 M [26]. In our experiments,
HU and Ara-C enhanced zygote formation at concentrations lower than those reported to in-hibit the synthesis of DNA or nucleic acid (Table 1). Accordingly, the finding that these drugs en-hanced zygote formation in the alga supports that the inhibition of both nDNA and cytDNA synthe-sis, levels of which are probably low, is involved in the induction of zygote formation.
Our investigation of the number of pyrenoids/
cell and pyrenoidal diameters throughout the stages of asexual and sexual reproduction indi-cated that arrest of pyrenoid production and en-largement of pyrenoidal size occurred during pregametogenesis. Furthermore, our results showed that administration of EB, AO and NB resulted in both a decrease in pyrenoid production and increase in pyrenoidal size in C. ehrenbergii, whereas APC administration caused only the pyrenoidal size to increase (Table 2). This result suggests that the inhibition of cytDNA synthesis is more essential to the induction of pregametes in
Fig. 3. Separate treatment of vegetative cells of m+and m−strains ofC.ehrenbergiiwith NB, APC or HU. Cells were grown
in the manner mentioned in Fig. 2. All photomicrographs are enlarged at the same magnification. Scale bar, 100mm. (A – D) M+
strain cells. (E – H) M−strain cells. (A and E) Cells grown in the absence of any inhibitors. (B and F) Cells grown in the presence
of 3×10−5 M NB. Pyrenoids are enlarged and pyrenoid numbers are decreased. (C and G) Cells grown in the presence of
the alga than that of nDNA synthesis. In our experiments, MC and HU caused only the increase of pyrenoidal size and Ara-C only the decrease of pyrenoid production (Table 2). From these results, we speculate that MC and HU function as selec-tive inhibitors of nDNA synthesis and Ara-C as a selective inhibitor of cytDNA synthesis in C. ehrenbergii. In fact, in HeLa cells, cytDNA synthe-sis was shown to be less affected by HU than nDNA synthesis [28]. In addition, Ara-C was re-ported to affect DNA synthesis in some strains of E.coliandBacillus subtilisat only huge concentra-tions such as 4 mM [13]. In our experiments, the concentration at which Ara-C enhanced zygote formation was also comparatively high, 0.1 – 0.3 mM (Table 1). Then, MC and HU caused the increase of pyrenoid number at concentrations high enough to decrease the growth rate, similar to APC (Table 2). Walne [29] reported that the pyrenoid number was increased inChlamydomonas cells treated with enough colchicine (5 mM) to inhibit cell division. Accordingly, the increase of pyrenoid number caused by administration of these chemicals may result from the inhibition of cell division.
Ara-C administration caused only a decrease of pyrenoid production, whereas all other cytDNA inhibitors tested (such as EB, AO and NB) evoked both a decrease of pyrenoid production and an increase of pyrenoidal size (Table 2). This result suggests that arrest of pyrenoid production is caused by the direct inhibition of cytDNA synthe-sis but enlargement of pyrenoidal size is not. Pre-vious studies [7,8] suggested that the replication cycles of nuclear and organellar DNAs were tightly coupled with each other. InSaccharomyces lactis, mtDNA synthesis occurred slightly before nDNA synthesis [7]. Hola et al. [30] recently indi-cated that the timing of nDNA synthesis depends on the appearance of a cytoplasmic signal, based on the finding that the two nuclei of permeabilized mammalian binucleate cells enter DNA synthesis coordinately in cell-free extracts of Xenopus eggs. All of this evidence taken together suggests that the inhibition of cytDNA synthesis causes the inhibition of nDNA synthesis directly or indi-rectly. We, therefore, consider that enlargement of pyrenoidal size caused by administration of EB, AO and NB results from the inhibition of nDNA synthesis evoked by the inhibition of cytDNA synthesis. In our experiments, nDNA inhibitors
actually resulted in enlargement of pyrenoidal size (Table 2). From these results, we conclude that differentiation into pregametes in C. ehrenbergii is caused by the inhibition of both cytDNA and nDNA synthesis, levels of which are probably low, and the inhibition of cytDNA synthesis may be an initial signal for the induction of pregametes.
In our experiments, inhibitors of cytDNA syn-thesis such as EB, AO, NB and Ara-C enhanced zygote formation in the presence of nitrate (Table 1). By contrast, inhibitors of nDNA synthesis such as APC, MC and HU enhanced it in both the presence and absence of nitrate (Table 1). This result suggests that the nitrogen source for cytDNA synthesis is the nitrate which is present in medium, whereas that for nDNA synthesis is both medium nitrate and nitrogen pooled within the cells. Nitrogen depletion in medium may cause the inhibition of cytDNA synthesis. Moreover, if the nitrogen within the cells was to be exhausted, the synthesis of nDNA may be inhibited. Accordingly, these results also support that the inhibition of cytDNA synthesis occurs prior to that of nDNA synthesis and may be an initial signal for zygote formation.
The present study showed that arrest of pyrenoid production and enlargement of pyrenoidal size in C.ehrenbergii may be caused by the inhibition of cytDNA and nDNA synthesis. Since pyrenoids were reported to contain predomi-nantly ribulose 1,5-bisphosphate carboxylase/ oxy-genase (Rubisco), which is a Calvin – Benson cycle enzyme [31 – 36], enlargement of pyrenoidal size probably indicates the enhancement of pyrenoid-localized Rubisco activity. Coleman [37] observed that pyrenoids were surrounded by plastid DNA (ptDNA) in Spirogyra and E. gracilis. This obser-vation supports that the inhibition of ptDNA synthesis modulates pyrenoid production and ac-tivity in C. ehrenbergii directly.
References
[1] J. Hamada, Studies on several environmental factors for zygote formation and germination in Closterium ehren
-bergii, Bot. Mag. Tokyo 91 (1978) 173 – 180.
[2] T. Hogetsu, M. Yokoyama, Light, a nitrogen-depleted medium and cell – cell interaction in the conjugation process ofClosterium ehrenbergii, Meneghini, Plant Cell Physiol. 20 (4) (1979) 811 – 817.
[4] J.A. Parsons, Mitochondrial incorporation of tritiated thymidine inTetrahymena pyriformis, J. Cell Biol. 25 (I) (1965) 641 – 646.
[5] T.E. Evans, Synthesis of a cytoplasmic DNA during the G2interphase ofPhysarum polycephalum, Biochem.
Bio-phys. Res. Commun. 22 (1966) 678 – 683.
[6] K. Chiang, N. Sueoka, Replication of chloroplast DNA inChlamydomonas reinhardiduring vegetative cell cycle: its mode and regulation, Proc. US Natl. Acad. Sci. 57 (1967) 1506 – 1513.
[7] D. Smith, P. Tauro, E. Schweizer, H.O. Halvorson, The replication of mitochondrial DNA during the cell cycle in Saccharomyces lactis, Proc. US Natl. Acad. Sci. 60 (1968) 936 – 942.
[8] R. Sager, Cytoplasmic Genes and Organelles, Academic press, New York, 1972, pp. 1 – 45.
[9] T. Ichimura, Sexual cell division and conjugation-papilla formation in sexual reproduction of Closterium strigo
-sum, Proceedings of the Seventh International Seaweed Symposium, 1971, pp. 208 – 214.
[10] T. Ichimura, Hybrid inviability and predominant sur-vival of mating type minus progeny in laboratory crosses between two closely related mating groups ofClosterium ehrenbergii, Evolution 37 (2) (1983) 252 – 260.
[11] J.R. Rosowski, R.W. Hoshaw, Staining algal pyrenoids with carmine after fixation in an acidified hypochlorite solution, Stain Technol. 45 (6) (1970) 293 – 298. [12] E.F. Gale, W. Cundliffe, P.E. Reynolds, M.H.
Rich-mond, M.J. Waring, The Molecular Basis of Antibiotic Action, Wiley, London, 1972, pp. 173 – 277.
[13] N.R. Cozzarelli, The mechanism of action of inhibitors of DNA synthesis, Ann. Rev. Biochem. 46 (1977) 641 – 668.
[14] B. Hudson, J. Vinograd, Catenated circular DNA molecules in HeLa cell mitochondria, Nature 216 (1967) 647 – 652.
[15] C.P. Hollenberg, P. Borst, E.F.J. Van Bruggen, Mito-chondrial DNA V. A 25-m closed circular duplex DNA
molecule in wild-type yeast mitochondria, Struct. Genet. complexity Biochim. Biophys. Acta 209 (1970) 1 – 15. [16] Z. Zhang, B.R. Green, T. Cavalier-Smith, Single gene
circles in dinoflagellate chloroplast genomes, Nature 400 (1999) 155 – 159.
[17] E.M. Denovan-Wright, A.M. Nedelcu, R.W. Lee, Com-plete sequence of the mitochondrial DNA of Chlamy
-domonas eugametos, Plant Mol. Biol. 36 (1998) 285 – 295. [18] U. Kessler, K. Zetsche, Physical map and gene organiza-tion of the mitochondrial genome from the unicellular green alga Platymonas (Tetraselmis) subcordiformis
(Prasynophyceae), Plant Mol. Biol. 29 (1995) 1081 – 1086.
[19] P.S. Perlman, H.R. Mahler, Molecular consequences of ethidium bromide mutagenesis, Nat. New Biol. 231 (1971) 12 – 16.
[20] V.R. Flechtner, R. Sager, Ethidium bromide induced selective and reversible loss of chloroplast DNA, Nat.
New Biol. 241 (1973) 277 – 279.
[21] L. Simpson, Effect of acriflavin on the kinetoplast of
Leishmania trarentolae, J. Cell Biol. 37 (1968) 660 – 682. [22] D.H. Smith, B.D. Davis, Mode of action of novobiocin
in Escherichia coli, J. Bacteriol. 93 (1967) 71 – 79. [23] F. Hanaoka, H. Kato, S. Ikegami, M. Ohashi, M.
Yamada, Aphidicolin does inhibit repair replication in HeLa cells, Biochem. Biophys. Res. Commun. 87 (1979) 575 – 580.
[24] S. Ikegami, T. Taguchi, M. Ohashi, Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-a, Nature 275 (1978) 458 – 460.
[25] H. Fukuda, A. Komamine, Relationship between trac-heary element differentiation and DNA synthesis in single cells isolated from the mesophyll ofZinnia elegans
— analysis by inhibitors of DNA synthesis, Plant Cell Physiol. 22 (1981) 41 – 49.
[26] J. Timpson, Hydroxyurea, Mutat. Res. 32 (1975) 115 – 132.
[27] G.V. Rama Reddy, M. Goulian, S.S. Hendler, Inhibi-tion of E. coli DNA polymerase II by ara-CTP, Nat. New Biol. 234 (1971) 286 – 288.
[28] C. Vesco, S. Penman, Purified cytoplasmic DNA from HeLa cells, resistance to inhibition by hydroxyurea, Biochem. Biophys. Res. Commun. 35 (1969) 249 – 257. [29] P. Walne, The effects of colchicine on cellular
organiza-tion inChlamydomonas. II. Ultrastructure, Am. J. Bot. 54 (1967) 564 – 577.
[30] M. Hola, M. Howard, F.N. Nawaz, S. Castleden, R.F. Brooks, Individual nuclei differ in their sensitivity to the cytoplasmic inducers of DNA synthesis: Implications for the origin of cell cycle variability, Exp. Cell Res. 229 (1996) 350 – 359.
[31] D.J. Griffiths, The pyrenoid, Bot. Rev. 36 (1970) 29 – 58. [32] D.J. Griffiths, The pyrenoid and its role in algal
metabolism, Sci. Prog. Oxf. 66 (1980) 537 – 553. [33] G. Lacoste-Royal, S.P. Gibbs, @Immunocytochemical
localization of ribulose-1,5-bisphosphate carboxylase, in pyrenoids and thylakoid region of the chloroplast of
Chlamydomonas reinhardtii, 83 (1987) 602 – 606. [34] R.M. Mckay, S.P. Gibbs, Immunocytochemical
localiza-tion of ribulose 1,5-bisphosphate carboxylase/oxygenase in light-limited and light-saturated cells of Chlorella pyrenoidosa, Protoplasma 149 (1989) 31 – 37.
[35] T. Osafune, S. Sumida, T. Ehara, E. Hase, Three-dimen-sional distribution of ribulose-1,5-bisphosphate carboxy-lase/oxygenase in chloroplasts of actively photosynthesizing cells of Euglena gracilis, J. Electron Microsc. 38 (5) (1989) 399 – 402.
[36] R. Michael, L. Mckay, S.P. Gibbs, Phycoerythrin is absent from the pyrenoid of Porphyridium cruentum: photosynthetic implications, Planta 180 (1990) 249 – 256. [37] A.W. Coleman, Diversity of plastid DNA configuration among classes of eukaryotenalgae, J. Phycol. 21 (1985) 1 – 16.