Brain Research 887 (2000) 174–177
www.elsevier.com / locate / bres
Short communication
Evidence for central
a
2-adrenergic modulation of rat pineal melatonin
synthesis
a ,
*
a a a,b¨ ¨
S.M. Mustanoja
, T. Hatonen , A. Alila-Johansson , Laakso
a
Department of Physiology, Institute of Biomedicine, University of Helsinki, Helsinki, Finland b
Neural Networks Research Centre, Helsinki University of Technology, Espoo, Finland
Accepted 29 August 2000
Abstract
This study was performed to distinguish central and peripherala2-adrenoceptors in the inhibition of rat pineal melatonin synthesis. The rats received lipo- or hydrophilic a2-adrenoceptor ligand injections at middark; after 1 or 2 h the pineal melatonin contents were measured. The lipophilic agonist medetomidine (100mg / kg s.c.) suppressed the melatonin contents significantly, while the hydrophilic agonists ST-91 and p-aminoclonidine (10 or 100 mg / kg i.v.) did not. The suppression by medetomidine was counteracted by the lipophilic antagonist yohimbine (0.3–3.0 mg / kg i.p.) but not by the hydrophilic antagonist L-659,066 (1–10 mg / kg i.v.). In conclusion, the suppression of nocturnal melatonin synthesis bya2-adrenoceptor agonists is mainly of central origin. 2000 Elsevier Science B.V. All rights reserved.
Theme: Endocrine and autonomic regulation
Topic: Neuroendocrine regulation: other
Keywords: a2-Adrenoceptor; Pineal gland; Melatonin; Medetomidine
Melatonin synthesis is under control of the central agonists reduce nocturnal pineal NAT activity [1] and nervous system, through pathways originating in the melatonin content [14] in rats and plasma melatonin levels biological clock, the hypothalamic suprachiasmatic nuclei in humans [9]. The site of thea2-adrenoceptors inducing (SCN), and ending at the peripheral sympathetic fibers the suppression of melatonin synthesis has not been innervating the pineal gland [12]. The information from defined. There area2-adrenoceptors in all the structures of the SCN is mediated through the hypothalamic paraven- the regulatory pathways mentioned above [10,11,13,25] as tricular nuclei (PVN), the intermediolateral cell column of well as in the pineal itself [22,23].
the thoracic spinal cord (IMLC) and the superior cervical Postsynaptic receptors on pinealocyte membranes are ganglia (SCG). not likely to be involved in the melatonin suppression in Locally in the gland, noradrenaline acts through post- vivo because in cultured pineal glands, a2-adrenoceptor synaptic b- and a1-adrenoceptors stimulating N-acetyl- agonists increase rather than decrease NAT activity and the transferase (NAT) activity [24], the enzyme mainly re- production of melatonin [1,15,21]. In addition, the a2 -sponsible of the nocturnal rise of melatonin synthesis. The adrenoceptor agonist medetomidine does not suppress synaptic mechanisms in the central regulatory pathways melatonin production stimulated by isoproterenol in vivo and in the ganglia are not well known. [14]. All other sites from the hypothalamic clock to the It has previously been shown that a2-adrenoceptor pineal presynaptic terminals should be considered as
possible targets of effective a2-adrenergic drugs.
The present experiments were carried out in order to distinguish between the central and peripheral a2
-adreno-*Corresponding author. Tel.: 1358-9-191-8534; fax: 1
358-9-612-ceptors in inhibiting rat pineal melatonin synthesis. Like
43160.
E-mail address: [email protected] (S.M. Mustanoja). other circumventricular organs, the pineal gland is outside
S.M. Mustanoja et al. / Brain Research 887 (2000) 174 –177 175
the blood–brain barrier [3]. Thus, by investigating the was collected into glass tubes and allowed to clot for about effects of hydrophilica2-adrenergic drugs not penetrating 1 h. Serum was separated by centrifugation and stored at the blood–brain barrier, it is possible to get information on 2248C for melatonin measurements.
whether the receptors at the pineal nerve terminals and / or In the third and forth experiments the effects of in the sympathetic ganglia have a significant role in the peripheral a2-adrenoceptor agonists ST-91 and p-amino-melatonin suppression by a2-adrenoceptor agonists, or clonidine on nocturnal pineal melatonin contents were whether the suppression is more centrally mediated. studied. Both drugs act mainly peripherally [6,7]. The rats Adult male Wistar rats were used in all experiments received i.v. injections of saline or one of the drugs (doses (weight about 300 g, age 8–10 weeks, 8–10 animals / 10 and 100 mg / kg). The samples were collected 60 min group). The rats were kept under controlled lighting (ST-91) or 120 min ( p-aminoclonidine) after the injec-conditions (12 h light / 12 h dark) from birth, and they were tions.
synchronized with the lighting regime (dark period from Melatonin was measured by radioimmunoassay [26]. 03:00 to 15:00 h, dim red light ,1 lux) at least 2 weeks The unspecific binding was 5–6% and the lower detection before each experiment. Illuminance during the light limit was smaller than the lowest standard (19.5 pg / ml). period was 100–150 lux at the level of the cages. The Intra-assay variability was 4–10%, and the interassay experiments began in the middle of the dark period at variability of 51 assays, including the assays of this study, 09.00 h and all procedures were performed in darkness during 24 months was 8–12% in the concentration range until the pineal glands were frozen. Temperature and of the samples.
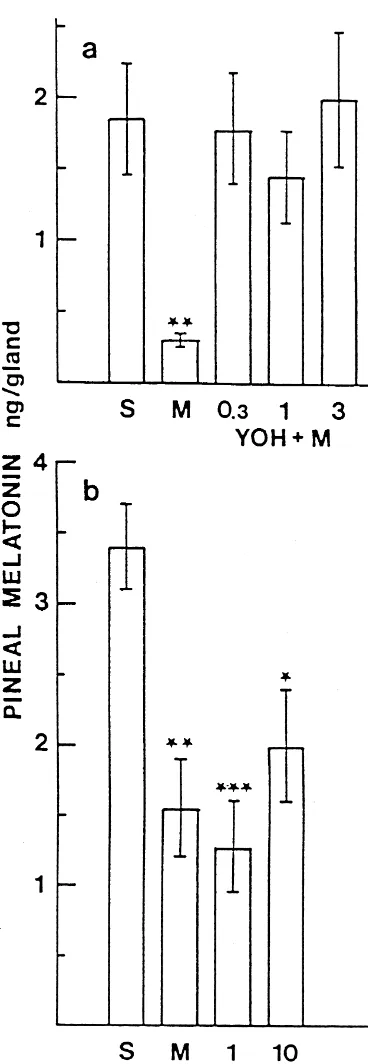
relative humidity in the animal departments were 23–268C The nocturnal pineal melatonin contents in saline in-and 48–60%, respectively. The rats were given food in-and jected rats were approximately 2.5–3.5 ng / gland in experi-water ad libitum. ments 2–4 (Fig. 1b, Table 1), but somewhat lower in The hydrophilic a2-adrenoceptor agonists used in the experiment 1, in which the rats originated in a different experiments were ST-91(2-(2,6-diethylphenylamino)-2-im- breeding colony (Fig. 1a). Medetomidine, the a2 -adreno-idazoline hydrochloride, Boehringer-Ingelheim, Biberach, ceptor agonist which acts both centrally and peripherally, Germany) and p-aminoclonidine hydrochloride (RBI, suppressed the melatonin contents significantly in both Natick, MA, USA) and the hydrophilic a2-adrenoceptor experiment 1 and 2 (Fig. 1a, b).
antagonist was L-659,066 ((2R-trans)-N-(2-(1,3,4,7,12 b- The serum melatonin concentrations measured in the hexahydro-29-oxo-spiro(2 H-benzofuro(2,3-a)quinolizine- second experiment correlated with pineal melatonin con-2,49- imidazolidin) - 39- yl)ethyl) methanesulphonamide tents (Pearson’s r50.90 with 95% confidence interval monohydrochloride, Merck, Rahway, NJ, USA).The lipo- 0.81–0.94, P,0.0001, n539). The mean serum levels philic control drugs which easily cross the blood–brain were: control group 82 pg / ml, medetomidine injected rats barrier were medetomidine hydrochloride (agonist, Orion without the antagonist 26 pg / ml, and medetomidine in-Pharma, Turku, Finland) and yohimbine hydrochloride jected rats pretreated with the lower or higher dose of (antagonist, RBI, Natick, MA, USA). The hydrophilic L-659,066 18 or 37 pg / ml, respectively. In all three drugs were injected into the tail vein (100ml) in order to medetomidine injected groups the serum levels were ensure rapid distribution, medetomidine was given sub- significantly lower than in the control group (P,0.01 or cutaneously (100 ml) and yohimbine intraperitoneally due 0.001, Tukey’s test). The finding suggests that the to the large volume needed (1 ml). medetomidine induced suppression of pineal melatonin In the first experiment the effect of thea2-adrenoceptor results from decreased synthesis rather than from increased antagonist yohimbine on medetomidine induced suppres- release from the gland.
sion of pineal melatonin levels was studied. Both drugs The effect of medetomidine was counteracted by yohim-easily cross the blood–brain barrier [5,20]. Rats were bine, the antagonist with central and peripheral actions. divided into five groups. Each group received two in- Even the lowest dose 0.3 mg / kg inhibited the jections with a 30 min interval: group 1 two injections of medetomidine induced suppression completely (Fig. 1a). saline, group 2 saline and 0.1 mg / kg medetomidine, L-659,066, the antagonist with only peripheral actions, was groups 3, 4 and 5 first received 0.3, 1.0 or 3.0 mg / kg unable to counteract the medetomidine induced melatonin yohimbine, respectively, and then medetomidine (0.1 mg / suppression; the highest dose (10 mg / kg) remained inef-kg). The rats were decapitated in an adjacent room 120 fective (Fig. 1b). In line with this finding, the peripherally min after the second injection, the pineal glands quickly acting a2-adrenoceptor agonists ST-91 and p-amino-removed, frozen on solid CO , and stored at2 2248C until clonidine did not suppress pineal melatonin contents the melatonin content was measured. (Table 1).
176 S.M. Mustanoja et al. / Brain Research 887 (2000) 174 –177
Table 1
Nocturnal pineal melatonin contents in male rats after injections of saline a
or peripherala2-adrenoceptor agonists
Treatment Dose Pineal melatonin (mg / kg) (ng / gland) content6S.E.M. (n)
SAL 2.78 60.24 (10)
ST-91 10 3.32 60.14 (10)
ST-91 100 2.66 60.17 (10)
ANOVA P50.0455 (no differences in Tukey’s test)
SAL 2.41 60.25 (9)
PAC 10 2.53 60.25 (9)
PAC 100 2.71 60.12 (10)
ANOVA NS (P.0.05)
a
SAL5saline, PAC5p-aminoclonidine. The injections were given in-travenously in the middle of the dark period, 60 min (ST-91) or 120 min (PAC) before sampling.
regulate the release of noradrenaline from the pineal nerve endings [4,17,18], and that melatonin synthesis can be stimulated by a2-adrenoceptor agonists in tissue culture [1,15,21]. Present results indicate that only central effects are seen when the drug is given through a systemic route in vivo.
There are several possible sites for the a2-adrenergic regulation of melatonin synthesis inside the blood–brain barrier. If the effect is not indirect and does not originate outside the main regulatory pathways, the synaptic trans-mission can be modulated in all three nuclei of the pathway: the SCN, the PVN, and the IMLC, because functionally significant a2-adrenoceptors have been de-tected in these structures [11,13]. The importance of the receptors in the regulation of melatonin synthesis has, however, not been studied.
The present results are in line with studies where a contribution ofa2-adrenoceptors has been suggested in the expression of circadian rhythms [8,19]. Like the immediate suppression of melatonin by a2-adrenoceptor agonists, phase shifts produced by the drugs resemble those pro-duced by light. Therefore, it is interesting that various experimental paradigms have failed to show any role for thea2-adrenoceptors in the melatonin suppression by light [1,16]. An explanation for this discrepancy has to be waited for, until information is available concerning the differences between the hypothalamic phase adjusting mechanisms and the mechanisms responsible of the
imme-Fig. 1. Nocturnal pineal melatonin contents in male rats after injections diate control of melatonin synthesis. of a2-adrenoceptor agonist medetomidine preceded by an injection of
saline ora2-adrenoceptor antagonists yohimbine (a) or L-659,066 (b). S5two injections of saline; M5saline1medetomidine (100 mg / kg); numbers under the columns refer to the doses of thea2-adrenoceptor
antagonists (mg / kg, YOH5yohimbine). The interval from the first to the Acknowledgements second injection was 30 min (a) or 15 min (b). The samples were
collected 2 h after the second injection. The results are means with
Medetomidine was provided by Farmos Group (Turku,
S.E.M.s of 8–10 rats / group. One-way ANOVAs: (a) P,0.001, (b) P,
Finland), ST-91 by Boehringer-Ingelheim (Biberach,
Ger-0.001. Tukey’s tests: * different from group S, P,0.05, ** P,0.01, ***
S.M. Mustanoja et al. / Brain Research 887 (2000) 174 –177 177
Pineal melatonin in rats: suppression by the selective alpha2-adreno-References
ceptor agonist medetomidine, Eur. J. Pharmacol. 326 (1997) 229– 236.
[1] L. Alphs, A. Heller, W. Lovenberg, Adrenergic regulation of the [15] S.M. Mustanoja, N. Back, A. Alila-Johansson, M.-L. Laakso,¨ reduction in acetyl coenzyme A:arylamine N-acetyltransferase ac- Melatonin release from rat pineal in vitro is stimulated by both the tivity in the rat pineal, J. Neurochem. 34 (1980) 83–90. a -adrenoceptor agonist medetomidine and the antagonist
2
[2] B.V. Clineschmidt, D.J. Pettibone, V.J. Lotti, H.B. Hucker, B.M. atipamezole, Eur. J. Pharmacol. 383 (1999) 75–82.
Sweeney, D.R. Reiss, E.V. Lis, J.R. Huff, J. Vacca, A peripherally [16] S.M. Mustanoja, T. Hatonen, A. Alila-Johansson, M.-L. Laakso,¨ ¨ acting alpha-2 adrenoceptor antagonist: L-659,066, J. Pharmacol. Evidence against alpha2-adrenoceptor involvement in the regulation Exp. Ther. 245 (1988) 32–40. of rat melatonin synthesis by ambient lighting, Neuroscience 92 [3] J. Dretzki, Licht- und elektronenmikroskopische Untersuchungen (1999) 967–973.
¨
zum Problem der Blut-Hirn-Schranke circumventricularer Organe [17] F. Pelayo, M.L. Dubocovich, S.Z. Langer, Regulation of norad-der Ratte nach Behandlung mit Myofer, Z. Anat. Entwickl.-Gesch. renaline release in the rat pineal through a negative feedback 134 (1971) 278–297. mechanism mediated by presynaptica-adrenoceptors, Eur. J. Phar-[4] W.J. Drijfhout, A.G. van der Linde, S.E. Kooi, C.J. Grol, B.H.C. macol. 45 (1977) 317–318.
Westerink, Norepinephrine release in the rat pineal gland: the input [18] K. Racke, M. Sommer, F. Burns, B. Hering, Differential effects of´ from the biological clock measured by in vivo microdialysis, J. electrical stimulation, blockade of neuronal amine uptake and Neurochem. 66 (1996) 748–755. activation ofa -adrenoceptors on the release of endogenous
norad-2
[5] J.W. Hubbard, S.L. Pfister, A.M. Biediger, T.C. Herzig, T.K. Keeton, renaline and 5-hydroxytryptamine from the isolated rat pineal gland, The pharmacokinetic properties of yohimbine in the conscious rat, Naunyn-Schmiedeberg’s Arch. Pharmacol. 343 (1991) 337–343. Naunyn-Scmiedeberg’s Arch. Pharmacol. 337 (1988) 583–587. [19] A.M. Rosenwasser, L.J. Vogt, M.W. Pellowski, Circadian phase
ˇ ˘ ´
[6] J. Jurcovicova, T. Le, L. Krulich, The paradox of a2 adrenergic shifting induced by clonidine injections in Syrian hamsters, Biol. regulation of prolactin (PRL) secretion. I. The PRL-releasing action Rhythm Res. 26 (1995) 553–572.
of thea2receptor agonists, Brain Res. Bull. 23 (1989) 417–424. [20] J.-M. Savola, H. Ruskoaho, J. Puurunen, J.S. Salonen, N.T. Karki,¨ [7] W. Kobinger, L. Pichler, Investigation into some imidazoline Evidence for medetomidine as a selective and potent agonist at
compounds, with respect to peripheral a-adrenoceptor stimulation a -adrenoceptors, J. Acton. Pharma. 5 (1986) 275–284. 2
and depression of cardiovascular centers, Naunyn-Schmiederberg’s [21] N.C. Schaad, D.C. Klein, a -adrenergic receptor activation poten-2
Arch. Pharmacol. 291 (1975) 175–191. tiates DBcAMP-stimulated rat pineal N-acetyltransferase ¨ ¨
[8] M.-L. Laakso, S.M. Mustanoja, T. Hatonen, A. Alila-Johansson, (E.C.2.3.1.87) activity, Soc. Neurosci. Abstr. 17 (1991) 355. Alpha2-adrenoceptor agonist medetomidine affects the melatonin [22] N.C. Schaad, D.C. Klein, Characterization of a-adrenergic
re-2
rhythm in rats, Neurosci. Lett. 238 (1997) 61–64. ceptors on rat pinealocytes, Endocrinology 130 (1992) 2804–2810. [9] A.J. Lewy, L.J. Siever, T.W. Uhde, S.P. Markey, Clonidine reduces [23] V. Simonneaux, M. Ebadi, D.B. Bylund, Identification and charac-plasma melatonin levels, J. Pharm. Pharmacol. 38 (1986) 555–556. terization ofa -adrenergic receptors in bovine pineal gland, Mol.
2D
[10] J.B. McCallum, N. Boban, Q. Hogan, W.T. Schmeling, J.P. Kam- Pharmacol. 40 (1991) 235–241.
pine, Z.J. Bosnjak, The mechanism ofa 2-adrenergic inhibition of [24] D. Sugden, Melatonin biosynthesis in the mammalian pineal gland, sympathetic ganglionic transmission, Anesth. Analg. 87 (1998) Experientia 45 (1989) 922–932.
503–510. [25] J.R. Unnerstall, T.A. Kopajtic, M.J. Kuhar, Distribution of a 2 [11] T. Miyazaki, H. Kobayashi, T. Tosaka, Presynaptic inhibition by agonist binding sites in the rat and human central nervous system: noradrenaline of the E.P.S.C. evoked in neonatal rat sympathetic analysis of some functional, anatomic correlates of the pharmacolog-preganglionic neurons, Brain Res. 790 (1998) 170–177. ic effects of clonidine and related adrenergic agents, Brain Res. Rev. [12] R.Y. Moore, Neural control of the pineal gland, Behav. Brain Res. 7 (1984) 69–101.
73 (1996) 125–130. [26] O. Vakkuri, J. Leppaluoto, O. Vuolteenaho, Development and valida-¨ [13] A. Morien, V.M. Cassone, P.J. Wellman, Diurnal changes in paraven- tion of a melatonin radioimmunoassay using radioiodinated tricular hypothalamic a1 and a2-adrenoceptors and food intake in melatonin as tracer, Acta Endocrinol. (Copenh.) 106 (1984) 152– rats, Pharmacol. Biochem. Behav. 63 (1999) 33–38. 157.
¨ ¨