www.elsevier.com / locate / bres
Research report
Auditory noise can prevent increased extracellular acetylcholine levels
in the hippocampus in response to aversive stimulation
*
¨
C.M. Thiel, C.P. Muller, J.P. Huston, R.K.W. Schwarting
¨ ¨ ¨
Institute of Physiological Psychology I, Heinrich-Heine-University of Dusseldorf, Universitats-str. 1, 40225 Dusseldorf, Germany Accepted 8 August 2000
Abstract
The intent of this study was to investigate neurochemical and behavioural effects of aversive stimulation and the impact of auditory background noise. Using in vivo microdialysis, hippocampal acetylcholine was extracted and subjected to HPLC analysis while male Wistar rats were exposed to aversive stimulation similar to that used in conventional procedures for aversive conditioning. Three groups of animals were used. Animals in the first group were exposed to a single tone / footshock pairing followed by a tone alone 2 h later. Animals in the second group served as controls and were only exposed to the tone without shock. A third group was exposed to the same tone / shock pairing and tone as the first group while being exposed to constant background noise during the whole experiment. The results showed, that the tone / shock combination led to pronounced behavioral and cholinergic activation. In contrast, exposure to background noise prevented the increase in hippocampal ACh levels to tone / shock stimulation. The unconditioned behavioural response, however, was not prevented suggesting that hippocampal ACh is not a necessary correlate of behavioural activation or arousal. A second experiment intended to investigate the effects of background noise in a shuttle box avoidance learning paradigm where rats were trained to avoid an aversive footshock, which was signalled by a tone. There, one group of rats was exposed to background noise during avoidance learning, and the other group was not exposed to noise. Whereas both groups learned to avoid the shock to some degree over training, the noise exposed animals did not show improvement in escape performance over the course of training, indicating that the noise hindered development of an adaptive response to the shock. In summary, our data indicate that background noise can prevent increased extracellular hippocampal ACh levels in response to an aversive stimulus, and can also lead to deficits in learning to escape from shock.
2000 Elsevier Science B.V. All rights reserved.
Keywords: Hippocampus; Acetylcholine; Microdialysis; Avoidance learning; Aversive stimuli; Background noise
1. Introduction the functional role of hippocampal ACh in animals ex-posed to various experimental manipulations. A number of Pharmacological, electrophysiological and lesion studies variables have been shown to be associated with activation have implicated the septohippocampal cholinergic system of hippocampal ACh. For example, some studies describe in a variety of functions ranging from arousal and atten- a correlation between locomotor activity and increased tion to learning, memory and emotion [5,6,8,13,14, hippocampal ACh levels [11,13]. It has also been found 18,24,43,49]. With the development of in vivo micro- that simple sensory stimulation, including novel tactile, dialysis and sensitive assays, especially high-performance visual and auditory stimuli, significantly increased hip-liquid chromatography with electrochemical detection pocampal ACh [21]. Augmentation of hippocampal ACh (HPLC–ED), it has become possible to monitor extracellu- levels has also been obtained by various aversive or lar acetylcholine (ACh) levels in freely moving animals. appetitive stimuli, and with neutral stimuli which had been Using such techniques, several studies have investigated paired with an aversive stimulus [1,2,12,15,20,31, 35,36,48]. With respect to learning, increased cholinergic activation was reported in the hippocampus of rats during
*Corresponding author. General and Physiological Psychology, Phillips- performance of a discrimination learning task [51]. Using a University of Marburg, Guttenburg-str. 18, 35032 Marburg, Germany.
simpler paradigm, namely habituation learning, we found
Tel.:149-6421-282-3639; fax:149-6421-282-3610.
increased hippocampal ACh levels when rats were exposed
E-mail address: [email protected] (R.K.W.
Schwart-ing). to a novel open field, but also when they showed
havioural habituation during re-exposure to the same open The rats were anaesthetised with a combination of Ketavet field on the following day. Smaller cholinergic increases (0.9 ml / kg) and Rompun (0.4 ml / kg), and were implanted were, however, also found in a control group, which was with guide canulas, which consisted of a 15 mm thin wall handled but not exposed to the open field, indicating that stainless steel canula (22 G) with a thread in the top [7,41]. aversive aspects of handling might have contributed to The canula was aimed above the ventral hippocampus (A: increased extracellular ACh levels in this task [47]. 26.0, L:64.8, V:23.2 mm) and was secured to the skull Therefore, the present study was intended to explore in with dental cement. After surgery, the animals were more detail hippocampal cholinergic activity in relation to handled daily and allowed to recover for at least 4 days aversive stimulation using a conditioning paradigm. A first before the beginning of microdialysis.
aim was to investigate the behavioural and neurochemical
effects of aversive stimulation (a footshock), which was 2.1.2. Dialysis procedure
paired with a neutral stimulus (a tone). Rats were exposed On the day preceding the experiment, a microdialysis to a single tone / shock pairing and a tone alone 2 h later, probe of concentric design (length of membrane: 4 mm) while extracellular hippocampal ACh levels and behav- was inserted into the guide canula. The probes were ioural activity were measured. A control group was only self-manufactured as described previously [41]. After exposed to the tone. Behavioural testing in rats often insertion the probe was connected to a microinfusion pump involves a constant masking background noise during the via a liquid swivel mounted on a balanced arm above the
1
experiment. Therefore, a second aim was to investigate cage. The probe was perfused with Ringer solution (Na
1 11 2
whether such background noise might influence behav- 147 mmol, K 4 mmol, Ca 2.25 mmol, Cl 155 mmol) ioural and neurochemical responses to aversive stimulation at room temperature. In order to obtain detectable levels of in the above conditioning procedure. Thus, a third group of ACh, 0.3 mM neostigmine was added to the Ringer rats was exposed to a single tone / shock pairing and 2 h solution. After probe insertion, the animal was placed later to a tone alone while being constantly exposed to individually into the experimental cage (26328328 cm) background noise. which was situated in a sound attenuated chamber. Two The results showed that background noise prevented the speakers were mounted on top of the cage for delivery of increase in hippocampal ACh levels to the tone / shock auditory stimuli. The animal was kept under a 12 h stimulation without, however, preventing the uncon- light / dark cycle (luminous density: 60 Lux) and had free ditioned behavioural response. We therefore performed a access to food and water.
second, behavioural experiment to investigate in more On the next day, dialysis was performed between 9.30– detail the consequences of enduring background noise. 14.00 h. Samples were taken manually every 10 min. After Assuming that the septohippocampal cholinergic system is collection of three baseline samples the animals were involved in learning, we asked whether the observed lack exposed to the following procedures:
of ACh activation in noise exposed animals would also
result in reduced learning. To keep conditions similar to 2.2. Experimental manipulations the above in vivo microdialysis study, we chose shuttle
box avoidance learning, a task, where rats are trained to
escape / avoid an aversive footshock, which is signalled by • Tone / shock without background noise (n57). Rats a tone. Half of the rats were exposed to constant back- were exposed to a single tone / shock pairing (tone: ground noise during avoidance learning, while a control 1600 Hz for 3 s, 115 dB, followed immediately by a group was exposed to the learning paradigm without 60 s 0.3 mA scrambled footshock; constant current background noise. We hypothesised, that an attenuated shock generator, 521 / C, Campden Instruments; pa-cholinergic hippocampal reactivity in noise exposed ani- rameters determined by piloting) according to an mals would result in impaired active avoidance learning. aversive conditioning procedure. Two hours later, the tone was presented again without shock. This 2 h time window was chosen since hippocampal ACh levels
2. Materials and methods were expected to be at baseline 2 h after the tone / shock administration.
2.1. Experiment 1: neurochemical and behavioural • Tone control group without background noise (n55).
effects of aversive stimulation and the effects of Animals were exposed to a 3 s 115 dB tone, which
background noise was repeated after 2 h.
• Tone / shock with background noise (n510). In this 2.1.1. Subjects and surgery group, animals were treated like those in group A, i.e. Male Wistar rats (240–370 g) obtained from the Tier- exposed to a single aversive tone / shock pairing and a
¨
before beginning of dialysis (i.e. 7.30 am) and lasted analysed by means of independent t-tests. A P-value of throughout the whole testing session. 0.05 was required for significance.
2.3. Experiment 2: effects of background noise on active 2.2.1. Behavioural data analysis
avoidance learning
Behaviour was videotaped and analysed subsequently with the help of a semi-automated computer system. The
following behavioural measures were analysed at stimulus 2.3.1. Subjects
relevant time points in blocks of 10 min corresponding to Twenty male Wistar rats weighing between 270 and 330 the dialysis samples. Locomotion was scored as the g at the beginning of the experiment were used. They were number of crossings of virtual lines dividing the cage into housed individually under standard laboratory conditions four quadrants. Rearing was scored as the number of times and allowed free access to food and water. All animals the rat reared up on its hindlimbs, irrespective of whether were handled daily for 3 days prior to behavioural testing. the animal showed on- or off-wall rearing. Since the most
obvious response to our shock was twitching, this be- 2.3.2. Apparatus
haviour was scored as the time which the animal spent The shuttle box was constructed of grey plexiglas and twitching or shaking the fore- and hindlegs in response to measured 66 cm333 cm wide339 cm high. The floor was the footshock. made of 2 mm diameter stainless steel rods spaced 1.5 cm
apart. The box was divided into two equal compartments 2.2.2. Neurochemical data analysis by a 5 cm high plexiglas barrier. Each compartment could Microdialysis samples were assayed for ACh levels be electrified separately through a shock scrambler (521 / using HPLC–ED [10,41]. The chromatographic separation C, Campden Instruments). A speaker was mounted in the of ACh was achieved by a 80 mm long column filled with centre on the top of the box for delivery of auditory Chromospher 5C18 and loaded with sodium-dodecylsul- stimuli.
fate. An enzyme reactor was attached to this column,
which was filled with LiChrosorb-NH2 (activated by 2.3.3. Procedure
glutardialdehyde), and which contained 80 units of acetyl- Rats were tested under two different conditions accord-cholinesterase and 40 units of choline oxidase co-valently ing to the microdialysis experiments: One group of ani-bonded to the stationary phase. Passing the enzyme mals, termed ‘noise’ (n510), was exposed to a constant reactor, ACh was converted to hydrogen peroxide, which broadband background noise (70 dB) which started 2 h was detected at a VT-03 electrochemical flowcell (potential before beginning of behavioural testing and which lasted set at 500 mV; ANTEC Decade detector). The mobile throughout the testing session. The other group of rats, phase was composed of 0.1 M K HPO2 4 and 1 mM termed ‘no noise’ was not exposed to background noise tetramethyl-ammonium-chloride adjusted to pH 8.0 with (n510).
KH PO and delivered at a flow-rate of 0.5 ml / min. The2 4 Rats were placed in the shuttle box and allowed to freely
detection limit for ACh was approximately 15 fmol / in- explore the apparatus for 180 s. Then, they received 20 jection. The neurochemical data, which were not corrected shuttle trials, where they were trained to terminate a shock for dialysis recovery, are presented and analysed in terms by jumping over a barrier to the adjoining compartment. of percentages of baseline. Each trial began with a 3 s 115 dB tone followed by a 0.3 mA scrambled footshock according to the microdialysis 2.2.3. Histological analysis experiment. If the animal crossed the barrier during the At the end of the experiment, the animals were deeply tone, the stimulus was terminated and no shock was anaesthetised with Nembutal and perfused transcardially delivered (avoidance response). If the animal crossed the with saline followed by 10% phosphate buffered formalin. barrier during shock delivery, an escape response was The brains were removed, sliced on a cryotome and stained measured. If the rat failed to cross, the shock was with cresyl violet for analysis of probe placement. Only terminated after 20 s (escape failure). After 30 s, the next animals with correct probe placements located within the trial was initiated.
ventral hippocampus were used for further data analysis
[38,47]. 2.3.4. Data analysis
2.3.5. Statistical analysis
Escape behaviour was analysed for both groups using ANOVA for repeated measures with the blocks of trials (4 levels) as factor. Post hoc analysis of significant effects was performed using Tukey tests. A P-value of 0.05 was required for significance.
3. Results
3.1. Experiment 1: neurochemical and behavioural
effects of aversive stimulation and the effects of background noise
Fig. 2. Extracellular hippocampal ACh levels (10 min samples,
The basal concentration of hippocampal ACh
means6S.E.M.) in animals that were exposed twice to a 115 dB tone only
(mean6S.E.M.) in dialysate was 0.5460.12 for animals
(3 s duration; dashed lines). All values are expressed as percentage of
exposed to the tone / shock stimulation without background preceding baseline activity (B1–B3). noise, 0.8460.08 pmol / 20 ml for animals exposed to the
tone without background noise and 0.8960.14 pmol / 20ml
for animals exposed to the tone / shock stimulation with tone increased extracellular hippocampal ACh levels background noise. There were no statistical differences in (ANOVA F(3,11)51.14, P50.376).
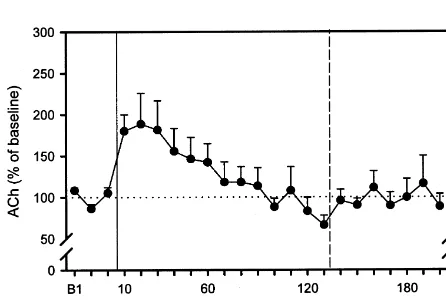
basal levels between these groups (ANOVA F(2,19)5 Extracellular ACh levels in animals that were exposed to 2.041, P50.157). a constant background noise during the conditioning Extracellular hippocampal ACh levels in animals, which procedure are shown in Fig. 3. Exposure to the tone / shock were exposed to the conditioning procedure without back- did not increase extracellular hippocampal ACh in these ground noise, are shown in Fig. 1. Exposure to tone / shock animals (ANOVA F(5,43)51.78, P50.138). Also, there stimulation significantly increased extracellular hippocam- was no effect of the tone alone presented 2 h later. pal ACh (ANOVA F(3,20)53.85, P50.025) to 180% Behavioural responses are presented in Table 1. Ani-during tone / shock application and to 189% in the sample mals, which were exposed to the tone / shock stimulation, collected after tone / shock exposure. Extracellular ACh responded to the shock with running and rearing on the values returned to baseline after approximately 1 h. The hind limbs. Note, that these behaviours displayed to the exposure to the tone 2 h later did not increase extracellular tone / shock stimulation did not differ quantitatively
be-ACh. tween animals that were exposed to noise and those that
Fig. 2 shows extracellular hippocampal ACh in control were not (t-tests, P.0.05). In both groups, there was no animals which were exposed to the tone without back- behavioural activation during presentation of the tone 2 h ground noise. Neither the first nor second exposure to the
Fig. 1. Extracellular hippocampal ACh levels (10 min samples, Fig. 3. Extracellular hippocampal ACh in animals that were exposed to a means6S.E.M.) in animals that were exposed to a tone / shock stimulation tone / shock stimulation (solid line) and a tone (dashed line) as animals in without background noise (3 s 115 dB tone, 60 s 0.3 mA scrambled Fig. 1. In contrast to those, rats were tested under conditions of constant footshock; solid line) followed by the tone only (dashed line) 2 h later. background noise (grey background) starting 2 h before and lasting All values are expressed as percentage of preceding baseline activity throughout and after administration of the stimuli. All values are
Table 1 latencies across the blocks of trials for rats which were not
a
Behavioural activation exposed to noise (n59) and noise exposed animals (n59). Tone / shock Tone Tone / shock A significant time effect for escape latencies was found in no noise no noise noise rats which were not exposed to background noise (ANOVA Locomotion 17.763.8 0.460.4 9.663.8 factor time F(3,24)54.02, P50.019), indicating that these
Rearing 3.661.3 0 3.161.5 animals improved in learning to escape from the foot-Twitching 29.662.8 0 25.264.6 shock. In contrast, rats which were exposed to noise did
a
The level of twitching and the amount of rearing and locomotion during not show a decrease in escape latencies with training the 10 min time interval of tone or tone / shock presentation. (ANOVA factor time F(3,24)50.57, P50.640). Note, that
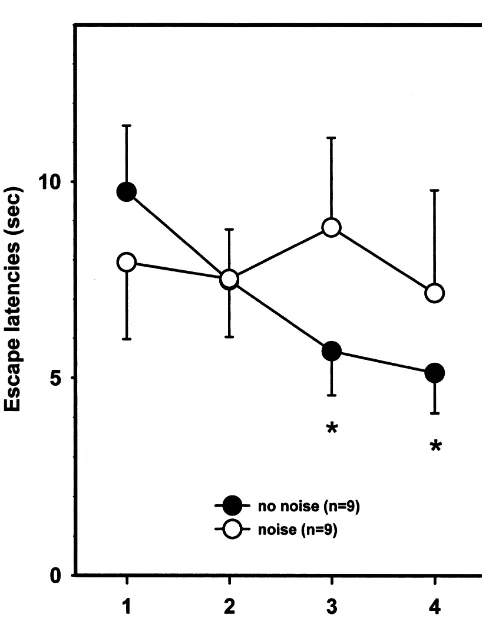
both groups of rats mostly escaped rather than avoided the later (data not shown). Animals in the tone control group shock in most trials. Nevertheless, the number of avoid-did not show measurable behavioural activation to the ance responses increased from the first to the last block of presentation of the tone. trials from 0.3 to 1.9 (animals not exposed to noise) and from 0.6 to 1.7 (animals exposed to noise; ANOVAs, no 3.2. Experiment 2: effects of background noise on active noise: F(3,24)56.97, P50.002; noise: F(3,24)53.08, P5 avoidance learning 0.046), but did not differ between the two groups. The number of escape failures did not change over trials and Two animals (one from each group) were excluded from did not differ between the two groups (data not shown). the analysis since they either jumped out of the apparatus
or sat on the barrier. Fig. 4 shows the mean escape
4. Discussion
The present results demonstrate increased extracellular hippocampal ACh levels and unconditioned behavioural activation in response to a single tone / shock stimulation. Whereas the unconditioned behavioural response to the tone / shock stimulus was also seen in rats that were exposed to background noise, there was no increase in extracellular ACh levels to the tone / shock stimulation in these animals, indicating reduced cholinergic septohip-pocampal responsiveness. An additional behavioural study which investigated the impact of background noise in a shuttle box demonstrated less efficient escape learning in noise exposed animals.
4.1. Neurochemical and behavioural effects of aversive
stimulation
4.1.1. Tone /shock without noise
The tone / shock stimulus led to an increase in extracellu-lar hippocampal ACh levels in animals that were not exposed to background noise. This confirms previous observations where increases in extracellular hippocampal ACh were reported during various forms of aversive stimulation including footshock [1,2,12,20,35,46]. Here, hippocampal ACh levels increased to the tone / shock combination, but not after presentation of the tone alone which was presented 2 h later. Possibly, the single tone / shock pairing was not sufficient for the tone to acquire
Fig. 4. Shuttle performance. Mean latency6S.E.M. to perform the correct
aversive value. The fact that these rats did not show any
response (barrier jump) in the shuttle task following the onset of the tone.
The latency is plotted in blocks of five trials. One group of animals (white behavioural reaction to the conditioned tone supports such
symbols, n59) was exposed to constant background noise 2 h before and an explanation. during shuttle avoidance whereas the other group (black symbols, n59)
was exposed to the shuttle avoidance without background noise.
Re-4.1.2. Tone without noise
sponses shown in less than 3 s reflect avoidance responses, whereas
The tone did not increase extracellular hippocampal
responses of longer latency are escape responses. * P,0.05 vs. first block
cholinergic activation to the tone / shock stimulation was exposed animals which might have influenced the ACh largely due to the shock (or the combination of tone and increase to the combined tone / shock stimulation. How-shock). In contrast, others reported behavioral activation ever, it is unlikely that the tone contributed significantly to and increases in hippocampal ACh to repeated long-lasting increased ACh levels induced by the tone / shock combina-auditory stimulation [21]. Thus, an combina-auditory stimulus, as tion, since the tone alone had no influence on ACh. such, may not be sufficient to activate hippocampal ACh,
unless it also induces behavioural activation. However, this 4.2.1.3. Pain threshold. Stress, including noise, can in-view is contradicted by the data obtained with animals duce hypoalgesia [19,45]. Although it cannot be complete-experiencing tone / shock under background noise, which ly excluded that the pain threshold was lower in rats are discussed below. exposed to background noise, the present behavioural observations argue against it. Thus, in both experiments, 4.1.3. Tone /shock with background noise control and noise exposed animals showed similar un-The finding, that background noise prevented the neuro- conditioned behavioural responses to the shock, indicating chemical but not the behavioural response to the tone / that both groups perceived the shock as aversive.
shock stimulation, indicates that hippocampal ACh activity
is not necessarily associated with unconditioned behaviour 4.2.2. Possible neurochemical mechanisms
per se. Although hippocampal ACh has often been linked While the impact of background noise on stimulus-to behavioural activity and arousal [1,11,33], there are evoked ACh activity has not been investigated before, indications that cholinergic hippocampal activation is not several studies have examined behavioural and neuro-invariably related to motor activity [32,39,41,47]. For chemical effects of noise per se. Noise has been shown to example, we previously demonstrated that behavioural act as a stressor with neuroendocrine and behavioural habituation was not accompanied by reduced hippocampal effects, including activation of adrenal steroid secretion, ACh levels [47]. Thus, the present results support the decreases of locomotor activity, consumatory behaviour, conclusion that hippocampal cholinergic activation is not a and emotional reactions [22,23,40,44]. Lai et al. found necessary concomitant of behavioural activation or arousal. increases in cholinergic muscarinic receptors and decreases in choline uptake in the hippocampus after noise exposure 4.2. Why did background noise prevent hippocampal [25,28]. Other studies showed that noise-induced changes
ACh increases to aversive stimulation? in hippocampal cholinergic activity may be mediated by corticotropin-releasing factor and endogenous opioids [25– 4.2.1. Methodological considerations 27]. Interestingly, these substances were also shown to decrease exploratory responses and to inhibit septohip-4.2.1.1. Basal ACh levels. It might be argued that the lack pocampal cholinergic activity, respectively [9,34]. Other of cholinergic activation to the tone / shock stimulation in endogenous substances which were found to reduce noise exposed animals indicates a ceiling effect, induced cholinergic activations, like galanin [16,42] or GABA [12], by higher basal ACh concentrations in noise exposed should also be taken into account. Possibly, our back-animals. This explanation is unlikely for the following ground noise might have induced physiological changes reasons. First, there were no significant differences be- via one of these mechanism, which prevented increased tween basal hippocampal ACh values in the three groups. hippocampal ACh levels in experiment 1 and reduced Second, basal extracellular ACh levels in the same mag- escape learning in experiment 2.
nitude as those observed in noise exposed animals here,
did not prevent increases in hippocampal ACh levels upon 4.3. Possible behavioural consequences of background open field exposure in a previous study [47]. Third, to noise
assess whether exposure to background noise induces long
both groups were principally able to associate the tone be carefully considered as a source of variation and with footshock, although, in both groups, the number of confounding.
avoidance responses after 20 training trials was still small. An obvious difference between control and noise exposed
animals was, however, observed in their escape latencies: Acknowledgements Rats that were exposed to background noise did not
improve their escape latencies with training, which might This work was supported by DFG grant Schw 559 / 2-2. indicate a deficit in learning to initiate an adequate
behavioural response to an aversive event. One possible explanation for the learning deficit is that the background
References
noise masked the conditioned tone stimulus, and thus hindered the development of an adequate escape reaction
[1] E. Acquas, C. Wilson, H.C. Fibiger, Conditioned and unconditioned
to the shock onset. While this cannot be ruled out entirely, stimuli increase frontal cortical and hippocampal acetylcholine it is unlikely to account for the results, since both groups release: effects of novelty, habituation and fear, J. Neurosci. 16 showed comparable avoidance responding, indicating that (1996) 3089–3096.
[2] A.M. Aloisi, F. Casamenti, C. Scali, G. Pepeu, G. Carli, Effects of
the tone acquired stimulus control in spite of background
novelty, pain and stress on hippocampal extracellular acetylcholine
noise.
levels in male rats, Brain Res. 748 (1997) 219–226.
There is some evidence, that cholinergic mechanisms
[3] A.F.T. Arnsten, P.S. Goldman Rakic, Noise stress impairs prefrontal
contribute to avoidance learning. For example, cholinergic cortical cognitive function in monkeys, Arch. Gen. Psychiatry 55 hippocampal lesions induce deficits in acquisition of (1998) 362–368.
[4] E.L. Bailey, D.H. Overstreet, A.D. Crocker, Effects of
intrahip-shuttle box avoidance [4], whereas pharmacological
en-pocampal injections of the cholinergic neurotoxin AF64A on
open-hancement of cholinergic function can improve shuttle-box
field activity and avoidance learning in the rat, Behav. Neural Biol.
avoidance learning [29,37]. Enhanced escape learning, in
45 (1986) 263–274.
the Morris water maze, was also shown in rats raised in an [5] M.G. Baxter, P.C. Holland, M. Gallagher, Disruption of decrements enriched environment which led to increased hippocampal in conditioned stimulus processing by selective removal of
hip-ChAT activity. Together, these studies suggest, that im- pocampal cholinergic input, J. Neurosci. 17 (1997) 5230–5236. [6] B.H. Bland, The physiology and pharmacology of hippocampal
paired escape learning in rats exposed to background noise
formation theta rhythms, Prog. Neurobiol. 26 (1986) 1–54.
might be mediated by changes in cholinergic mechanisms
[7] F. Boix, M. Pfister, J.P. Huston, R.K.W. Schwarting, Substance P
(see also [3]). decreases extracellular concentrations of acetylcholine in neos-Functionally, one source of explanation may lie in the triatum and nucleus accumbens in vivo: possible relevance for the
concept of ‘learned inattention’, which subsumes a number central processing of reward and aversion, Behav. Brain Res. 63 (1994) 213–219.
of paradigms, such as latent inhibition and conditioned
[8] L.S. Brito, G.N. Brito, Locomotor activity and one-way active
blocking, all of which demonstrate that pre-exposure to a
avoidance after intrahippocampal injection of neurotransmitter
an-conditioned stimulus, and contextual stimuli can influence tagonists, Braz. J. Med. Biol. Res. 23 (1990) 1015–1019. the degree to which an organism can acquire a new [9] D.R. Britton, G.F. Koob, J. Rivier, W. Vale, Intraventricular
cortico-UCS-CS connection [17,30,50]. Since the background tropin-releasing factor enhances behavioral effects of novelty, Life Sci. 31 (1982) 363–367.
noise in the above experiments contains elements of the
[10] G. Damsma, D. Lammerts van Bueren, B.H.C. Westerink, A.S.
conditioned tone stimulus, its interference with escape
Horn, Determination of acetylcholine and choline in the femtomole
learning and perhaps its suppression of the cholinergic range by means of HPLC, a post-column enzyme reactor, and activation could reflect a latent inhibition-like phenom- electrochemical detection, Chromatographia 24 (1987) 827–831.
enon. [11] J. Day, G. Damsma, H.C. Fibiger, Cholinergic activity in the rat hippocampus, cortex and striatum correlates with locomotor activity:
Together, the experiments have demonstrated that
back-an in vivo microdialysis study, Pharmacol. Biochem. Behav. 38
ground noise can: (a) prevent an increase in extracellular
(1991) 723–729.
hippocampal ACh levels in response to an aversive [12] L. Dazzi, C. Motzo, A. Imperato, M. Serra, G.L. Gessa, G. Biggio, stimulus; and (b) can impair escape learning. Perhaps, this Modulation of basal and stress-induced release of acetylcholine and
reduced cholinergic hippocampal responsiveness to aver- dopamine in rat brain by abecarnil and imidazenil, two axioselective gamma-aminobutyric acid A receptor modulators, J. Pharmacol.
sive stimulation impairs learning in tasks which require the
Exp. Ther. 273 (1995) 241–247.
initiation of an adequate behavioural response to such
[13] S.B. Dunnett, A.T. Wareham, E.M. Torres, Cholinergic blockade in
aversive events. Finally, it may be important to note that a prefrontal cortex and hippocampus disrupts short-term memory in number of behavioral studies routinely employ background rats, Neuroreport 1 (1990) 61–64.
noise as a masking stimulation. The present neurochemical [14] S.E. File, L.E. Gonzalez, N. Andrews, Endogenous acetylcholine in the dorsal hippocampus reduces anxiety through actions on nicotinic
and behavioural consequences of constant background
and muscarinic(1) receptors, Behav. Neurosci. 112 (1998) 352–359.
noise have implications for such experimental designs,
[15] C.A. Ghiani, L. Dazzi, E. Maciocco, G. Flore, G. Maira, G. Biggio,
since background noise can have powerful neurochemical Antagonism by abecarnil of enhanced acetylcholine release in the rat and behavioral effects, which may interact with both, brain during anticipation but not consumption of food, Pharmacol.
¨
[16] P. Girotti, R. Bertorelli, G. Fisone, T. Land, U. Langel, S. Consolo, [34] T. Mizuno, F. Kimura, Medial septal injection of naloxone elevates T. Bartfai, N-terminal galanin fragments inhibit the hippocampal acetylcholine release in the hippocampus and induces behavioral release of acetylcholine in vivo, Brain Res. 612 (1993) 258–262. seizures in rats, Brain Res. 713 (1996) 1–7.
[17] J.A. Gray, P.M. Moran, G. Grigorian, S.L. Peters, A.M.J. Young, [35] K. Nail-Boucherie, N. Dourmap, R. Jaffard, J. Costentin, Contextual M.H. Joseph, Latent inhibition: the nucleus accumbens connection fear conditioning is associated with an increase of acetylcholine revisited, Behav. Brain Res. 88 (1997) 27–34. release in the hippocampus of rat, Cogn. Brain Res. 9 (2000) [18] J.J. Hagan, J.D. Salamone, J. Simpson, S.D. Iversen, R.G.M. Morris, 193–197.
´ ¨
Place navigation in rats is impaired by lesions of medial septum and [36] O.G. Nilsson, P. Kalen, E. Rosengren, A. Bjorklund, Acetylcholine diagonal band but not nucleus basalis magnocellularis, Behav. Brain release in the rat hippocampus as studied by microdialysis is Res. 27 (1988) 9–20. dependent on axonal impulse flow and increases during behavioural [19] F.J. Helmstetter, P.S. Bellgowan, Hypoalgesia in response to sensi- activation, Neuroscience 36 (1990) 325–338.
tization during acute noise stress, Behav. Neurosci. 108 (1994) [37] F. Pavone, F. Capone, M. Battaglia, M. Sansone, Shuttle-box
177–185. avoidance learning in mice: improvement by combined glucose and
[20] A. Imperato, S. Puglisi-Allegra, P. Casolini, L. Angelucci, Changes tacrine, Neurobiol. Learn. Mem. 69 (1998) 204–210.
in brain dopamine and acetylcholine release during and following [38] G. Paxinos, C. Watson, The Rat Brain in Stereotaxic Coordinates, stress are independent of the pituitary-adrenocortical axis, Brain Academic Press, Sydney, 1986.
Res. 538 (1991) 111–117. [39] G. Pepeu, P. Blandina, The acetylcholine, GABA, glutamate triangle [21] F.M. Inglis, H.C. Fibiger, Increases in hippocampal and frontal in the rat forebrain, J. Physiol. Paris 92 (1998) 351–355.
cortical acetylcholine release associated with presentation of sensory [40] F. Petty, G. Kramer, L. Wilson, Y.L. Chae, Learned helplessness and stimuli, Neuroscience 66 (1995) 81–86. in vivo hippocampal norepinephrine release, Pharmacol. Biochem. [22] M.R. Irwin, D.S. Segal, R.L. Hauger, T.L. Smith, Individual Behav. 46 (1993) 231–235.
behavioral and neuroendocrine differences in responsiveness to [41] M. Pfister, F. Boix, J.P. Huston, R.K. Schwarting, Different effects audiogenic stress, Pharmacol. Biochem. Behav. 32 (1989) 913–917. of scopolamine on extracellular acetylcholine levels in neostriatum [23] R.J. Katz, K.A. Roth, B.J. Carroll, Acute and chronic stress effects and nucleus accumbens measured in vivo: possible interaction with on open field activity in the rat: implications for a model of aversive stimulation, J. Neural Transm. Gen. Sect. 97 (1994) 13–25. depression, Neurosci. Biobehav. Rev. 5 (1981) 247–251. [42] J.K. Robinson, A. Zocchi, A. Pert, J.N. Crawley, Galanin microin-[24] J.S. Kim, E.D. Levin, Nicotinic, muscarinic and dopaminergic jected into the medial septum inhibits scopolamine-induced acetyl-actions in the ventral hippocampus and the nucleus accumbens: choline overflow in the rat ventral hippocampus, Brain Res. 709 effects on spatial working memory in rats, Brain Res. 725 (1996) (1996) 81–87.
231–240. [43] R.J. Rodgers, J.C. Cole, Effects of scopolamine and its quaternary [25] H. Lai, Acute exposure to noise affects sodium-dependent high- analogue in the murine elevated plus-maze test of anxiety, Behav.
affinity choline uptake in the central nervous system of the rat, Pharmacol. 6 (1995) 283–289.
Pharmacol. Biochem. Behav. 28 (1987) 147–151. [44] K.A. Roth, R.J. Katz, Stress, behavioral arousal, and open field [26] H. Lai, M.A. Carino, Effects of noise on high-affinity choline uptake activity — a reexamination of emotionality in the rat, Neurosci.
in the frontal cortex and hippocampus of the rat are blocked by Biobehav. Rev. 3 (1979) 247–263.
intracerebroventricular injection of corticotropin-releasing factor [45] E.A. Stein, J.M. Hiller, E.J. Simon, Effects of stress on opioid antagonist, Brain Res. 527 (1990) 354–358. receptor binding in the rat central nervous system, Neuroscience 51 [27] H. Lai, M.A. Carino, Opioid receptor subtypes mediating the noise- (1992) 683–690.
induced decreases in high-affinity choline uptake in the rat brain, [46] T. Tajima, H. Endo, Y. Suzuki, H. Ikari, M. Gotoh, A. Iguchi, Pharmacol. Biochem. Behav. 42 (1992) 553–558. Immobilization stress-induced increase of hippocampal acetylcho-[28] H. Lai, M.A. Carino, Y.F. Wen, Repeated noise exposure affects line and of plasma epinephrine, norepinephrine and glucose in rats,
muscarinic cholinergic receptors in the rat brain, Brain Res. 488 Brain Res. 720 (1996) 155–158.
(1989) 61–64. [47] C.M. Thiel, J.P. Huston, R.K.W. Schwarting, Hippocampal acetyl-[29] A. Loizzo, S. Palazzesi, S. Loizzo, M. Battaglia, M. Sansone, choline and habituation learning, Neuroscience 85 (1998) 1253–
Effects of low doses of physostigmine on avoidance learning and 1263.
¨
EEG in two strains of mice, Behav. Brain Res. 81 (1996) 155–161. [48] C.M. Thiel, C.P. Muller, J.P. Huston, R.K.W. Schwarting, Effects of [30] R.E. Lubow, Latent inhibition as a measure of learned in attention: appetitive and aversive stimuli on cholinergic activity in the rat
some problems and solutions, Behav. Brain Res. 88 (1997) 75–84. forebrain. Society for Neuroscience, 465.5, 1998.
[31] G.P. Mark, P.V. Rada, T.J. Shors, Inescapable stress enhances [49] O.S. Vinogradova, E.S. Brazhnik, V.F. Kitchigina, V.S. Stafekhina, extracellular acetylcholine in the rat hippocampus and prefrontal Acetylcholine, theta-rhythm and activity of hippocampal neurons in cortex but not the nucleus accumbens or amygdala, Neuroscience 74 the rabbit-IV. Sensory stimulation, Neuroscience 53 (1993) 993–
(1996) 767–774. 1007.
[32] D. Mitsushima, C. Yamanoi, F. Kimura, Restriction of environmen- [50] I. Weiner, S. Feldon, The switching model of latent inhibition: an tal space attenuates locomotor activity and hippocampal acetyl- update of neural substrates, Behav. Brain Res. 88 (1997) 11–26. choline release in male rats, Brain Res. 805 (1998) 207–212. [51] Y. Yamamuro, K. Hori, J. Tanaka, H. Iwano, M. Nomura, Septo-[33] T. Mizuno, Y. Endo, J. Arita, F. Kimura, Acetylcholine release in the hippocampal cholinergic system under the discrimination learning rat hippocampus as measured by the microdialysis method correlates task in the rat: a microdialysis study with the dual-probe approach, with motor activity and exhibits a diurnal variation, Neuroscience 44 Brain Res. 684 (1995) 1–7.