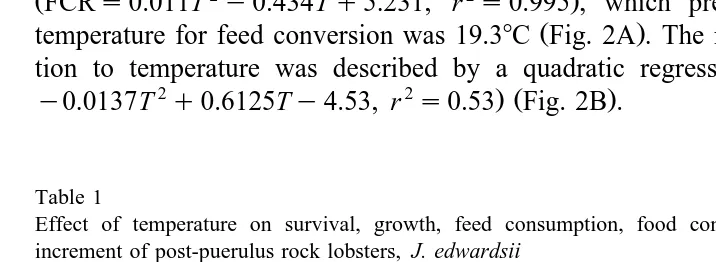www.elsevier.nlrlocateraqua-online
The effect of temperature on survival, growth,
feeding and metabolic activity of the southern rock
lobster, Jasus edwardsii
C.W. Thomas
), B.J. Crear, P.R. Hart
Tasmanian Aquaculture and Fisheries Institute, UniÕersity of Tasmania, Marine Research Laboratories,
Nubeena Crescent, Taroona, TAS, 7053, Australia
Accepted 20 October 1999
Abstract
Ž .
This study investigated the effect of temperature 18, 20, 22 and 248C on growth, feeding and metabolism of post-puerulus lobsters as part of a larger study of the culture potential of farming
Ž .
the southern rock lobster, Jasus edwardsii. There was no significant difference P)0.05 in the
Ž . Ž .
specific growth rates SGR at 18, 20 or 228C; however, growth decreased significantly P-0.05
Ž 2
at 248C. The growth response was described by a quadratic regression SGRs y0.031T q
2 .
1.261Ty10.884, r s0.77 , which predicted the optimum temperature for growth to be 20.68C. Lobsters held at 228C had smaller intermoult periods than lobsters at 188C; however, a decreased moult increment at 228C meant that growth rate did not increase at that temperature. The decreased growth at 248C compared to 228C was evidenced by a longer intermoult period. Temperature affected survival of lobsters, with survival at 248C being significantly lower ŽP-0.05 than at 18. 8C. There were no significant differences P)0.05 in survival at 18, 20 orŽ .
Ž .
228C. The response of feed conversion ratio FCR to temperature was described by a quadratic
Ž 2 2 .
regression FCRs0.011T y0.434Tq5.231, r s0.995 , which suggested that the optimum
Ž .
temperature for feed conversion was 19.38C. Oxygen consumption rates MO2 increased with increasing temperatures between 188C and 228C and declined at 248C; whilst total ammonia
Ž .
nitrogen TAN excretion increased with increasing temperature. The relationships were described
2 Ž 2 .
by regression equations: MO s y0.044T q1.91Ty18.553 r s1.0 ; TANs0.127Ty1.174 2
Ž 2 .
r s0.91 . A temperature of 248C appears to be above the upper thermal tolerance of juvenile J.
)Corresponding author. Tel.:q61-03-62-277-273; fax:q61-03-62-277-298.
Ž .
E-mail address: [email protected] C.W. Thomas .
0044-8486r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved. Ž .
Ž .
edwardsii. Lobsters held at this temperature showed reduced survival compared to those at 188C ,
Ž .
reduced growth compared to those at 228C and appeared to be suffering from respiratory stress Žmoult-related mortalities, low Q10 between 228C and 248C . Overall, the results indicate that. juvenile J. edwardsii can be cultured at 228C without adversely affecting their growth, survival or feed efficiency with optimal temperature range between 19 and 218C. q2000 Elsevier Science
B.V. All rights reserved.
Keywords: Jasus edwardsii; Temperature; Growth; Respiration; Feed conversion ratio; Ammonia excretion
1. Introduction
The southern rock lobster, Jasus edwardsii, forms the basis of an important fishery in southern Australia and New Zealand. Research has shown that the Australian and New
Ž .
Zealand stocks are the same Booth et al., 1990 . J. edwardsii commands a high market price, identifying them as a potential aquaculture species. Typical of most spiny lobsters, they have a long and complex early life history. The planktonic phyllosoma stage is spent dispersed by oceanic currents for a period of 8–16 months before settling as
Ž .
puerulus in shallow coastal waters Kennedy, 1990 . Presently, it is not feasible to commercially produce puerulus in hatcheries; therefore, initial industry development is focussing on the collection and ongrowing of recently settled puerulus. One of the important variables that will affect the efficiency of J. edwardsii culture is temperature. Temperature is one of the major environmental factors affecting the growth of
Ž .
crustaceans reviewed by Hartnoll, 1982 . Growth rate has been shown to increase with increasing temperature to a maximum, before declining near the upper thermal limits
ŽChittleborough, 1975 . Elevated temperature could potentially reduce the culture period,. Ž
which would be important for the economic viability of J. edwardsii culture Hooker et
.
al., 1997 . The optimum temperature range suggested for culture of J. edwardsii is
Ž .
between 18–208C Booth and Kittaka, 1994 . However, considerable variation in results has been obtained at similar temperatures. It is unclear whether this variation is a result
Ž
of different husbandry practices or from intraspecific variations Sastry and Vargo,
. Ž .
1977 . For example, Bunter and Westaway 1993 found reduced survival of juvenile
Ž .
lobsters at 188C compared to lower ambient temperatures 10–168C in Tasmania.
Ž .
However, Manuel 1991 found that 188C did not adversely effect survival of the same species collected in southern New Zealand. Previous studies also indicated that growth
Ž . Ž .
rate was reduced at 228C Hollings, 1988; Manuel, 1991 ; while Hooker et al. 1997 reported increased mortalities associated with water temperatures of 23.38C. Defining the upper thermal limits will be important for site selection and the most appropriate culture temperature. In addition, few data are available on the influence of temperature on feed consumption, food conversion ratio, oxygen consumption or ammonia excretion of juvenile J. edwardsii. This will have important implications for culture in regards to feeding costs and systems management.
The aim of this study was to determine the effect of elevated temperature on survival, growth, feeding, oxygen consumption and ammonia excretion of post-puerulus J.
edwardsii, and to identify the upper thermal limit and the most efficient temperature for
2. Materials and methods
Pueruli of J. edwardsii were collected from experimental collectors located at Bicheno on the east coast of Tasmania. They were maintained in a recirculating system at 188C before the commencement of the trials.
Trials were conducted in a flow-through system consisting of 4=280-l reservoir
Ž .
tanks and 12=33-l black fibreglass tanks diameter 430 mm, depth 300 mm . Incoming water was filtered to 400 mm before entering the reservoir tanks. The reservoir tanks were maintained at precise temperatures of 18, 20, 22 or 248C using 1.2–2.4 kW titanium heaters. Temperatures in the tanks were monitored at 10-min intervals by a
Ž .
Vemco minilog data logger TR data loggers . Temperatures were also manually checked each day. Each heated reservoir tank was connected to three replicate experi-mental tanks. The flow to each tank enabled a complete water exchange every 30–40 minutes. An 8-h light:16 h dark photoperiod was maintained with light intensity of 1
mmol sy1
my2
at the water surface.
Ž .
Thirteen post-pueruli initial mean weight"SEs0.99"0.01 g were stocked into each 33-l tank and they were temperature-acclimatised from 188C over 5 days before the first weight measurement. During the first week after the initial weight measurement, mortalities were replaced with temperature-acclimatised animals of similar weight. These mortalities were assumed to be due to stresses associated with handling and were not included in the final mortality data.
The juvenile lobsters were fed once daily with either freshly opened mussels cultured
ŽMytilus edulis planulatus or dry growout pellets for penaeids Penaeus japonicus .. Ž .
Previous studies indicated that fast growth and high survival could be achieved on a
Ž .
mixed diet of fresh mussels and dry pellets Crear et al., 1999 . An estimation of daily food consumption was obtained via a visual estimate of food remaining in the tanks before morning cleaning. Food was provided slightly in excess and was based on the previous days’ consumption. Feed consumption was calculated on dry matter consumed per wet weight biomass and expressed as a percentage of wet lobster body weight per day. Samples of mussels and the dry diet were dried to give an estimate of moisture content. To take into account mortalities, the number of lobster days of feeding was
Ž .
calculated based on survivors at each weighing and used to calculate daily weight gain
Ž .
and feed intake for each lobster. Food conversion ratios FCR were calculated as the
Ž .
estimated dry weight of feed consumed per day g per lobster wet weight increase per
Ž .
day g .
Lobsters were weighed to the nearest 0.01 g at days 0, 29, 59 and 92. Animals were dried on absorbent paper for 30 s to remove excess water before weight measurement.
Ž . Ž .
Specific growth rate SGR , percentage weight gain % WG , moult increment and moult interval were used to evaluate growth. Moult interval and moult increment calculations were based on data collected between the second and third weight measure-ments, as there were no mortalities during that period. These parameters were defined
Ž .) Ž
as: SGRs ln final weightyln initial weight 100rnumber of days; % WGs final
.)
At the completion of the growth trial, lobsters were starved for a period of 60 h before oxygen consumption trials at the experimental temperatures. The experimental system described above was supplied with 0.2 mm filtered seawater. A total of 48
Ž .
animals 12 per treatment in the intermoult stage were selected and weighed and placed
Ž .
into brown glass BOD bottles 755 ml . Animal weights ranged between 2.4 and 7.8 g.
Ž .
Twelve respirometers three per treatment had no animals and served as controls. The respirometers were immersed in the experimental tanks that acted as temperature baths. High oxygen saturation levels within the respirometers were maintained during the 16-h acclimatisation period by providing flow-through filtered seawater. An initial oxygen concentration measurement was recorded before the respirometers were sealed. Oxygen concentration was recorded after 60 min. The respirometers were then provided with flow-through water for 3 h to reoxygenate the water before the experimental procedure was repeated. During the measuring period, the oxygen concentration did not
y1 Ž y1.
fall below 4.0 mg l . Standard oxygen consumption MO2mg min was determined by the equation:
the volume of water in respirometer ml and T is the elapsed time in minutes.
At the completion of the oxygen consumption trial, 20 ml water samples were taken
Ž
from the respirometers and stored aty188C until total ammonia nitrogen TANsNH3 q.
qNH4 analyses were conducted. Water from the control respirometers was used to determine initial ammonia concentration. TAN was measured using the
phenol–hypo-Ž .
chlorite method using procedures outlined by Parsons et al. 1984 . Ammonia excretion
Ž y1 y1.
TANymg TANrg h was determined from the following equation:
TANyTAN )V
Ž
f i.
TANs
W)T
where TAN is the ammonia as nitrogen in the sample at the end of the measuringf
period in mg ly1, TAN is the ammonia as nitrogen in the sample at the beginning of the i
sampling period in mg ly1
, V is the volume of water in the container in litres, W is the weight of the lobster in grams and T is the time of the measuring period in minutes.
Q10 values for oxygen consumption and ammonia excretion were determined using the following equation:
10rT2yT1 M2
Q10s
ž /
M1
where M and M1 2soxygen consumption at temperatures T and T , respectively.1 2 All data were tested by ANOVA with comparisons of means following ANOVA using the Scheffe post-hoc test. Survival data were arcsine-transformed before analysis
ŽSokal and Rohlf, 1995 . The growth and survival data were plotted and curvilinear.
ammonia excretion. Regressions were tested for significance by analysis of variance. The optimal response to temperature was predicted as the upper or lower asymptote of
Ž .
the best-fitting quadratic response curve Lellis and Russell, 1990 .
3. Results
Ž .
The final mean weight and SGR at 228C were significantly higher P-0.05 than at
Ž .
248C Table 1 . No significant differences in growth were determined among 18, 20 or
Ž 2
228C. The quadratic regression of temperature on SGR SGRs y0.031T q1.261Ty
2 . Ž .
10.884, r s0.77 Fig. 1A predicted the optimum temperature for growth to be 218C. The intermoult period decreased as temperature increased, with lobsters at 228C
Ž . Ž .
having a significantly lower P-0.05 intermoult period to those at 188C Table 1 . At
Ž .
248C, the intermoult period increased again and was significantly higher P-0.05 than at 228C. The relationship between temperature and intermoult period was described by a
Ž 2 2 . Ž
quadratic regression Intermoult periods1.134T y48.056Tq532.99, r s0.61 Fig.
.
1B . No significant difference was detected in percentage moult increment at the four
Ž .
temperatures Table 1 . The relationship between percentage moult increment and
Ž 2
temperature was described by a quadratic regression Moult increments0.625T y
2 .
30.55Tq433.3, r s0.99 showing that as temperature increased, there was a reduction
Ž .
in moult increment Fig. 1C .
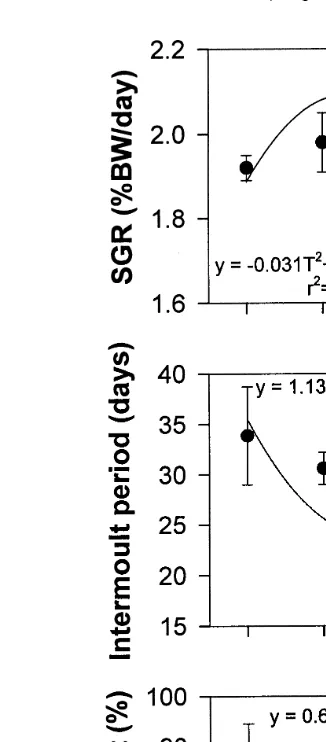
The effects of temperature on the FCR and feed consumption are shown in Table 1
Ž .
and Fig.2. No significant differences P)0.05 in FCR or feed consumption were detected. The response of FCR to temperature was described by a quadratic regression
ŽFCRs0.011T2y0.434Tq5.231, r2s0.995 , which predicted that the optimum.
Ž .
temperature for feed conversion was 19.38C Fig. 2A . The response of feed
consump-Ž
tion to temperature was described by a quadratic regression Feed consumptions
2 2 . Ž .
y0.0137T q0.6125Ty4.53, r s0.53 Fig. 2B .
Table 1
Ž .
Effect of temperature on survival, growth, feed consumption, food conversion ratio FCR and moult increment of post-puerulus rock lobsters, J. edwardsii
Ž .
Values are expressed as mean "SE . Values in the same row with same superscript are not significantly
Ž .
Total moults 30.33"1.86 29.67"1.45 46.33"4.10 23.67"2.03
Ž .
Moult increment % 86.50"8.50 72.00"1.00 63.67"3.68 60.00"3.00
a a,b b a
Ž .
Intermoult period days 33.85"4.85 30.60"1.60 19.80"0.76 34.69"1.55
Ž .
Ž . Ž . Ž .
Fig. 1. Growth of J. edwardsii versus temperature mean"SE . A Specific growth rate. B Intermoult
Ž .
period. C Moult increment.
Temperature affected survival of lobsters, with survival at 248C being significantly
Ž . Ž . Ž .
lower P-0.05 than at 188C Table 1 . There were no significant differences P)0.05 in survival at 18, 20 or 228C. Most mortalities at 248C occurred as lobsters approached the moult, whilst most mortalities at the other temperatures occurred due to cannibalism immediately after the moult.
Ž .
As expected, body weight significantly influenced P-0.01 oxygen consumption
Ž . Ž .
rate MO2 of lobsters at all temperatures Table 2 . The exponent b value of the
Ž y1.
Log -transformed linear regressions relating oxygen consumption10 mgrmin to body
Ž .
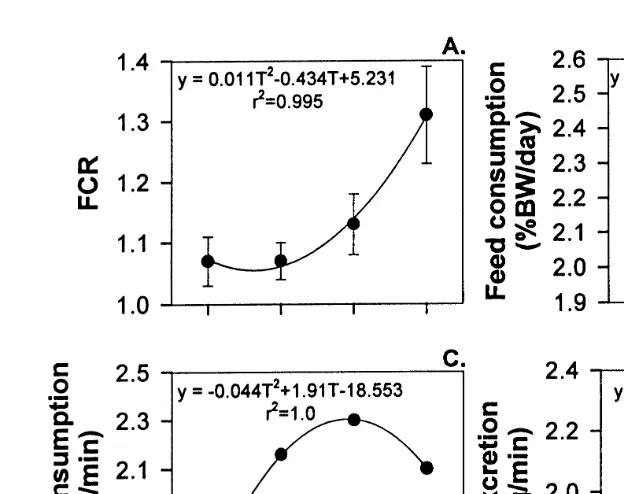
Fig. 2. Feeding, oxygen consumption rate and ammonia excretion rate of J. edwardsii versus temperature
Žmean"SE . A Feed conversion ratio. B Feed consumption % BW. Ž . Ž . Ž rday . C Oxygen consumption rates.. Ž . Ž .D Total ammonia nitrogen TAN excretion rates.Ž .
at 208C and 228C, b was less than 1 and weight-specific rate decreased with increasing body weight. To take into account the oxygen consumption variation due to body weight, the oxygen consumption data were weight-standardised at each temperature
ŽTable 3 . The standardised oxygen consumption data were then plotted against tempera-.
Ž .
ture Fig. 2C . There was a significant affect of temperature on oxygen consumption
ŽFs5727, P-0.01 , which was described by a quadratic regression. ŽMO2s
Table 2
Ž y1.
Linear regressions describing the relationship between total oxygen consumption MO2: mg min , total
Ž y1. Ž .
ammonia excretion TAN:mg min and body weight W: g of J. edwardsii at four temperatures 2
Ž .
Temperature 8C n Regression model r F P
18 19 Log MO2s1.515 Log10Wy3.138 0.74 48.7 -0.0001
20 18 Log MO2s0.860 Log10Wy2.597 0.80 63.9 -0.001
22 16 Log MO2s0.692 Log10Wy2.431 0.50 12.1 -0.004
24 23 Log MO2s1.091 Log10Wy2.741 0.72 54.8 -0.001
18 10 Log TANs0.794 Log10Wq0.229 0.75 18.3 -0.005
20 11 Log TANs0.865 Log10Wq0.185 0.74 19.9 -0.003
22 10 Log TANs0.187 Log10Wq0.772 0.28 3.07 0.118
Table 3
The standardised weight-specific oxygen consumption and ammonia excretion rates of J. edwardsii at each temperature
The rate data were standardised using the regression equations describing the relationship between body
Ž .
weight and oxygen consumptionrammonia excretion Table 2 . The Q10 values for each temperature range
Ž28C are also shown..
y0.044T q1.91Ty18.553, rs1.0 . Oxygen consumption increased rapidly with temperature up to 228C, with a decline at 248C. The Q10 values for oxygen consumption
Ž . Ž .
decreased with increasing temperatures Table 3 , ranging from 3.7 18–208C to 0.6
Ž22–248C ..
Ž y1.
The Log -transformed linear regressions relating TAN excretion10 mgrmin to
Ž .
body weight g at each temperature are shown in Table 2. The ammonia excretion rate
Ž .
was influenced by body weight at 188C and 208C P-0.01 , but not at 228C or 248C. The ammonia excretion data were standardised as for the oxygen consumption data
ŽTable 3 and then plotted against temperature Fig. 2D . Ammonia excretion increased. Ž .
Ž .
significantly Fs19.14, P-0.05 with temperature and was described by a linear
Ž 2 .
equation TANs0.127T–1.174, r s0.91 . The Q10 value for ammonia excretion was
Ž .
low 1.1 as the temperature increased from 188C to 208C, but was higher at the upper
Ž .
temperature ranges Table 3 .
4. Discussion
Previous studies have suggested that the optimum temperature range for the culture of
Ž
J. edwardsii is between 188C and 208C Hollings, 1988; Manuel, 1991; Booth and
. Ž .
Kittaka, 1994 . Overall, the calculated optimum temperatures for growth 20.68C and
Ž .
feed efficiency 19.38C from this study support this range. The results of this study also indicate that lobsters collected from the east coast of Tasmania can be cultured at
Ž .
elevated temperatures up to at least 228C after a period of acclimation without affecting growth or survival. The reduced growth, survival and declining oxygen consumption at 248C are indications that J. edwardsii is approaching its upper thermal limits.
Low growth and survival had previously been reported at 228C for J. edwardsii
Ž .
collected from New Zealand Hollings, 1988; Manuel, 1991 . It has been shown that crustaceans of the same species, but collected from geographically different locations,
Ž
exhibit differences in response to the same environmental conditions Sastry and Vargo,
.
Ž .
However, the poor performance at 228C recorded by Hollings 1988 may also be related to the reduced water quality and temperature fluctuations noted in the trial. Lellis and
Ž .
Russell 1990 suggested that the thermal preferences for growth of Panulirus argus may differ with development stage. The effects of temperature on J. edwardsii at other development stages also need to be examined.
Growth rate is a function of moulting frequency and the weight increment with each moult. Typically, there is a reduction in the intermoult period and the moult increment
Ž .
as temperature increases Hartnoll, 1982 . Both these components of growth were strongly influenced by temperature in this trial and were reflected in the growth rates observed at 188C and 228C. Although the intermoult period was reduced at 228C, growth rate did not increase because of the decreased moult increment. A reduced intermoult period and moult increment were also observed in P. longipes as the temperature
Ž .
increased from 208C to 268C Chittleborough, 1975 , although the reduction in moult increment was not enough to prevent increased growth at 268C. Temperature did not
Ž . Ž
affect the moult increments of P. interruptus 228C and 288C or P. cygnus 278C and
.
308C and the observed accelerated growth at the higher temperatures was driven by
Ž .
reduced intermoult periods Serfling and Ford, 1975; Lellis and Russell, 1990 .
As temperature approached the upper thermal limit of J. edwardsii, the effect of temperature on the components of growth varied. Growth was reduced at 248C, because the intermoult period increased, while the moult increment stayed the same at 228C. A similar result was observed with P. longipes as the temperature increased from 268C to
Ž . Ž .
298C Chittleborough, 1975 . Conversely, Manuel 1991 found that the reduced growth of juvenile J. edwardsii at higher temperatures was due to a smaller moult increment,
Ž .
with the intermoult period not being affected. Lellis and Russell 1990 found that moult increment decreased and intermoult period increased at higher temperatures. The effect of temperature on the components of growth appear to vary with lobster species, as
Ž .
noted for many crustaceans Hartnoll, 1982 .
Food consumption generally increases with temperature in response to increased
Ž .
metabolic activity Wyban et al., 1995 , although it was not apparent in this study. The growth rates achieved in this study exceed those reported in other studies of J.
Ž
edwardsii Hollings, 1988; Rayns, 1991; Bunter and Westaway, 1993; Hooker et al.,
.
1997 . Growth rates of around 2% BWrday and FCRs between 1.1 and 1.3 support the nutritional suitability of a mixed diet containing fresh mussels and prawn dry growout
Ž .
pellets for post-puerulus J. edwardsii Crear et al., 1999 .
The oxygen consumption rate of J. edwardsii is similar to that for other decapod
Ž
crustaceans of comparable size Weins and Armitage, 1961; McLeese, 1964; Chen and
.
Kou, 1996 . Oxygen consumption rates increased with increasing temperature with a decrease occurring at 248C. Similar respiratory responses for crustaceans at elevated
Ž
temperatures have been reported Rutledge and Pritchard, 1981; Zoutendyk, 1989; Varo
. Ž .
et al., 1991 . Vernberg 1983 described this decline in respiration at elevated tempera-tures as a strategy for metabolic regulation in energy conservation.
The positive correlation between increasing respiration rate and weight in this study
Ž
has been well documented for other crustaceans Wolvekamp and Waterman, 1960;
. Ž .
Cockcroft and Wooldridge, 1985 . Weymouth et al. 1944 gave the average value of b
Ž .
and found that they typically ranged between 0.286 and 0.877 at temperatures 8.5–
Ž . Ž .
17.88C. The b values from this study 0.86 208C and 0.69 228C are within the
Ž .
generally accepted range. However, b values at 188C and 248C are high, cf. Rao 1957 who found b of 1.05 for Metapenaeus monoceros.
Ž
Q10 typically varies between 2 and 3 near optimal temperatures Wolvekamp and
.
Waterman, 1960 and the Q10 for oxygen consumption recorded for this study for
Ž
18–228C fits within this range. A similar Q10 of 2.3 was recorded for larger 186–2180
. Ž .
g J. edwardsii between 178C and 218C Crear, 1998 . The increase in metabolic rate could not be sustained at 248C, resulting in a Q10 value of less than 1 for the 22–248C temperature range. Q10 generally decreases as temperature moves towards the thermal
Ž . Ž .
maximum Varo et al., 1991 . Zainal et al. 1992 also reported Q10 values less than 1 for squat lobsters, Munida sarsi and Munida rugosa, at the temperature maximum.
Mortalities at 248C appeared to be stress-related, mostly occurring during the
Ž .
pre-moult period. Penkoff and Thurberg 1982 have shown that the American lobster,
Homarus americanus, has an increased oxygen requirement during the pre-moult period.
Additionally, the availability of oxygen decreases as water temperature increases. This suggests that the mortalities at 248C in this study may have been due to respiratory failure caused by the inability of lobsters to meet the increased oxygen requirements of moulting. Respiratory stress leading to mortality at the moult has been reported for the Western rock lobster, P. cygnus, and the South African rock lobster, J. lalandii, when
Ž .
lobsters were cultured at low oxygen levels Chittleborough, 1975; Beyers et al., 1994 . Under intensive culture conditions, stocking levels will be governed by the systems capacity to provide oxygen for metabolic activity. The implications for systems design are that animals maintained at 228C will consume approximately 40% more oxygen than
Ž
those at 188C. However, water holds 6.5% less oxygen at 228C 35 ppt and 100%
. Ž .
saturation . The equations Figs. 1 and 2 relating temperature, body weight and oxygen consumption can assist in determining the appropriate stocking levels and flow rates at a particular temperature based on the oxygen requirements. The effects of activity and feeding on oxygen consumption will require further investigation.
Ammonia excretion rates increased in response to increasing temperature, a response
Ž
that has been documented for crustaceans Regnault, 1987; Villarreal and Rivera 1993;
.
Chen and Kou, 1996; Crear, 1998 . Ammonia excretion was influenced by body weight
Ž . Ž .
particularly at the lower temperatures. The exponent b at 188C 0.794 and 208C 0.865
Ž
were consistent with b values of 0.88 for Xiphopenaeus kroyeri Carvalho and Phan,
. Ž .
1997 and 0.75 for P. japonicus Marangos et al., 1990 . However, b values were low at 228C and 248C. Ammonia excretion accounts for approximately 80% of the nitrogenous
Ž .
excretion in J. edwardsii Crear, 1998 . Ammonia has been shown to decrease growth and cause mortality in aquatic organisms, and recommended levels of total ammonia are
y1Ž .
generally below 0.5 mg l Forteath, 1990 . Therefore, the rate of ammonia production in intensive recirculating systems is of primary concern for lobster culturists. These preliminary results will assist in calculating the ammonia excretion rates of small J.
edwardsii and the biofiltration requirements for ammonia removal. Feeding has been
Ž .
In conclusion, this study shows that a temperature range of between 198C and 218C is optimal for post-puerulus J. edwardsii in terms of survival, growth and FCR. At higher temperatures, the measured performance criteria were reduced and the upper thermal limit appeared to be near 248C. Culturists would need to consider the economic advantages of lobsters reaching market size in the shortest possible time against the increased costs associated with heating water. In addition, lobsters at higher tempera-tures have greater respiratory requirements and excrete greater amounts of ammonia. If lobsters are to be cultured at elevated temperatures in intensive recirculating systems, then there will be even greater reliance on water treatment, to ensure water quality is not limiting growth. Finally, if lobsters are to be grown in flow-through systems, or in cages
Ž .
in the sea Hollings, 1988 , these results indicate that it will be necessary to select sites where summer water temperatures do not rise above 228C.
Acknowledgements
Ž .
We wish to thank Kirsten Rough Tuna Boat Owners Association and Dr. John
Ž .
Gunn CSIRO for temperature data loggers and Mr. Alan Beech for technical assis-tance. The project was partially funded by the Fisheries Research and Development
ŽProject No. 98.305 ..
References
Beyers, C.J., De, B., Wilke, C.G., Goosen, P.C., 1994. The effects of oxygen deficiency on growth, intermoult period, mortality and ingestion rates of aquarium-held juvenile rock lobster Jasus lalandii. S. Afr. J. Mar. Sci. 14, 79–87.
Booth, J.D., Kittaka, J., 1994. Growout of juvenile spiny lobsters. In: Phillips, B.F., Cobb, J.S., Kittaka, J.
ŽEds. , Spiny Lobster Management. Blackwell Scientific Fishing News Book, pp. 424–445..
Booth, J.D., Street, R.J., Smith, P.J., 1990. Systematic status of the rock lobster Jasus edwardsii from New Zealand and J. noÕaehollandie from Australia. N. Z. J. Mar. Freshwater Res. 24, 239–249.
Bridges, C.R., Brand, A.R., 1980. Oxygen consumption and oxygen-independence in marine crustaceans. Mar. Ecol. Prog. Ser. 2, 133–141.
Ž .
Bunter, O., Westaway, C., 1993. Growth rates of juvenile lobsters. Austasia Aquaculture 7 1 , 46–49. Carvalho, P.S.M., Phan, V.N., 1997. Oxygen consumption and ammonia excretion of Xiphopenaeus kroyeri
Ž .
Heller Penaeidae in relation to mass temperature and experimental procedures. Shrimp oxygen uptake and ammonia excretion. J. Exp. Mar. Biol. Ecol. 209, 143–156.
Chen, J., Kou, T., 1996. Effects of temperature on oxygen consumption and nitrogenous excretion of juvenile Macrobrachium rosenbergii. Aquaculture 145, 295–303.
Chittleborough, R.G., 1975. Environmental factors affecting growth and survival of juvenile western rock
Ž .
lobsters Panulirus longipes Milne-Edwards . Aust. J. Mar. Freshwater Res. 26, 177–196.
Cockcroft, A.C., Wooldridge, T., 1985. The effects of mass, temperature and moulting on the respiration of
Ž . Ž .
Macropetasma africanus Balss Decapoda: Penaeoidea . Comp. Biochem. Physiol. 81A 1 , 143–148. Crear, B., Thomas, C., Hart, P., 1999. An evaluation of the suitability of artificial prawn diets for the southern
rock lobster, Jasus edwardsii. World Aquaculture ’99. Book of Abstracts. The Annual International Conference and Exposition of the World Aquaculture Society, 26 April–2 May 1999, Sydney, Australia. World Aquaculture Society, p. 183.
Forteath, N., 1990. In: A Handbook on Recirculating Systems for Aquatic Organisms. Fishing Industry Training Board of Tasmania, p. 72.
Ž .
Hartnoll, R.G., 1982. Growth. In: The Biology of Crustacea. Bliss, D.E. Ed. , Embryology, Morphology and Genetics 2 Academic Press, pp. 111–196.
Hollings, T., 1988. In: Aquaculture of the NZ rock lobster. New Zealand Fishing Industry Training Board, p. 51.
Hooker, S.H., Jeffs, A.G., Creese, R.G., Sivaguru, K., 1997. Growth of captive lobsters Jasus edwardsii
ŽHutton. ŽCrustacea: Palinuridae in north-eastern New Zealand. Mar. Freshwater Res. 48, 903–909.. Ž .
Kennedy, B., 1990. Juvenile crays: where do they settle and when? Fishing Today 13 6 , 22–23.
Lellis, W.A., Russell, J.A., 1990. Effect of temperature on survival, growth and feed intake of postlarval spiny lobsters, Panulirus argus. Aquaculture 90, 1–9.
Manuel, S., 1991. Factors affecting the growth rate of juvenile Jasus edwardsii reared in captivity. MSc Thesis, University of Otago, Dunedin, New Zealand, 129 pp.
Marangos, C., Alliot, E., Brogen, C., Ceccaldi, H.J., 1990. Nycthemeral variations of ammonia excretion in
Ž .
Penaeus japonicus Crustacea, Decapoda, Penaeidae . Aquaculture 84, 383–391.
McLeese, D.W., 1964. Oxygen consumption of the lobster Homarus americanus Milne-Edwards. Helgol. Wiss. Meeresunters. 10, 7–18.
Parsons, T.R., Maita, Y., Lali, C.M., 1984. In: A Manual of Chemical and Biological Methods for Seawater Analysis. Permagon Press, Oxford, p. 173.
Penkoff, S.J., Thurberg, F.P., 1982. Changes in oxygen consumption of the American Lobster, Homarus americanus, during the molt cycle. Comp. Biochem. Physiol. 72A, 621–622.
Rao, K.P., 1957. Oxygen consumption as a function of size and salinity in Metapenaeus monoceros Fab. from marine and brackish water environments. J. Exp. Biol. 35, 307–313.
Regnault, M., 1987. Nitrogen excretion in marine and freshwater Crustacea. Biol. Rev. 62, 1–24.
Rutledge, P.S., Pritchard, A.W., 1981. Scope for activity in the crayfish Pacifastacus leniusculus. Am. J. Physiol. 240, R87–R92.
Sastry, A.N., Vargo, S.L., 1977. Variations in the physiological response of crustacean larvae to temperature.
Ž .
In: Vernberg, F.J., Calabrese, A., Thurberg, F.P., Vernberg, W.B. Eds. , Physiological Response of Marine Biota to Pollutants. Academic Press, New York, pp. 401–424.
Serfling, S.A., Ford, R.F., 1975. Laboratory culture of juvenile stages of the California spiny lobster Panulirus
Ž .
interruptus Randall at elevated temperatures. Aquaculture 6, 377–387.
Sokal, R.R., Rohlf, F.J., 1995. In: Biometry: The Principles and Practice of Statistics in Biological Research. W.H. Freeman and Company, New York, p. 887.
Varo, I., Taylor, A.C., Navarro, J.C., Amat, F., 1991. Effects of temperature and oxygen tension on oxygen consumption rates of nauplii of different Artemia strains. Mar. Ecol. Prog. Ser. 76, 25–31.
Ž .
Vernberg, F.J., 1983. Respiratory adaptions. In: Vernberg, F.J., Vernberg, W.B. Eds. , The Biology of Crustacea 8 Academic Press, New York, pp. 1–42, Environmental Adaptations.
Villarreal, H., Rivera, J.A., 1993. Effect of temperature and salinity on the oxygen consumption of laboratory
Ž .
produced Penaeus californiensis postlarvae. Comp. Biochem. Physiol. 106A 1 , 103–107.
Weins, A.W., Armitage, K.B., 1961. The oxygen consumption of the crayfish Orconectes immunis and Orconectes nais in response to temperature and to oxygen saturation. Physiol. Zool. 34, 39–54. Weymouth, F.W., Crimson, J.M., Hall, V.E., Belding, H.S., Field, J., 1944. Total and tissue respiration in
relation to body weight; a comparison of the kelp crab with other crustaceans and with mammals. Physiol. Zool. 17, 50–71.
Ž .
Wolvekamp, N.R., Waterman, T.H., 1960. Respiration. In: Waterman, T.H. Ed. , The Physiology of Crustacea 1 Academic Press, New York, pp. 35–91.
Wyban, J., Walsh, W.A., Godin, D.M., 1995. Temperature effects on growth, feeding rate and food conversion
Ž .
of the Pacific white shrimp PenaeusÕannamei . Aquaculture 138, 267–279.
Zainal, K.A.Y., Taylor, A.C., Atkinson, R.J.A., 1992. The effect of temperature and hypoxia on the respiratory
Ž .
physiology of the squat lobsters, Munida rugosa and Munida sarsi Anomura, Galatheidae . Comp.
Ž .
Biochem. Physiol. 101A 3 , 557–567.