APETALA3-nuclease hybrid protein: a potential tool for
APETALA
3 target gene mutagenesis
Patricia Lariguet, Christophe Dunand, Michel Herzog, Gilles Vachon *
Laboratoire de Ge´ne´tique Mole´culaire des Plantes,CNRS UMR5575,Uni6ersite´ Joseph Fourier,CERMO B.P.53,
F-38041Grenoble Cedex9,France
Received 7 April 1999; received in revised form 10 May 1999; accepted 31 May 1999
Abstract
Direct target genes of homeotic genes are largely unknown in plants. The class B homeotic geneAPETALA3 (AP3) is required for petal and stamen identities. TheAP3 gene encodes a MADS-domain containing protein which forms heterodimers in vitro with the second class B homeotic proteinPISTILLATA(PI). Here, we describe a new strategy that can be used to isolate mutants of genes that are immediate targets of AP3 or AP3/PI. The strategy is based on providing a nuclease activity to AP3 by translationally fusing the FN nuclease domain of the FokI restriction enzyme. In electro-mobility shift assays, AP3-FN/PI
heterodimers display the same binding specificity for CArG-box elements as AP3/PI heterodimers, although with a lower affinity. Transgenic lines carrying theAP3-FNfusion gene under control of the 35S promoter were obtained. The 35S::AP3-FNconstruct
is able to partially suppress theap3-1 mutant phenotype showing that the AP3 part of the hybrid protein is functional in vivo. When crossed with the DNA-break repair deficient mutantu6h1, offspring were obtained that showed, to various degrees, a lack
of fertility consistent with the role ofAP3 in gamete development. The mutant phenotypes are inherited to the next generation. This is the first report of a strategy designed to create mutants of genes directly regulated by a homeotic gene. © 1999 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:APETALA3; Homeotic genes;FokI enzyme; Hybrid protein; Target genes
www.elsevier.com/locate/plantsci
1. Introduction
Homeotic mutations lead to the conversion of a particular body part or organ into another. Home-otic mutants have been described in both animals
and plants. In Arabidopsis thaliana, homeotic
mu-tations in flower development have been
inten-sively studied. Flowers of Arabidopsis are
composed of four concentric whorls of organs with, from the outer to the innermost whorl, four sepals, four petals, six stamens and two fused carpels. The ‘ABC’ model [1,2] describes how three genetic functions called A, B and C, each represented by one or several genes, specify each four whorl identity by overlapping and
combina-torial actions. The class A genes function in whorls 1 and 2, the class B in whorls 2 and 3, the
class C in whorls 3 and 4. Nearly all Arabidopsis
homeotic genes belong to the family of MADS-do-main transcription factors [3]. The MADS doMADS-do-main encodes a 56 amino acid domain homologous to the DNA-binding domain of several yeast and mammalian proteins and recognises in vitro
CArG-box containing sequences (CC(A/T)6GG)
[4].
Homeotic genes are thought to specify the iden-tity of a body structure by controlling the localised expression of subordinate target genes, which are themselves responsible for specific morphogenesis. Very little information is available concerning the identity and function of genes acting downstream in plant homeotic pathways and even less is known about target genes directly regulated by
* Corresponding author. Tel.: +33-476635658; fax: + 33-476514336.
E-mail address:[email protected] (G. Vachon)
homeotic proteins. It is of importance to identify these direct target genes in order to identify func-tions necessary to translate the positional informa-tion delivered by homeotic genes in various
morphogenetic programmes. Molecular
ap-proaches have been employed in animals and plants to identify target cDNAs. Direct incubation of chromatin with homeotic proteins such as UL-TRABITHORAX (UBX) in Drosophila [5,6] or
AGAMOUS (AG) inArabidopsis[7] has permitted
the isolation of several cDNAs. Another indirect approach, based on the expression pattern of known MADS-box genes, has led to the identifica-tion of several cDNAs expressed in flowers whose expression pattern is homeotic-dependent [8]. The main disadvantage of these molecular approaches is that none of them allows the isolation of the corresponding mutant. Genetic assays, such as enhancer trap methods, could be used to identify mutants and genes whose expression patterns in vivo are consistent with homeotic regulation. Al-though these genetic methods have important
ad-vantages, their shortcoming concerns the
identification of directly regulated targets: any gene that is expressed in an homeotic pattern-like manner will probably be affected by the homeotic mutation, but in many cases the effect will be indirect [9].
To date, no mutant of genes immediately down-stream of a homeotic gene has been reported in plants and no approach is available to allow the isolation of such mutants. We present here a new strategy that can be used to fill this gap and create mutants of genes acting directly downstream of a
homeotic gene, in this case APETALA3 (AP3).
AP3, a class B gene, together with PISTILLATA
(PI), the second class B gene, are sufficient to
provide sepal and stamen identities, in combina-tion with the A and C class genes respectively
[10 – 13]. Loss of function of AP3 and PI, have
very similar phenotypes and lead to flowers with petals converted into sepals and stamens into
carpelloid organs [14]. AP3 is necessary for
main-taining transcriptionally its own gene [11]. It is not known whether this autoregulation is direct. In
addition, AP3 is also regulated
post-transcription-ally [11]. When co-synthesised in vitro, AP3 and
PI proteins interact and only AP3/PI
het-erodimers, but not AP3 and PI homodimers, bind specifically to CArG elements [15,16]. This interac-tion is mediated through the I region of the AP3
protein. Recently, Sablowski and Meyerowitz [17] have designed a powerful molecular genetic
method that led to the isolation of a cDNA,NAP
(for NAC-LIKE ACTIVATED BY AP3/PI),
which is an immediate target of AP3/PI. To date,
NAP is the only known direct target gene of a
plant homeotic gene and could play a role in the transition between growth by cell division and cell expansion in stamens and petals.
The strategy designed to obtain mutants of genes that are immediate targets of the AP3 is based on the fusion of a domain with nuclease
activity to AP3. This strategy, outlined below,
allowed the isolation of several mutant lines af-fected in stamen and petal development showing various degrees of sterility and can be applied to other plant transcription factors.
2. Materials and methods
2.1. Plant material and growth conditions
Plants were grown either in a 16 h light/8 h dark
cycle at 22°C in growth cabinets or in soil in the greenhouse. When necessary, surface sterilised seeds were growth on MSAR [18] supplemented
with kanamycin (50 mg/ml) in Petri dishes. To
make transgenic lines, A. thaliana ecotype
Was-sileskija (WS) was transformed with A. tumefa
-ciens strain C58 using the vacuum infiltration method [19]. Selection of primary transformants was performed using the Basta selection as de-scribed by Bechtold et al. [19]. Further selection was with kanamycin in Petri dishes. The C19 control line was provided by Nicole Bechtold (INRA, Versailles, France) and is resistant to both
Basta and kanamycin. Theu6h1 mutant seeds were
kindly sent to us by Dr David Mount (University of Arizona, Tucson, Arizona).
2.2. Fusion of AP3 and FN
The AP3 cDNA was amplified using primer
pairs AP3-1 (5%
-CATGACATGTGGCATATG-GCGAGAGGGAAGATC-3%) and AP3-2 (5%
-CGGGATCCTTACTCGAGTTCAAGAAGATGG
AA-3%) to replace theAP3 stop codon by an XhoI
site (underlined) using a pGEM3Z plasmid
con-taining AP3 cDNA as template (kindly provided
Tech-nology, Pasadena, CA). The FN domain was
am-plified using primer pairs FN-1 (5%
-CGAGCTC-GAGCAACTAGTCAAAAGTGAA-3%) and FN-2
(5%
-CGGGATCCTCATTAAAAGTTTATCTCG-CC-3%) using pRRSfokIRas template (kindly
pro-vided by Dr Chandrasegaran, Johns Hopkins University, Baltimore, MD). Amplification
prod-ucts were cloned into the pMOSBlue plasmid
(Pharmacia) and sequenced. The gene fusion was
realised by cloning the XhoI – BamHI FNfragment
into the XhoI – BamHI-cut pMOSBlue plasmid
harbouring the XhoI-containing AP3 cDNA
in-sert. The fusion gene was then cloned into the pSPUTK plasmid and a pCGN18-derived plasmid containing a Basta resistance gene in addition to the kanamycin resistance gene.
2.3. Protein synthesis in reticulocyte lysates
Expression of AP3-FN, AP3 and PI proteins was
performed as described [15,16] using the TNT kit
(Promega). 35S-labelling of the proteins was done
according to the manufacturer. EMSA and nucle-ase assays were carried out using unlabelled proteins.
2.4. Electromobility shift and nuclease acti6ity
assays
Electromobility shift assays (EMSA) were per-formed essentially as previously described [15]. In Fig. 2c experiment, probe A was a HindIII – BamHI fragment derived from the promoter of the Arabidopsis AP3 gene [15] and was radiolabelled by end-filling using Klenow enzyme in the pres-ence of radiolabelled nucleotide. Oligo B is a HindIII – BamHI fragment carrying a mutated binding site of the MADS-domain Serum Re-sponse Factor [20].
The nuclease activity test (Fig. 2c) was per-formed under the same conditions as the binding
reactions for EMSA except that 2 mM MgCl2was
added to the reaction. After incubation of the probe with the lysates for 4 h in the presence of 2
mM MgCl2, a phenol/chloroforme treatment was
applied. The DNA was precipitated and loaded on an agarose gel. A plasmid carrying the probe A [15] was used as the probe. To radiolabel the plasmid, the unique NdeI site, located approxi-mately 200 bp away from probe A, was end-filled using Klenow enzyme in the presence of radiola-belled nucleotide.
2.5. PCR and RT-PCR
The presence of the full-length fusion gene in T2 lines was determined by PCR using genomic DNA
and primers AP3-1 and FN-2 as described above.
A 1319 bp amplification product is expected. Reverse transcription was performed using an oligo(dT) primer from total RNA isolated from
inflorescences. For AP3, PCR was performed
us-ing primers AP3-1 and AP3-2 and should give rise to a 730 bp product. Contamination by genomic
DNA would give a 1656 bp AP3 amplification
product which was not observed (see Fig. 3). For
AP3-FN, primers AP3-1 and FN-2 were used which
should give rise to a 1319 bp amplification
product. AP3 and AP3-FN amplification products
were sequenced to confirm the specificity of the amplification.
Controls with no reverse transcription step gave no amplification product (data not shown). The expression of adenine phosphoribosyltransferase
(APT) was chosen as a control [21] and primers to
sites inside the coding sequence (forward primer:
5%-TCCCAGAATCGCTAAGATTGCC-3%;
re-verse primer: 5%-CCTTTCCCTTAAGCTCTG-3%)
were employed which should give rise to a 479 bp
fragment. TheAPTfragment amplification in Fig.
3 was performed separately from AP3-FNor AP3
cDNA amplifications and mixed afterwards in a 1:1 ratio before loading onto an agarose gel. 30
PCR cycles were applied for AP3, AP3-FN and
APT amplifications
2.6. Complementation of the ap3-1 mutant
F1 plants heterozygous for both theap3-1 allele
and the transgene were allowed to self-fertilize and kanamycin-resistant progeny were recovered. F2 plants (129) were analysed for their ability to
complement the ap3-1 mutation at 22°C.
Thirty-seven plants homozygous for the ap3-1 allele and
heterozygous for the transgenic insert were as-sayed for their phenotype and the genotype was confirmed in the progeny.
2.7. Selection of 35S::AP3-FN u6h1 lines
Selection of u6h1 homozygous lines were
per-formed on F3 families using the root growth
UV-sensitivity test as described [22]. F3 lines
on MSAR supplemented with kanamycin to select for the presence of the transgene. The F4 and F5
generations of the 35S::AP3-FN, 35S::AP3-FN
u6h1, C19 (transgenic control line) and C19 u6h1
lines were grown on soil in the greenhouse or
growth cabinets without prior selection on
kanamycin. Visual inspection of flowers was per-formed to identify the F4 mutants.
3. Results
3.1. Strategy for isolating mutants of genes directly regulated by AP3
The type IIS FokI restriction enzyme is
com-posed of two distinct and separable domains, one for the specific recognition of DNA and the other,
called FN, for the endonuclease activity that
cleaves the DNA nearby the recognition site (9 bp for one strand and 13 bp for the other), regardless
of the DNA sequence. The FN domain has been
fused to various heterologous DNA-binding do-mains or proteins [23 – 25]. However, only the
Sp1-FN hybrid protein has been used in vivo in
transient expression experiments in animal cells. In
these cells, Sp1-FN induces single-strand breaks
(SSBs) and double-strand breaks (DSBs) of a
target vector [26]. No hybrid protein with the FN
domain has been expressed in a whole organism. Our approach to isolate mutants of AP3 direct target genes is based on conferring a nuclease
activity to AP3 by translationally fusing the FN
domain to the entire AP3 protein. Fig. 1 illustrates the design of the experiment. By expressing the
AP3-FN hybrid protein in plants, DNA cleavages
in AP3 target gene(s) could occur in cells of whorls 2 and 3 (where PI is present) and could induce misrepair by homologous or non-homologous
re-pair pathways [27 – 29]. Since AP3 expression is
detected early at floral stage 3 (defined in [30]), before stamen primordium specification, precur-sors of pollen-mother cells will carry a mutation.
This mutation will therefore be transmitted
through pollen to offspring, thereby creating mu-tants after two generations in the case of a
reces-sive mutation. The efficiency of the
AP3-FN protein in vivo should be increased in a
mutant deficient in DNA repair mechanisms (see below).
3.2. Fusion of AP3 to FN and acti6ity of AP3-FN in 6itro
AP3 and FN were PCR-amplified so that a first
XhoI site replaced the AP3 stop codon and a
second XhoI site was placed at the 5% end and in
frame of the FN domain (see Section 2). The
AP3-FN fusion gene was cloned in the eukaryotic
pSPUTK expression plasmid in order to express the hybrid protein in reticulocyte lysates as de-scribed for AP3 and PI [15]. In vitro transcription
and translation in the presence of 35S-methionine
demonstrated that the expected AP3-FN hybrid
protein was produced (Fig. 2a). The ability of the
AP3-FN/PI complex to bind specific target
se-quences was investigated in vitro. AP3-FN or AP3
were synthesised by cotranslation with PI and
analysed using a fragment of the AP3 promoter
(probe A) by electrophoretic mobility shift assays (EMSA). As shown in Fig. 2b, probe A was
shifted when AP3/PI heterodimers were present in
the reaction. The binding of AP3/PI was competed
away by increasing concentrations of unlabelled probe A but not by a probe containing a mutated
CArG element (oligo B) [20]. When AP3-FN/PI
heterodimers were present in the binding reaction, probe A was also shifted. The intensity of the shift was lower compared to the shift obtained with
AP3/PI heterodimers. Nevertheless, the binding of
AP3-FN/PI to probe A was competed away by
Fig. 1. The rationale of the AP3-FNstrategy. When expressed
constituvely in vivo, the AP3- FNfusion protein should form
dimers with PI in second (petal) and third (stamen) whorl precursor cells. Upon binding of the AP3-FN/PI heterodimer,
DNA cleavage of target genes by FNoccur which can induce
non-labelled probe A, but not by oligo B. We
conclude that AP3-FN/PI heterodimers have
re-tained the ability to bind specifically in vitro to CArG elements.
The nuclease activity assay was performed with
reticulocyte lysates containing AP3/PI, AP3-FN/
PI heterodimers or AP3-FN in the presence of
Mg2+ which is required for F
N activity. The
sin-gular need for AP3 and PI to be co-translated in a eucaryotic expression system to form an active heterodimer [15,16] renders the cleavage test chal-lenging since it is performed with crude extracts containing endogenous nuclease activities. We have optimised the incubation time, lysate
vol-ume range and Mg2+ concentrations that
min-imised these endogenous activities although they could not be totally abolished as shown in Fig.
2c where the intensity of the probe (P) is lower
after incubation with AP3/PI lysate for instance.
Nevertheless, the intensity of a probe A-contain-ing fragment decreased significantly when
incu-bated with increasing amounts of AP3-FN/PI
lysate but remained constant with AP3/PI or
AP3-FN lysates. This result indicates that AP3-FN
shows a specific nuclease activity only when PI is present in vitro and is consistent with the specific binding shown in Fig. 2b. In the case of a DSB activity, a 200 bp fragment was expected to ap-pear (see Section 2) but was not visible under our
conditions presumably because the FN domain is
present as a single copy in the heterodimer (see Section 4) and induces SSBs in the target DNA which becomes more sensitive to endogenous nu-cleases.
Fig. 2.
Fig. 2. DNA-binding and nuclease activity of the AP3-FN/PI
heterodimer. (a) Expression of AP3, AP3-FNand PI in
locyte lysates. Proteins were synthesised using the TNT reticu-locyte lysate system (Promega). Labelled 35S-methionine in
vitro translation reactions demonstrated that the expected polypeptides were produced. The number of methionines in these proteins is AP3, 4; AP3-FN, 10; PI, 10. Molecular
weight predicted from the sequence of the proteins are indi-cated. (b) DNA-binding activity of the AP3-FN/PI
het-erodimer. AP3 or AP3-FN were cosynthesized with PI in
vitro. Reticulocyte lysates were incubated with the radiola-belled CArG-box-containing fragment referred as probe A in [15]. AP3-FN/PI heterodimers bind to probe A although with
a lower affinity than AP3/PI heterodimers. Competition was done with two concentrations (×50 and ×200) of non-ra-dioactive probe A indicated as ‘A’ in the figure (filled trian-gles) or a fragment carrying a mutated binding site of the MADS-domain Serum Response Factor referred as oligo B in [20] and indicated as ‘B’ in the figure (open triangles). When synthesised separately and mixed afterward, AP3/PI het-erodimers displayed a drastically reduced binding activity (data not shown). A control with a lysate containing a pSPUTK without insert is included (L). The probe (P) shown on the left lane was not incubated with the lysate. (c) Nucle-ase activity of the AP3-FN/PI heterodimer. A radiolabelled
probe A-carrying fragment was incubated for 4 h in the presence of 2 mM MgCl2with increasing concentrations (1, 2
and 3ml) of reticulocytes lysates containing AP3/PI, AP3-FN/
PI or AP3-FNas indicated. After a phenol/chloroforme
treat-ment, the DNA was precipitated and loaded on an agarose gel. A decrease in probe intensity is detected specifically with AP3-FN/PI but not with AP3/PI nor AP3-FN lysates. The
probe (P) shown on the left lane was not incubated with the lysate. A decrease of the intensity with AP3/PI, AP3-FN
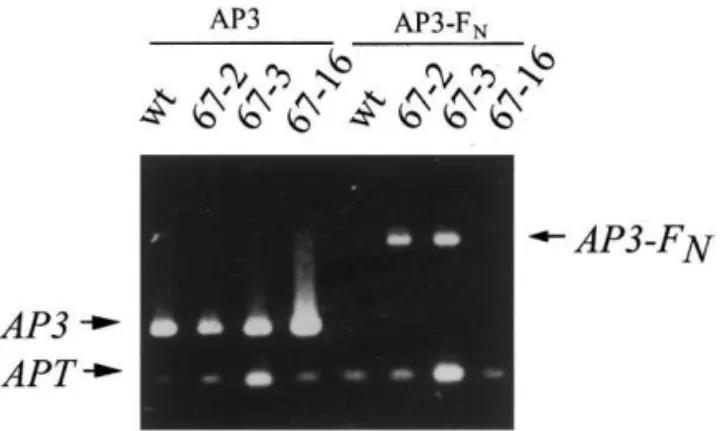
Fig. 3. RT-PCR amplification of AP3 and AP3-FN cDNAs.
Total RNA from inflorescences was used for reverse tran-scription using an oligo(dT) primer. PCR amplifications was performed with primers AP3-1 and FN-2 to amplify the entire
AP3-FNfusion gene and with primers AP3-1 and AP3-2 (see
Section 2) to amplify the entireAP3 cDNA. The expression of adenine phosphoribosyltransferase (APT) was used as a control. AP3 expression in all three lines is similar to the wildtype expression. While no expression was detected in the wild-type, anAP3-FN amplification product was observed at
the expected size (1.3 kb) in lines 67-2 and 67-3. A weakly detectable, higher molecular weight product, whose specificity is unclear, is detected in line 67-16.
second-whorl organs are found instead of petals and third whorl organs are abnormal stamens
producing no pollen [31,32]. The AP3-FN
trans-genic line 67-2 that segregates for one T-DNA
insertion was crossed with ap3-1 homozygous
plants. F2 plants that were resistant to kanamycin
were observed. If AP3-FN fully complemented the
ap3-1 mutation, all F2 kanamycin-resistant lines
would be expected to form fertile flowers with four fully developed petals while a segregation of 3
(wild-type) to 1 (ap3-1) plant phenotypes would
indicate a failure of the hybrid protein to comple-ment the mutant. All F2 plants were fertile at 22°C. However, one quarter of the plants was distinguishable from the rest of the population. Firstly, although these plants made flowers with white petals instead of green second whorl organs
in ap3-1 flowers (Fig. 4a), the petals were short
compared to wild-type (Fig. 4b and c, respec-tively). Secondly, stamens did not release high amounts of pollen relative to wild-type stamens (compare Fig. 4b and c) which led to the forma-tion of siliques containing fewer seeds than wild-type siliques.
We conclude from these results that AP3-FN
can suppress the ap3-1 phenotype and therefore is
able, at least partially, to fulfil AP3 functions in
vivo.
3.5. Analysis of 35S::AP3-FN progeny
Several thousands of 35S::AP3-FN plants were
observed in T2, T3 and T4 generations. No defects were observed in flowers or in the overall develop-ment of these plants. This lack of abnormal
phe-notype could indicate that the FN domain was not
functional in vivo or alternatively that plant cells were able to repair efficiently DNA breaks
gener-ated by FN. To test the latter alternative, line 67-2
was crossed with the DNA-break repair deficient
mutant u6h1[33,34]. Upon crossing, two
35S::AP3-FN u6h1 plants were identified in the F2
population. The misrepaired breaks of AP3-FN
would occur for the first time in these F2 plants.
Therefore, it was necessary to propagate
35S::AP3-FNu6h1 plants for two additional
gener-ations (i.e. F4 generation) to detect potential reces-sive mutants in the offspring.
About 3000 F4 plants deriving from each of the
following genotypes: 35S::AP3-FN, 35S::AP3-FN
u6h1, C19 (transgenic control) and C19u6h1 were
3.3. 35S::AP3-FN transgenic lines
The AP3-FN fusion gene was cloned in a
pCGN18-derived plasmid [11] downstream of the constitutive cauliflower mosaic virus 35S promoter and transgenic lines were obtained. The presence of the fusion gene was assessed by PCR for three lines: 67-2, 67-3 and 67-16. All lines had integrated the full length gene fusion (data not shown). The
expression of the endogenous AP3 and of the
transgene were assessed by RT-PCR using total RNA isolated from inflorescences (Fig. 3). The
endogenous AP3 mRNA level in these lines was
comparable to the wild-type level. The AP3-FN
mRNA was detected in two of the lines and its
abundance was similar than the endogenous AP3
mRNA level. This results indicate that theAP3-FN
transgene is expressed in transgenic lines 67-2 and 67-3.
3.4. Complementation of the ap3-1 mutant by 35S::AP3-FN
To determine whether the AP3-FN protein was
functional in vivo, we attempted to genetically
complement the ap3-1 mutant with the 35S::AP
3-FN construct. ap3-1 is a weak,
Fig. 4. Complementation ofap3-1. (a)ap3-1 flower. Second and third whorl organs are short green sepaloid organs and short abnormal green staminoid organs respectively. (b)ap3-1 35S::AP3-FN. Short white petals are visible. Stamens are nearly as tall
as wild-type stamens with yellow anthers releasing some pollen grains. (c) Wild-type flower (ecotype Ler). Plants were grown on soil at 22°C under long-day photoperiod.
Fig. 5. Phenotype of mutants isolated in the progeny of 35S::AP3-FN u6h1 plants. Shown are inflorescences and flowers of F5
plants. (a) Wild-type immature flower, ecotype WS. Petals are small and narrow. Stamens are small and yellow with no visible released pollen grains. The stigmatic tissue on top of pistil is underdevelopped. (b) Wild-type mature flower, ecotype WS. Petals are large and bent backwards (one petal was manually removed). Stamens are tall and pollen is being released. Stigmatic tissue is fully developed. (c – d) Inflorescence and individual flower respectively from mutantSS1. (c) The inflorescence shows almost wild-type flowers. Siliques seen on the picture did not contain seeds. (d) Petals are slightly shorter than wild-type. Stamens produce pollen but flowers are sterile. Pistil is underdevelopped. (e – f) Inflorescence and individual flower respectively from mutant
visually inspected. Plants were sown and grown under the same conditions. The C19 control plants showed no abnormal phenotype neither in their general growth or floral development. C19
u6h1 plants behaved similarly indicating that,
from the initial cross to the F4 plants, the u6h1
background had no indirect effect on genotype or phenotype of the plants during their growth on soil and selections on kanamycin when cultured
in Petri dishes. Conversely, while 35S::AP3-FN
lines in a wild-type background were normal as described above, 19 plants in the F4 progeny of
the 35S::AP3-FN u6h1 plants showed, to various
degrees, a lack of fertility. These 19 plants were isolated from the progeny of six individual F3 families out of 17 tested. Four phenotypic classes were distinguished: completely sterile (CS, seven plants), severely sterile (SS, seven plants), aborted flower bud (AFB, four plants) and unbent petals (UP, one plant). When seeds were produced (classes SS, AFB and UP), plants in the F5 gen-eration were observed and showed the same phe-notype as F4 mutants, therefore indicating that the mutations are transmitted.
3.6. Analysis of the mutants
Mutants of class CS produced no progeny which prevented their further analysis. Mutants of class SS show severe sterility with normal flow-ers. Fig. 5 illustrates representative phenotypes of
mutants SS1, SS2 and SS4 (Fig. 5 c – d, e – f and
g – h, respectively). In these plants, flowers have normal pollen-releasing stamens. The sterility phenotype seems therefore to be the consequence of a non-functional pollen. Petals are slightly shorter than wild-type. Mutants of class AFB develop unopened flowers. The sterility is often total until very late in plant development when the last four to five flowers make short siliques
containing few seeds. Mutants AFB9 and AFB10
illustrate this class (Fig. 5 i – j and k – l, respec-tively). For both mutants, the sterility comes ob-viously from the fact that stamen development is
impaired. Mutant UP17 was the only member of
class UP with partial sterility homogenous along the plant.
Most of the mutants were isolated in a
green-house under shortday (SD) conditions. We
looked at the phenotype of mutant classes SS, AFB and UP under longday (LD) conditions. For most mutants, especially mutants AFB, the
penetrance of the phenotypes was incomplete un-der LD conditions. The phenotype of SS mutants was less sensitive to day length.
To ensure that the sterility phenotype was not
related to the presence of the AP3-FN hybrid
protein in the u6h1 background, mutants SS1 and
SS2 were crossed with wild-type plants and
off-spring were observed without selection on
kanamycine. F1 plants showed a partial sterility suggesting that the mutations are semi-dominant. In both F2 populations, sterile plants were ob-served in a 3:1 to 2:1 ratio depending on the penetrance of the phenotype. Therefore, the sterility phenotype is not dependent on the
pres-ence of the transgene in the u6h1 background.
Furthermore, offsprings of F5 plants showing a SS phenotype were sown on MSAR medium con-taining kanamycine. Several families showed a kanamycine-sensitive phenotype indicating that the SS phenotype was not dependent on the pres-ence of the transgene.
4. Discussion
The identification of direct target genes of homeotic genes has been a difficult task in both plants and animals [9]. Even more difficult is the isolation of mutants of these target genes and none has been described in plants. We report here a strategy design to isolate such mutants. This strategy, based on the use of a fusion of
AP3 to the FokI nuclease domain (FN), has led
to the isolation of mutants showing defects in the development of third and second whorl organs. Several lines of evidence indicate that these mu-tants were created by the specific action of
AP3-FN/PI heterodimers.
4.1. Acti6ity of AP3-FN
The EMSA experiments performed in this study indicate that the I region of the AP3 protein, which constitutes a key molecular deter-minant for specific dimer formation [15,16,12], is
still accessible in vitro to PI in the AP3-FN
hy-brid protein. The evidence that this AP3-FN/PI
interaction also occurs in vivo is provided by the
partial complementation of ap3-1. However,
con-stitutive expression of AP3-FN in plant is
distin-guishable from AP3 one: 35S::AP3 transgenic
fourth whorl together with a carpel-to-stamen homeotic transformation [11]. Such a
transfor-mation is not observed in 35S::AP3-FN lines.
This is strikingly similar to the wild-type
pheno-type reported for 35S::AP3-GUS lines [35] in
which the GUS gene was fused to the entire
AP3 gene in a manner similar to the FN domain.
GUS staining in 35S::AP3-GUS flowers is indeed
restricted to second and third whorls. The au-thors postulated that specific destabilization fac-tors of the AP3-GUS fusion may be operating to degrade the protein in floral domains where it is not normally expressed. The relatively low
abundance of the AP3-FN transcript, despite the
use of the 35S promoter, might indicate that
AP3 mRNA, rather than AP3 protein, is
un-stable when fused to a heterologous sequence. In previous in vitro studies, only DNA-bind-ing proteins workDNA-bind-ing as homodimers have been
fused to FN [23 – 25] resulting in hybrid
homod-imers with two FN domains. On the opposite,
only one copy of the FN domain is present in
the AP3-FN/PI heterodimer. On account of
crys-tallographic studies with the FokI enzyme, it was
concluded that FokI binds to its target sequence
as a monomer, raising the question of how a single domain could introduce DSBs in the target DNA [36]. However, recent biochemical
data indicate a role for the FN domain in
dimer-ization and show that the presence of only one
functional FN domain induces SSBs rather than
DSBs in the target DNA [37,38]. Thus the
AP3-FN/PI heterodimer is expected to introduce
mainly SSBs in the target DNA. The degrada-tion of the probe shown in Fig. 2c in the
pres-ence of AP3-FN/PI specifically is likely to be an
indirect consequence of these SSBs in the DNA which becomes a substrate for lysate
exonucle-ases. The Sp1-FN pointer lacking the Sp1
DNA-binding domain that can presumably be recruited as monomer also introduces SSBs in the target DNA in vivo [26] but its activity has not been studied in vitro.
The activity of FN in vivo is strongly
sug-gested by the obtention of mutants affected in
floral development in the progeny of 35S::AP
3-FN u6h1 plants but not in 35S::AP3-FN plant
progeny. The necessity of placing the AP3-FN
hybrid protein in the u6h1 background might
reflect an efficient repair system in wild-type
plant cells. This observation indicates that the success of this strategy is certainly determined in
part by the DNA repair/DNA cleavage ratio in
plant cells and suggests that this ratio is high in the wild-type background. In animal cells, the
transiently expressed Sp1-FN protein introduces
SSBs and DSBs in as much as 10% of the target plasmid but the efficiency of repair in vivo has not been studied [26]. The knowledge of DNA-break repair pathways is not fully elucidated in plants [39]. DSBs have been shown to be
re-paired either via homologous recombination
which is increased by a one to two orders of magnitude [27,28] with misrepair leading in some cases to deletions, either via non-homologous end joining largely associated with deletions and ‘filler’ DNA insertions [29]. SSB repair in plants
is mostly unknown. The u6h1 mutant used in
this study is sensitive to DSB-inducing agents such as bleomycine [34]. It is also deficient in T-DNA integration [34]. A model for T-DNA integration into the plant DNA has been pro-posed. It involves a series of SSBs of the plant DNA and integration of the T-DNA by illegiti-mate recombination [40]. Therefore, it is possible
that, after DSBs or SSBs by AP3-FN nearby a
cis-regulatory target element, deletions appear
within this region, especially in the u6h1
background.
4.2. Mutant phenotypes
It is noteworthy that sterility is a common
phenotype for the isolated mutants. The AP3-FN
strategy allows the mutagenesis of AP3/PI target
genes prior to gamete formation. When ex-pressed constitutively, AP3 protein induces a floral phenotype uniquely in flowers in the
pres-ence of PI [11]. If AP3-FN protein follows the
same extent, e.g. slightly for the SS class and severely for the AFB class. This observation
might reflect the fact that most AP3/PI target
genes are common to second and third whorl
organs as suggested previously for NAP [17]. In
the case of NAP, although no mutant has been
isolated so far, studies using NAP antisense and
sense transgenic lines indicate a role for NAP in
fertility [17]. It will be of interest to analyse the
NAP locus in the mutants described to
deter-mine whether a NAP mutant has been isolated.
With conventional mutagenesis agents like EMS, plants with easy scorable phenotypes such as albinos are observed at a frequency of 1 – 5% [41]. No albino plants were observed suggesting
that the FN domain induced few or no
non-spe-cific mutations under our conditions. This is consistent with the requirement of PI for AP3 function [11]. Alternatively, we cannot exclude
that only AP3-FN low expression lines were
re-covered because, when highly expressed, FN is
lethal to the plant. Interestingly, we were unable
to obtain transformants with a 35S::AG-FN
con-struct.
One possible drawback of this method is that mainly weak mutations would be produced as the hybrid protein is likely to induce mutations
mainly in cis-regulatory elements. Hence, weak
phenotypes would be observed since a strong loss-of-function mutation is unlikely. Neverthe-less, the strategy described here can be applied to any transcription factor such as LEAFY in floral development or factors that are expressed in embryogenesis that would create mutations early in plant development.
So far, the genes that act downstream in plant homeotic pathways encode proteins with various regulatory functions: transcription factors [42], a putative kinase [7], a protein probably involved in the transition between growth by cell division and cell expansion [17]. Clearly, the genetic path-ways controlled by homeotic genes are complex. Unconventional approaches, such as that devel-oped by Sablowski and Meyerowitz [17] or the one described here, will be useful to unravel these processes.
Acknowledgements
We are indebted to Dr Patricia Villain for suggesting the critical use of DNA-repair
mu-tants. We are grateful to Dr Elliot Meyerowitz
for the gift of AP3 cDNA, Dr Chandrasegaran
for the gift of pRRSfokIR, Dr Jose´-Luis
Riech-mann for the gift of probe A-containing plasmid,
AP3 and PI cDNA-containing pSPUTK plamids
and his help for EMSA experiments, Dr Hong Ma for the gift of oligo B-containing plasmid
and AG cDNA, Dr Leslie Sieburth for the gift
of pCGN18 plasmid and Dr Reinhart Hehl for the gift of the Basta resistance gene. We wish to thank Nicole Bechtold help with the vacuum infiltration and for the gift of the C19 control line. We also thanks Dr Jean-Marc Bonneville, Dr Pierre Carol, Dr Jean-Gabriel Valay and Dr
Gaynor Green for critical reading of the
manuscript. We thank Jean-Pierre Alcaraz for
sequencing AP3-FN products and Mireille
Ro-cipon for her photographic expertise. This work was supported by the Centre National de la Recherche Scientifique and the Ministe`re de l’Ed-ucation Nationale, de la Recherche et de la Technologie. P.L. was supported financially by a predoctoral fellowship from the Ministe`re de l’Education Nationale, de la Recherche et de la Technologie. C.D. was supported financially by an Allocation de Formation et Reconversion fel-lowship from the Ministe`re de l’Emploi et de la Solidarite´.
References
[1] E.S. Coen, E.M. Meyerowitz, The war of the whorls: genetic interactions controlling flower development, Na-ture 353 (1991) 31 – 37.
[2] D. Weigel, E.M. Meyerowitz, The ABCs of floral home-otic genes, Cell 78 (1994) 203 – 209.
[3] H. Sommer, J. Beltran, P. Huijser, et al., Deficiens, a homeotic gene involved in the control of flower morpho-genesis in Antirrhinum majus: the protein shows homol-ogy to transcription factors, EMBO J. 9 (1990) 605 – 613. [4] O. Shore, A.D. Sharrowcks, The MADS box family of transcription factors, Eur. J. Biochem. 229 (1995) 1 – 13. [5] A.P. Gould, J.J. Brookman, D.I. Strutt, R.A.H. White, Targets of homeotic gene control inDrosophila, Nature 348 (1990) 308 – 311.
[6] Y. Graba, D. Aragnol, P. Laurenti, et al., Homeotic control in Drosophila; the scabrous gene is an in vivo target of Ultrabithorax proteins, EMBO J. 11 (1992) 3375 – 3384.
[7] T. Ito, N. Takahashi, Y. Shimura, K. Okada, A serine/
[8] H. Ma, M.F. Yanofsky, E.M. Meyerowitz, AGL
1-AGL6, an Arabidopsis gene family with similarity to floral homeotic and transcription factors genes, Genes and Dev. 5 (1991) 484 – 495.
[9] Y. Graba, D. Aragnol, J. Pradel, Drosophila Hox complex downstream targets and the function of home-otic genes, Bioassay 19 (1997) 379 – 388.
[10] T. Jack, L.L. Brockman, E.M. Meyerowitz, The home-otic geneAPETALA3 ofArabidopsis thalianaencodes a MADS box and is expressed in petals and stamens, Cell 68 (1992) 683 – 697.
[11] T. Jack, G.L. Fox, E.M. Meyerowitz, Arabidopsis
homeotic gene APETALA3 ectopic expression: tran-scriptional and posttrantran-scriptional regulation determine floral identity, Cell 76 (1994) 703 – 716.
[12] B.A. Krizek, E.M. Meyerowitz, Mapping the protein regions responsible for the functional specificities of the
Arabidopsis MADS domain organ-identity proteins, Proc. Natl. Acad. Sci. USA 93 (1996) 4063 – 4070. [13] B.A. Krizek, E.M. Meyerowitz, TheArabidopsis
home-otic genes APETALA3 andPISTILLATAare sufficient to provide the B class organ identity function, Devel-opment 122 (1996) 11 – 22.
[14] J.L. Bowman, D.R. Smyth, E.M. Meyerowitz, Genes directing flower development in Arabidopsis, Plant Cell 1 (1989) 37 – 52.
[15] J.L. Riechmann, B.A. Krizek, E.M. Meyerowitz, Dimerization specificity of Arabidopsis MADS domain homeotic proteins APETALA1, APETALA3, PISTIL-LATA, and AGAMOUS, Proc. Natl. Acad. Sci. USA 93 (1996) 4793 – 4798.
[16] J.L. Riechmann, M. Wang, E.M. Meyerowitz, DNA-binding properties of Arabidopsis MADS domain homeotic proteins APETALA1, APETALA3, PISTIL-LATA and AGAMOUS, Nucl. Acids Res. 24 (1996) 3134 – 3141.
[17] R. Sablowski, E.M. Meyerowitz, A homolog of NO APICAL MERISTEM is an immediate target of the floral homeotic genes APETALA3/PISTILLATA, Cell 92 (1998) 93 – 103.
[18] C. Koncz, J. Schell, The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector, Mol. Gen. Genet. 204 (1986) 383 – 396.
[19] N. Bechtold, J. Ellis, G. Pelletier, In planta Agrobac
-terium mediated gene transfer by infiltration of adult
Arabidopsis thaliana plants, C. R. Acad. Sci. France 316 (1993) 1194 – 1199.
[20] H. Huang, Y. Mizukami, Y. Hu, H. Ma, Isolation and characterization of the binding sequences for the product of the Arabidopsis floral homeotic gene AGA
-MOUS, Nucl. Acids Res. 21 (1993) 4769 – 4776. [21] R. Cowling, Y. Kamiya, H. Seto, N.P. Harberd,
Gib-berellin dose-response regulation of GA4 gene tran-script levels in Arabidopsis, Plant Physiol. 117 (1998) 1195 – 1203.
[22] M.E. Jenkins, G.R. Harlow, Z. Liu, M.A. Shotwell, J. Ma, D. Mount, Radiation-sensitive mutants of Ara
-bidopsis thaliana, Genetics 140 (1995) 725 – 732.
[23] Y.G. Kim, J. Cha, S. Chandrasegaran, Hybrid restric-tion enzymes: zinc finger fusions to Fok I cleavage
do-main, Proc. Natl. Acad. Sci. USA 93 (1996) 1156 – 1160.
[24] Y.G. Kim, S. Chandrasegaran, Chimeric restriction en-donuclease, Proc. Natl. Acad. Sci. USA 91 (1994) 883 – 887.
[25] Y.G. Kim, P.S. Kim, A. Herbert, A. Rich, Construc-tion of a Z-DNA-specific restricConstruc-tion endonuclease, Proc. Natl. Acad. Sci. USA 94 (1997) 12875 – 12879. [26] J.-S. Lee, C.-H. Lee, J.H. Chung, Studying the
recruit-ment of Sp1 to theb-globin promoter wtith an in vivo method: protein position identification with nuclease tail (PIN*POINT), Proc. Natl. Acad. Sci. USA 95 (1998) 969 – 974.
[27] M. Chiurazzi, A. Ray, J.F. Viret, et al., Enhancement of somatic intrachromosomal homologous recombina-tion inArabidopsis by the HO endonuclease, Plant Cell 8 (1996) 2057 – 2066.
[28] H. Puchta, B. Dujon, B. Hohn, Two different but re-lated mechanisms are used in plants for the repair of genomic double-strand breaks by homologous recombi-nation, Proc. Natl. Acad. Sci. USA 93 (1996) 5055 – 5060.
[29] V. Gorbunova, A.A. Levy, Non-homolgous DNA end joining in plant cells is associated with deletions and filler DNA insertions, Nucl. Acids Res. 25 (1997) 4650 – 4657.
[30] D.R. Smyth, J.L. Bowman, E.M. Meyerowitz, Early flower development in Arabidopsis, Plant Cell 2 (1990) 755 – 767.
[31] J.L. Bowman, D.R. Smyth, E.M. Meyerowitz, Genetic interactions among floral homeotic genes in Arabidop
-sis, Development 112 (1991) 1 – 20.
[32] R. Sablowski, E.M. Meyerowitz, Temperature-sensitive splicing in the floral homeotic mutant apetala3-1, Plant Cell 10 (1998) 1453 – 1464.
[33] G.R. Harlow, M.E. Jenkins, T.S. Pittalwala, D.W. Mount, Isolation of u6h1, an Arabidopsis mutant hy-persensitive to ultraviolet light and ionizing radiation, Plant Cell 6 (1994) 227 – 235.
[34] R.V. Sonti, M. Chiurazzi, D. Wong, et al., Arabidopsis
mutants deficient in T-DNA integration, Proc. Natl. Acad. Sci. USA 92 (1995) 11786 – 11790.
[35] B. McGonigle, K. Bouhidel, V. Irish, Nuclear localiza-tion of the Arabidopsis APETALA3 and PISTILLATA
homeotic gene products depends on their simultaneous expression, Genes Dev. 10 (1996) 1812 – 1821.
[36] D.A. Wah, J.I.S. Bitinaite, A.K. Aggarwal, Structure of FokI has implications for DNA cleavage, Proc. Natl. Acad. Sci. USA 95 (1998) 10564 – 10569.
[37] J. Bitinaite, D.A. Wah, A.K. Aggarwal, I. Schildkraut,
FokI dimerization is required for DNA cleavage, Proc. Natl. Acad. Sci. USA 95 (1998) 10570 – 10575.
[38] D.A Wah, J.A. Hirsch, L.F. Dorner, I. Schidkraut, A.K. Aggarwal, Structure of the multimodular endonu-clease FokI bound to DNA, Nature 388 (1997) 97 – 100.
[39] A.B. Britt, DNA damage and DNA repair in plants, Ann. Rev. Plant Physiol Plant Mol. Biol. 47 (1996) 75 – 100.
[40] B. Tinland, F. Schoumacher, V. Gloeckler, A.M. Bravo-Angel, B. Hohn, The Agrobacterium tumefaciens
Bravo-Angel, B. Hohn, The Agrobacterium tumefaciens
virulence D2 protein is responsible for precise integration of T-DNA into the plant genome, EMBO J. 17 (1995) 3585 – 3595.
[41] J. Lightner, T. Caspar, Seed mutagenesis of Arabidopsis, in: J. Martinez-Zapater, J. Salinas, (Eds.), Methods in
Molecular Biology, vol. 82, Arabidopsis Protocols, Hu-mana Press Inc, pp. 91 – 103.
[42] B. Savidge, S.D. Rounsley, M.F. Yanofsky, Tem-poral relationship between the transcription of twoAra
-bidopsis MADS box genes and the floral organ identity genes, Plant Cell 7 (1995) 721 – 733.