Isolation and characterization of a cDNA clone encoding
asparagine synthetase from root nodules of
Elaeagnus umbellata
Ho Bang Kim, Sang Ho Lee, Chung Sun An *
Department of Biology,Seoul National Uni6ersity,Seoul151-742,South Korea
Received 15 September 1998; received in revised form 24 June 1999; accepted 28 June 1999
Abstract
A cDNA clone encoding asparagine synthetase (AS) was isolated from a root nodule cDNA library ofElaeagnus umbellataby competitive hybridization. The clone, pEuNOD-AS1, coded for 585 amino acid residues with molecular weight of 65.8 kDa and pI value of 6.12. Expression of AS was highly enhanced in the root nodule, and its expression pattern during nodule development was very similar to that ofnifH, showing highest level 6 – 8 weeks after inoculation and decreased thereafter. In situ hybridization result showed AS transcripts were strongly detected in the infected cells of fixation zone, wherenifH transcripts were also detected. These results suggest its expression may be under metabolic control rather than developmental control of the root nodule. Genomic Southern hybridization revealed the presence of at least two AS genes in the genome ofE.umbellata. © 1999 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Asparagine synthetase; cDNA;Elaeagnus umbellata; In situ hybridization; Nodule-enhanced expression; Root nodule
www.elsevier.com/locate/plantsci
1. Introduction
Actinorhizal root nodules are nitrogen-fixing symbioses involving the actinomyceteFrankia and roots of dicotyledonous plants belonging to eight different families and 25 genera. Most are capable of high rates of nitrogen fixation comparable to those found in legumes [1]. Actinorhizal root nod-ules resemble lateral roots with a central vascular system and originate from pericycle, while legumi-nous root nodules resemble shoots with peripheral vascular systems and originate from cortex [2]. Due to the presence of apical meristem activity, its nodule lobes show indeterminate growth pattern and thus consist of four different zones, which are meristem zone, prefixation zone, fixation zone and senescence zone [3]. For these reasons, the acti-norhizal root nodules may be useful system to study many aspects of plant development.
Ammonia fixed by dinitrogenase in root nodule should be transported to other parts of the plant through xylem after assimilation into amino acids. The type of amino acids to be transported through xylem are different according to plant species, developmental stage or environmental conditions. Generally symbiotic legumes and actinorhizal plants can be divided into amide transporters (temperate legumes and actinorhizal plants such as
Datisca, Elaeagnus, Myrica, etc.) and the ureide
transporters (tropical legumes and actinorhizal plants such as Alnus, etc.) [4]. Until now, studies of molecular aspects of enzymes related with am-monia assimilation in root nodules of actinorhizal plants were concentrated into Alnus, a ureide transporter. Recently, cDNA clones encoding glu-tamine synthetase, a component of GS/GOGAT enzyme complex, and acetylornithine transami-nase, an enzyme related with citrulline biosynthe-sis, from the root nodule of Alnus glutinosa were isolated and their expression patterns were charac-terized [5].
* Corresponding author. Tel.: +82-2-880-6678; fax: + 82-2-872-6881.
E-mail address:[email protected] (C.S. An)
Asparagine (Asn) is a major amino acid form transporting nitrogen in plants faced with condi-tions of excess ammonia (for example, germina-tion, growth on fertilizers and nitrogen fixation) and limitation of carbon source [6,7]. Asn is an ideal amino acid form for transport of reduced nitrogen, because of its higher N:C ratio (2:4) than glutamine (2:5) and its stability [8]. The synthesis of Asn is mediated by asparagine synthetase (AS, EC 6.3.5.4), which catalyzes the ATP-dependent transamination reaction transferring the amide group of glutamine (or ammonia) to aspartate, resulting in the formation of glutamate and Asn while hydrolyzing ATP to AMP and PPi;
L-Asp+L-Gln+ATP
L-Asn+L-Glu+AMP+PPi.
Two different types of AS have been described in
E. coli and yeast; a Gln-dependent form and an ammonia-dependent form [9,10]. Although all plant ASs studied up to date appear to be Gln-de-pendent form, AS in maize roots can use ammonia as a substrate effectively under the condition of excess ammonia [11]. Biochemical study of AS has been hampered by its extremely low stability, con-taminating asparaginase activity and specific non-protein inhibitors [12]. Molecular and genetic studies have been used to circumvent the difficulty of biochemical study. Significant progress has been made in understanding expression and regulation of AS through the isolation and characterization of cDNA and/or genomic clones from non-nodu-lating plants such as asparagus [13], Arabidopsis
[14], broccoli [15] and maize [16], and especially from legume plants such as pea [17],Lotus japoni
-cus [18], alfalfa [19], soybean [20] and broad bean [21]. Nothing, however, has been known on the expression and regulation of AS from any acti-norhizal plants.
In this paper, a cDNA clone encoding AS was isolated from the root nodule cDNA library of E.
umbellata, a amide transporter [4], by competitive
hybridization and its molecular biological aspects were characterized. Expression pattern of AS in different tissues was investigated by Northern hy-bridization. Expression pattern of AS during nod-ule development and distribution of AS transcripts in the root nodule were analyzed by RT-PCR (Reverse Transcriptase-mediated Polymerase Chain Reaction) and in situ hybridization, respec-tively. This is a first report for isolation and
characterization of cDNA clone encoding enzyme related with ammonia assimilation in the root nodule of amide transporting actinorhizal plants.
2. Materials and methods
2.1. Bacterial strain and plant material
Frankia strain EuIK1, a symbiont of E. umbel
-lata root nodule, was used to nodulate E. umbel
-lata seedlings. Culture methods for E. umbellata
seedlings and the Frankia strain were described previously [22]. RNAs for construction of nodule cDNA library were isolated from root nodules at various developmental stages 6 months after inoc-ulation. Uninoculated seedlings were used to iso-late RNAs of leaf and root. To study gene expression level during nodule development, nod-ules of 4, 6, 8, and 10 weeks after inoculation were harvested and stored at −80°C until used. For in situ hybridization, nodules of 8 – 10 weeks after inoculation were used.
2.2. Isolation of DNA and RNA
The method of Doyle and Doyle [25] for isola-tion of genomic DNA was modified to isolate total RNA from leaves, roots and nodules of E. umbel
-lata. Plant tissues were ground in liquid nitrogen. Polyvinypolypyrrolidone (PVPP) was added dur-ing grinddur-ing with liquid nitrogen to remove pheno-lic compounds. CTAB (cetyltrimethyl ammonium bromide) extraction buffer (10 – 12 ml/g tissue) was added to the tissue powder. The homogenate was incubated at 60°C for 10 min and extracted with phenol and chloroform. The supernatant was pre-cipitated with cold IPA and washed with washing buffer [76% (v/v) EtOH, 10 mM ammonium ac-etate]. Dried pellet was dissolved with nuclease-free water. Total RNA was differentially precipitated with lithium chloride from total nu-cleic acids and treated with RNase-free DNase to remove genomic DNA. Poly(A)+RNA for nodule
cDNA library construction was purified from total RNA using oligo d(T) cellulose column (Boehringer Mannheim, Mannheim, Germany) ac-cording to the methods of Ausubel et al. [24]. Genomic DNA was isolated from E. umbellata
2.3. Construction and screening of a cDNA library
cDNA was synthesized from 5 mg of poly(A)+
nodule RNA using cDNA synthesis Kit (Strata-gene, La Jolla, CA, USA) according to manufac-turer’s guide. The synthesized cDNA was inserted into ZAP express vector (Stratagene) and recombi-nant phage DNA was in vitro packaged using Gigapack III Packaging Extract (Stratagene) ac-cording to manufacturer’s guide. Phage DNAs from about 10 000 plaques per petri dish (r=4.5 cm) were transferred to nylon membranes (Amer-sham, Berckinghamshire, UK) and cross-linked by UV-treatment. Ten phage blots were competitively hybridized with nodule cDNA probe and an ex-cess of total RNA from roots and leaves according to Mangiarotti et al. [27]. The cDNA probe was synthesized from 2 to 5mg poly(A)+nodule RNA.
2.4. Cloning and sequence analysis
Positive phage clones from primary and sec-ondary competitive screening were changed into phagemid clones according to in vivo excision protocol of manufacturer (Stratagene). Phagemid DNA was deleted unidirectionally with exonucle-ase III and S1 nucleexonucle-ase by using double-stranded Nested Deletion Kit (Pharmacia Biotech, Uppsala, Sweden) based on the protocol of Henikoff [28]. The nucleotide sequences were determined by dideoxynucleotide chain termination method [29] using Taq polymerase (Promega, Madison, WI, USA) and T7 DNA polymerase (USB, Cleveland, OH, USA). The sequences were analyzed using PC-GENE (Release 6.01; IntelliGenetics, Moun-tain View, CA, USA), DNASIS/PROSIS (V6.01; Hitachi Software Engineering, Tokyo, Japan) and BLAST search program [30,31].
2.5. DNA and RNA blot analysis
For DNA analysis, genomic DNAs from leaves
of E. umbellata (10 mg) digested with several
re-striction enzymes (EcoRI, HindIII and BglII) were electrophoresed on 0.8% agarose gel, and transferred to Hybond-N membrane (Amersham) by capillary blotting method [26]. For RNA analy-sis, total RNAs from leaves, roots and nodules of
E.umbellata (10mg) were electrophoresed on a 1%
glyoxal gel and transferred to Hybond-N
mem-brane (Amersham) by capillary blotting method [26]. The blots were hybridized overnight with
32P-labelled AS probe under following condition;
6×SSC, 5×Denhardt’s solution, 0.5% SDS (Sodium Dodecyl Sulfate) at 65. The hybridized blots were washed at 65°C with gradually decreas-ing salt concentration to 0.5×SSC, 0.1% SDS, and exposed to X-ray film.
2.6. RT-PCR
RT-PCR method was used to analyze expres-sion pattern of AS gene in the course of nodule development. Two PCR primers [upper primer (position 1715 – 1737); 5% -TTCTGGAAGGGCTG-CACTAGGAG-3%, lower primer (position 1913 –
1937); 5%
-TCCCCATCAGGCATAGAATCCAT-T-3%] were designed to specifically amplify 3% UTR (untranslated region) of AS cDNA clone. Total RNAs (1 mg) from each developmental stage were
used as template for reverse transcription after RNase-free DNase (Promega) treatment. To assess nitrogenase activity during nodule development, 618 bp between position 88 and position 705 of
nifH ORF encoding nitrogenase reductase [32] was amplified. Transcripts of PUB (polyubiquitin) were also amplified as an indirect RT-PCR control using two primers specific to 3% UTR of PUB
cDNA clone [23]. PCR cycling conditions for AS (nifH and PUB) were 95°C for 5 min for initial denaturation followed by 95°C for 1 min, 62°C (67°C for nifH) for 1 min and 72°C for 1 min (30 cycles) with 10 min final extension at 72°C. Am-plified PCR products were electrophoresed on agarose gel, transferred to nylon membrane, and probed with inserts of AS, PUB and nifH clone. The hybridization signals of the blot were quantified with an image densitometer (Bio-Rad).
2.7. In situ hybridization
Tissue preparation was performed essentially as described by Cox and Goldberg [33]. Nodules were fixed overnight in FAA [50% (v/v) EtOH, 5% (v/v) glacial acetic acid, 10% (v/v) formaldehyde] under constant vacuum, dehydrated through a graded ethanol series, and embedded in Paraplast (Oxford, St. Louis. Mo. USA). Tissue sections (10
mm thick) were applied to precleaned slide glasses
Burlingame, CA, USA). Pretreatment, hybridiza-tion and washing of slides were performed essen-tially as described by McKhann and Hirsch [34], except for addition of RNase (30 mg RNase A/
NTE buffer 1 ml) in the course of washing. Anti-sense and sense RNA probes for in situ hybridization were prepared from linearized plas-mids with digoxigenin (DIG)-11-rUTP (Boehringer Mannheim) according to manufactur-er’s instruction. Hybridization was performed at 42°C for 16 h. After posthybridization treatment and incubation with antidigoxigenin conjugated with alkaline phosphatase, color development was allowed in a dark cabinet for approximately 1 – 2 day(s) with substrate, and stopped by immersing the slides in TE (pH 8.0). The sections were dehy-drated through a graded ethanol series and then mounted with Permount (Fisher Scientific, Fair Lawn, NJ, USA).
3. Results and discussion
3.1. Isolation of a cDNA clone encoding AS from
root nodule of E. umbellata
A cDNA library using 5 mg poly(A)+ RNA
from root nodule ofE. umbellata was constructed to isolate nodule-specific/enhanced cDNA clones. Primary plaque forming units (pfu) of the cDNA library were 5.5×105 with 95% recombination
efficiency and average size of the cDNA insert was about 1.5 kb. The 105phage clones were
competi-tively hybridized with 32P-labelled single-strand
nodule cDNA probe and an excess of total RNAs from roots and leaves, resulting in isolation of 117 putative nodule-specific/enhanced clones. A clone with a 0.85 kb insert showed nucleotide sequence homology with previously reported AS and cross-hybridized with 37 AS homologues out of 117 clones. Full nucleotide sequence of a cDNA clone with 2 kb insert out of 37 clones, which named pEuNOD-AS1, was determined and analyzed.
3.2. Nucleotide and deduced amino acid sequence
analysis of EuNOD-AS1
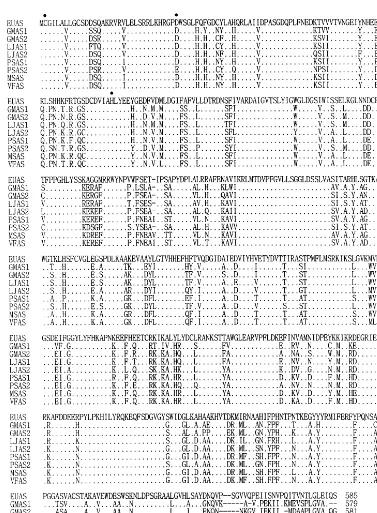
Asparagine synthetase clone, pEuNOD-AS1, coded for 585 amino acid residues (Fig. 1) with molecular weight of 65.8 kDa and pI (isoelectric point) value of 6.12. The deduced amino acid
sequence of EuNOD-AS1 showed overall sequence identities of 74 – 88, 50, 53 and 55% with those of other plant species, human, yeast (Asn2), and E.
coli (AsnB), respectively. Gln-dependent AS (AsnB) and ammonia-dependent AS (AsnA) were known in E. coli [9,35] and the deduced amino acid sequence of EuNOD-AS1 showed much higher sequence identity with the former (55%) than with the latter (14%).
The multiple alignment of deduced amino acid sequence of EuNOD-AS1 with those of previously reported nodule-forming leguminous plants showed many conserved sequence motifs through-out the overall sequence, except for highly variable regions in C-terminus (Fig. 1). A highly conserved region among AS and glutamine aminotrans-ferases (E. coli and yeast) is a region of six amino acid residues, His30, Arg31, Gly32, Pro33, Asp34, and
Ala35 [36]. The proline residue is not conserved in
the glutamine amidotransferases, whereas the ala-nine residue is not conserved in plant AS. The glutamine-binding domain of purF-type glutamine amidotransferases is thought to reside in the cata-lytic triad, Cys2, Asp29, and His102 of N-terminus
[36]. These residues reside to positions 2, 34, and 104, respectively, in all known plant AS.
The N-terminus of EuNOD-AS1 contains the invariant Cys2 residue, which has been shown
through site-directed mutagenesis of the human AS gene to be essential for Gln-dependent AS activity [37]. However, the variation of histidine consisting of catalytic triad in yeast Asn2 and E.
coli AsnB raises questions about whether this
residue of the catalytic triad is absolutely required for Gln-dependent AS activity. Further, there are several other conserved regions that may play structural or functional roles for ATP- and/or aspartate-binding sites. Along with the result showing higher sequence identity with Gln-depen-dent form than ammonia-depenGln-depen-dent form, the presence of glutamine-binding domain on EuNOD-AS1 strongly supports that EuNOD-AS1 uses glutamine as a substrate.
3.3. Expression pattern of EuNOD-AS1
The expression pattern of AS in leaf, root and nodule ofE. umbellatawas examined by Northern hybridization with full insert of pEuNOD-AS1 as
a probe. As shown in Fig. 2, AS mRNA was specifically detected in nodule as a 2.2 kb tran-script size. But, AS mRNAs of leaf and root were also very weakly detected after longer exposure (data not shown).
Fig. 1. Multiple alignment of amino acid sequence deduced from nucleotide sequence of pEuNOD-AS1 with ASs of nodule-form-ing leguminous plants usnodule-form-ing Clustal W. Filled circles indicate conserved amino acid residues forpurF-type Gln-binding domain. Identical residues are indicated by dots. Dashes indicate gaps introduced to maximize sequence similarity. The sources of AS polypeptide sequences used in this alignment are: EUAS1,E.umbellata(Genbank Acc. No. AF061740); GMAS1 and GMAS2,
Glycine max(U77679 and U77678); LJAS1 and LJAS2,Lotus japonicus(X89409 and X89410); PSAS1 and PSAS2,Pisum sati6um
Fig. 2. Expression of AS in different organs ofE.umbellata. Blot containing 10 mg total RNA per lane were hybridized
with the 32P-labeled insert of pEuNOD-AS1 (A). Gel was
stained with ethidium bromide before blotting to ensure equal loading of RNA (B). Arrows indicate the positions of rRNA bands. L, leaf; R, root; N, nodule.
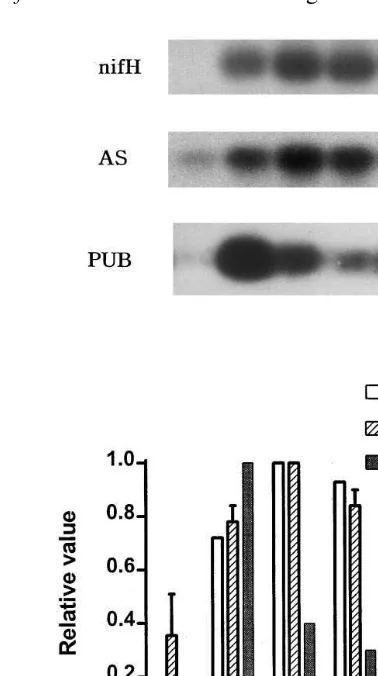
GOGAT [43]. Expression of nifH and AS was decreased 10 WAI, when PUB was highly ex-pressed (Fig. 3). Polyubiquitin is closely related with senescence of cells, tissues, organs, and entire organisms [44].
The spatial expression of AS in E. umbellata
root nodule was determined by in situ hybridiza-tion of longitudinal sechybridiza-tions of nodules with DIG-labeled antisense and sense RNA probes, respectively (Fig. 4A, B, C and F). In the course of in situ hybridization, antisense RNA probe of
nifH was used as a marker gene to confirm
in-Fig. 3. RT-PCR analysis of the expression of AS and PUB during nodule development.nifH transcripts encoding nitro-genase reductase were amplified as a marker gene to assess nitrogenase activity during nodule development. All total RNAs used in this experiment were treated with RNase-free DNase. RT-PCR products separated on agarose gel were transferred on nylon membrane, and probed with32P-labeled
DNAs containingnifH ofFrankia EuIK1 [32] and inserts of pEuNOD-AS1 and pEuNOD-PUB1 [23]. The hybridization signals were quantified using an image densitometer (BIO-RAD). Data are the mean values of two independent experi-ments. Error bars indicate standard deviation. R, root; N4, 4 weeks after inoculation (WAI); N6, 6 WAI; N8, 8 WAI; N10, 10 WAI.
Asn is a major nitrogen-containing compound in the xylem sap of temperate legumes such as alfalfa and pea. An increased AS activity has been correlated with the accumulation of Asn in alfalfa root nodules [39]. AS activity has been known to be increased in the root nodules of plant species transporting fixed nitrogen product into amide forms [19,40,41].
Fig. 4. In situ localization of AS transcript in longitudinal sections ofE.umbellataroot nodules. Antisense probe ofnifH was also used as a marker gene to identify infected cells in fixation zone. Antisense and sense RNA probes for in situ hybridization were prepared from linearized plasmids with digoxigenin (DIG)-11-rUTP (Boehringer Mannheim) according to manufacturer’s instruction. Boxed regions of panel a and d were detailed on panel b and e, respectively. Arrows indicate hybridization signals with purple color. A, longitudinal section probed with antisense probe of AS; B, detailed view of fixation zone of panel A; C, sense probe of AS; D, antisense probe ofnifH; E, detailed view of fixation zone of panel D; F, sense probe ofnifH. MZ, meristem zone; FZ, fixation zone; VS, vascular system; PD, periderm; IC, infected cell; UC, uninfected cell. Bars of panel A, C, D and F=250
mm, bars of panel B and E=50mm.
fected and nitrogen-fixing cells in the root nodule [45,3] (Fig. 4D and E). In situ hybridization using AS antisense RNA probe showed AS transcripts were detected only at the infected cells completely filled with Frankia hyphae in fixation zone (Fig. 4A and B), where nifH transcripts were also de-tected (Fig. 4D and E). No hybridization signal was detected with AS and nifH sense RNA probe (Fig. 4C and F).
Shi et al. [19] showed that AS mRNA was detected in ineffective nodule of alfalfa, although the expression level was much lower than that of effective nodule. AS transcripts were also detected in both infected and uninfected cells of the symbi-otic zone and in the nodule parenchyma of alfalfa
root nodule. From these data, Shi et al. [19] suggested that initial signal for AS expression in alfalfa nodule is unrelated to the presence of func-tional nitrogenase. On the other hand, our data showed that the expression pattern of AS during nodule development was very similar with that of
hybridization result, GS, key enzyme involved in primary ammonia assimilation in root nodule, were strongly expressed in the infected cells of fixation zone of Alnus glutinosa nodule, indicating that GS is under metabolic control [5].
3.4. AS genes in the genome of E. umbellata
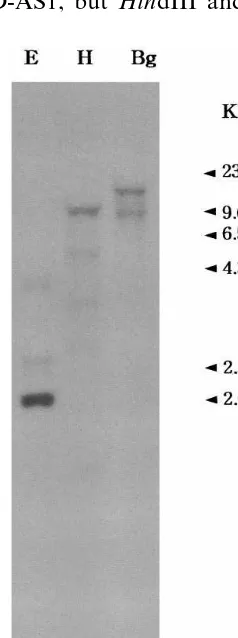
A genomic Southern hybridization was per-formed using full length cDNA insert of pE-uNOD-AS1 as a probe under high stringency condition (Fig. 5). Total genomic DNA was di-gested with restriction enzymes, EcoRI, HindIII and BglII. As shown in Fig. 5, several strong or weak hybridization signals were detected in each lane; 2.0, 2.5, and 3.7 kb EcoRI fragments, 1.5, 3.0, 3.5, 5.5, and 9.6 kb HindIII fragments and 8.0, and 15.0 kb BglII fragments. An EcoRI re-striction site is located on the nucleotide sequence of pEuNOD-AS1, but HindIII and BglII site are
not located on the sequence (data not shown). Along with result that another cDNA clone (pE-uNOD-AS2) showing different digestion pattern was isolated, this hybridization pattern indicates that AS may be encoded by at least two genes. Two different AS cDNA clones have been also isolated from pea [17], trefoil [18] and soybean [20].
In conclusion, we showed that a high expression level of AS in the root nodule of E. umbellata is closely related with nitrogen-fixation. Accordingly, we suggest its role as to further assimilate excess ammonia made by nitrogen fixation in infected cells, and its expression may be under metabolic control rather than developmental control of the root nodule.
Acknowledgements
This work was supported by Non Directed Re-search Fund (D 0189), Korea ReRe-search Founda-tion (1996). The authors would like to thank Dr Ann M. Hirsch for her kind help in in situ hybridization.
References
[1] D.D. Baker, W.B.C.R. Schwintzer, Introduction, in: J.D. Tjepkema (Ed.), The Biology of Frankia and Acti-norhizal Plants, Academic Press, New York, 1990, pp. 1 – 13.
[2] W. Newcomb, S.M. Wood, Morphogenesis and fine structure of Frankia (Actinomycetes): The microsym-biont of nitrogen-fixing actinorhizal root nodules, Int. Rev. Cytol 109 (1987) 1 – 88.
[3] A. Ribeiro, A.D.L. Akkermans, A. Van Kammen, T. Bisseling, K. Pawlowski, A nodule-specific gene encoding a subtilisin-like protease is expressed in early stages of actinorhizal nodule development, Plant Cell 7 (1995) 785 – 794.
[4] K.R. Schubert, Products of biological nitrogen fixation in higher plants: synthesis, transport, and metabolism, Ann. Rev. Plant Physiol. 37 (1986) 539 – 574.
[5] C. Guan, A. Ribeiro, A.D.L. Akkermans, Y. Jing, A. Van Kammen, T. Bisseling, K. Pawlowski, Nitrogen metabolism in actinorhizal nodules of Alnus glutinosa: expression of glutamine synthetase and acetylornithine transaminase, Plant Mol. Biol. 32 (1996) 1177 – 1184. [6] K.A. Sieciechowicz, K.W. Joy, R.J. Ireland, The
metabolism of asparagine in plants, Phytochemistry 27 (1988) 663 – 671.
[7] A.A. Urquhart, K.W. Joy, Use of phloem exudate tech-nique in the study of amino acid transport in pea plants, Plant Physiol. 68 (1981) 750 – 754.
Fig. 5. Genomic Southern hybridization of AS. Genomic DNAs (10 mg) digested with restriction enzymes were
sepa-rated on 1% agarose gel and corresponding nylon membrane was probed with 32P-labeled insert of pEuNOD-AS1 under
[8] P.J. Lea, B.J. Miflin, Transport and metabolism of as-paragine and other nitrogen compounds within the plant, in: P.K. Stumpf, E.E. Conn (Eds.), The Biochemistry of Plants, Academic press, New York, 1980, pp. 569 – 607. [9] J. Felton, S. Michaelis, A. Wright, Mutations in two
unlinked genes are required to produce asparagine aux-otrophy inEscherichia coli, J. Bacteriol. 142 (1980) 221 – 228.
[10] F. Ramos, J.M. Wiame, Two asparagine synthetases in
Saccharomyces cere6isiae, Eur. J. Biochem. 108 (1980)
373 – 377.
[11] I. Stulen, G.F. Israelstam, A. Oaks, Enzymes of as-paragine synthesis in maize roots, Planta 146 (1979) 237 – 241.
[12] G. Tjaden, G.M. Coruzzi, Glutamine and asparagine biosynthesis: the regulation of genes for enzymes along a common nitrogen-metabolic pathway, in: D.P.S. Verma (Ed.), Control of Plant Gene Expression, CRC, Boca Raton, 1993, pp. 459 – 470.
[13] K.M. Davies, G.A. King, Isolation and characterization of a cDNA for harvest-induced asparagine synthetase from Asparagus officinalis L, Plant Physiol. 102 (1993) 1337 – 1340.
[14] H.M. Lam, S.S.Y. Peng, G.M. Coruzzi, Metabolic regu-lation of the gene encoding glutamine-dependent as-paragine synthetase in Arabidopsis thaliana, Plant Physiol. 106 (1994) 1347 – 1357.
[15] C.G. Downs, B.J. Pogson, K.M. Davies, E.C. Amira, An asparagine synthetase cDNA clone (GenBank X84448) from broccoli (PGR95-016), Plant Physiol. 108 (1995) 1342.
[16] C. Chevalier, E. Bourgeois, D. Just, P. Raymond, Metabolic regulation of asparagine synthetase gene ex-pression in maize (Zea mays L.) root tips, Plant J. 9 (1996) 1 – 11.
[17] F. Tsai, G.M. Coruzzi, Dark-induced and organ-specific expression of two asparagine synthetase genes in Pisum sati6um, EMBO J. 9 (1990) 323 – 332.
[18] R.N. Waterhouse, A.J. Smyth, A. Massonneau, I.M. Prosser, D.T. Clarkson, Molecular cloning and charac-terisation of asparagine synthetase fromLotus japonicus: dynamics of asparagine synthesis in N-sufficient condi-tions, Plant Mol. Biol. 30 (1996) 883 – 897.
[19] L. Shi, S.N. Twary, H. Yoshioka, R.G. Gregerson, S.S. Miller, D.A. Samac, J.S. Gantt, P.J. Unkefer, C.P. Vance, Nitrogen assimilation in alfalfa: Isolation and characterization of an asparagine synthetase gene show-ing enhanced expression in root nodules and dark-adapted leaves, Plant Cell 9 (1997) 1339 – 1356.
[20] C.A. Hughes, H.S. Beard, B.F. Matthews, Molecular cloning and expression of two cDNAs encoding as-paragine synthetase in soybean, Plant Mol. Biol. 33 (1997) 301 – 311.
[21] H. Kuster, U. Albus, M. Fruhling, S.A. Tchetkova, L.A. Tikhonovitch, A. Puhler, A.M. Perlick, The asparagine synthetase gene VfAS1 is strongly expressed in the nitro-gen-fixing zone of broad bean (Vicia faba L.) root nod-ules, Plant Sci. 124 (1997) 89 – 95.
[22] S.C. Kim, C.D. Ku, M.C. Park, C.H. Kim, S.D. Song, C.S. An, Isolation of symbiotic Frankia EuIK1 strain from root nodule ofElaeagnus umbellata, Korean J. Bot. 36 (1993) 177 – 182.
[23] H.B. Kim, Structures and Expression Patterns of cDNA Clones Encoding Asparagine Synthetase, Chitinase, and Polyubiquitin from the Root Nodule of Elaeagnus um
-bellata, Ph.D. dissertation, Seoul National University, Seoul, 1998.
[24] F.M. Ausubel, R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, K. Struhl, Current Protocols in Molecular Biology, John Wiley and Sons, New York, 1987, pp. 4.5.1 – 4.5.3.
[25] J.J. Doyle, J.I. Doyle, Isolation of plant DNA from fresh tissue, Focus 12 (1990) 13 – 15.
[26] J. Sambrook, E.F. Fritsch, T. Maniatis, Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 1989 (Chapter 1, 2, 7, 8, 9 and 10).
[27] G. Mangiarotti, S. Chung, C. Zucker, H.F. Lodish, Selection and analysis of cloned developmentally-regu-lated Dictyostelium discoideum genes by hybridization competition, Nucl. Acids Res. 9 (1981) 947 – 963. [28] S. Henikoff, Unidirectional digestion with exonuclease
III creats targetted breakpoint for DNA sequencing, Gene 28 (1984) 351 – 359.
[29] F. Sanger, S. Nicklen, A.R. Coulson, DNA sequencing with chain terminating inhibitors, Proc. Natl. Acad. Sci. USA. 74 (1977) 5463 – 5467.
[30] W.R. Pearson, D.J. Lipman, Improved tools for biologi-cal sequence comparison, Proc. Natl. Acad. Sci. USA. 85 (1988) 2444 – 2448.
[31] S.F. Altschul, W. Gish, W. Miller, E.W. Meyers, D. Lipman, Basic local alignment search tool, J. Mol. Biol. 215 (1990) 403 – 410.
[32] H.B. Kim, C.S. An, Nucleotide sequence and expression of nifH,D from Frankia EuIK1 strain, a symbiont of
Elaeagnus umbellata, Physiol. Plant. 99 (1997) 690 – 695. [33] K.H. Cox, R.B. Goldberg, Analysis of plant gene expres-sion, in: C.H. Shaw (Ed.), Plant Molecular Biology: A Practical Approach, IRL press, Oxford, 1988, pp. 1 – 35. [34] H.I. Mackhann, A.M. Hirsch, In situ localization of specific mRNAs in plant tissues, in: B.R. Glick, J.E. Thompson (Eds.), Methods in Plant Molecular Biology and Biotechnology, CRC press, Boca Raton, 1993, pp. 179 – 205.
[35] R. Humbert, R.D. Simoni, Genetic and biochemical studies demonstrating a second gene coding for as-paragine synthetase inEscherichia coli, J. Bacteriol. 142 (1980) 212 – 220.
[36] B. Mei, H. Zalkin, A cysteine-histidine-aspartate cata-lytic triad is involved in glutamine amide transfer func-tion in purF-type glutamine amidotransferases, J. Biol. Chem. 264 (1989) 16613 – 16619.
[37] G. Van Heeke, S.M. Schuster, The N-terminal cysteine residue of human asparagine synthetase is essential of glutamine-dependent activity, J. Biol. Chem. 264 (1989) 19475 – 19478.
[38] M.J. Boland, J.F. Hanks, P.H.S. Reynolds, D.G. Blevins, N.E. Tolbert, K.R. Schubert, Subcellular orga-nization of ureide biogenesis from glycolytic intermedi-ates and ammonium in nitrogen-fixing soybean nodules, Planta 155 (1982) 45 – 51.
II. Asparagine synthesis, Biochem. Cell Biol. 66 (1988) 1349 – 1354.
[40] B.J. Miflin, J.V. Cullimore, Nitrogen assimilation in the legume Rhizobium symbiosis: a joint endeavour, in: D.P.S. Verma, T. Hohn (Eds.), Genes Involved in Mi-crobe-Plant Interactions, Springer-Verlag, Vienna, 1984, pp. 129 – 178.
[41] P.H. Reynolds, D.G. Blevins, M.J. Boland, K.R. Schu-bert, D.D. Randall, Enzymes of ammonia assimilation in legume nodules: a comparison between ureide- and amide-transporting plants, Physiol. Plant. 55 (1982) 255 – 260.
[42] R.V. Klucas, Studies on soybean nodule senescence, Plant Physiol. 54 (1994) 612 – 616.
[43] R.G. Groat, C.P. Vance, Root nodule enzymes of am-monia assimilation in alfalfa (Medicago sati6aL.): devel-opmental patterns and response to applied nitrogen, Plant Physiol. 67 (1981) 1198 – 1203.
[44] W.R. Belknap, J.E. Garbarino, The role of ubiquitin in plant senescence and stress responses, Trends Plant Sci. 1 (1996) 331 – 335.
[45] K. Pawlowski, A.D.L. Akkermans, A. Van Kammen, T. Bisseling, Expression ofFrankia nif genes in nodules of
Alnus glutinosa, Plant Soil 170 (1995) 371 – 376.