I
n the time between germinating and establishing phototrophic growth, seedlings rely on nutrients stored in the seed. In the Gramineae, aleurone cells help mobilize these reserves by syn-thesizing and secreting a variety of hydrolytic enzymes that par-ticipate in the breakdown of endosperm starch, protein, lipid and cell walls. In addition, aleurone cells contain stored phytin, the seed’s reserves of myo-inositol, phosphorous and mineral cations, such as K1and Mg21. T
hese are also released into the endosperm
after germination. Aleurone cells eventually die a few days after germination. Reserve mobilization by aleurone cells is strongly correlated with gibberellin (GA) produced de novo by the embryo, and with a decline in abscisic acid (ABA) content of the endosperm1. The relative ease with which aleurone tissue can be isolated from other cell types, combined with the specificity of the responses of these cells to GA and ABA, has made aleurone a model system for studying the cell and molecular biology of these hormones.
GA and ABA influence a range of other events during growth and development2,3, and studies of Arabidopsis mutants defective in GA and ABA signalling4
have led to the identification of some components of these signalling pathways. These recent develop-ments in GA and ABA signalling in aleurone are reviewed here, together with insight gained from molecular genetic studies with Arabidopsis (see also Refs 5–8).
How do aleurone cells sense GA and ABA?
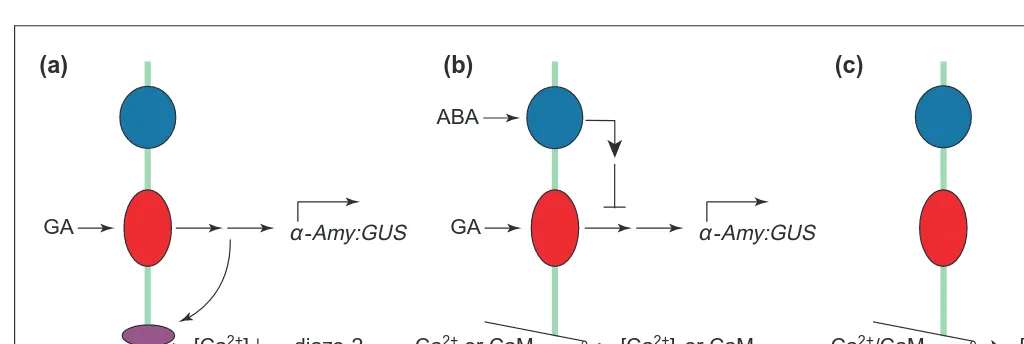
Evidence from two different experimental approaches suggests that GAs are perceived at the aleurone plasma membrane. First, GA4covalently coupled to Sepharose beads is membrane-imper-meant, but nevertheless stimulates high-level a-amylase gene expression and protein secretion in aleurone protoplasts9. Second, microinjection of GA into aleurone protoplasts does not stimulate a-amylase gene expression, and it is only when GA is present in the protoplast incubation medium that they respond10
. Other stud-ies11,12also support a plasma membrane site of GA action (Fig. 1). Microinjection of ABA into GA-treated barley aleurone proto-plasts does not appreciably inhibit GA-induced expression of an Amy:GUS reporter or a-amylase production, whereas ABA in the incubation medium does10. Thus, ABA inhibition of GA-action is mediated by perception at the plasma membrane. However, induc-tion of the ABA-responsive Em:GUS in barley aleurone proto-plasts can be achieved either by microinjecting ABA or by adding it to the medium6,13, suggesting that in this response ABA is per-ceived inside the protoplast (Fig. 1). Stomatal guard cells also appear to have internal and external sites of ABA perception14.
The current challenge is to identify GA and ABA receptors unequivocally. There has been some progress in the identification of GA binding proteins in aleurone, although no ABA-binding proteins have been found in this tissue.
Gibberellin and abscisic acid
signalling in aleurone
Alison Lovegrove and Richard Hooley
The plant hormones gibberellin and abscisic acid regulate gene expression, secretion and cell death in aleurone. The emerging picture is of gibberellin perception at the plasma membrane whereas abscisic acid acts at both the plasma membrane and in the cytoplasm – although gib-berellin and abscisic acid receptors have yet to be identified. A range of downstream-signalling components and events has been implicated in gibberellin and abscisic acid signalling in aleu-rone. These include the Gasubunit of a heterotrimeric G protein, a transient elevation in cGMP,
Ca21
-dependent and Ca21
-independent events in the cytoplasm, reversible protein phosphory-lation, and several promoter cis-elements and transcription factors, including GAMYB. In parallel, molecular genetic studies on mutants of Arabidopsis that show defects in responses to these hormones have identified components of gibberellin and abscisic acid signalling. These two approaches are yielding results that raise the possibility that specific gibberellin and abscisic acid signalling components perform similar functions in aleurone and other tissues.
Fig. 1. Simple scheme depicting the perception of gibberellin (GA)
and abscisic acid (ABA) in aleurone cells. GA is perceived by a receptor (red) at the plasma membrane (green line) and induces
a-amylase gene expression via a signal transduction pathway (arrows). ABA inhibition of this response is mediated by an ABA receptor at the plasma membrane (dark blue) and a signal trans-duction pathway (arrows and lines), which represses GA sig-nalling. ABA stimulation of Em:GUS expression is via an intracellular ABA receptor (pale blue).
Trends in Plant Science
GA gene expressionα-Amylase
Em:GUS expression ABA
GA-binding proteins at the plasma membrane
The development of a method for isolating large quantities of highly purified aleurone plasma membrane, combined with the use of a high specific-activity 125I-labelled GA-photoaffinity probe, led to the detection of two plasma membrane polypeptides of ~18 kDa and 68 kDa (Ref. 15). Photoaffinity labelling of these was com-peted by biologically active GA4, GA1and unlabelled photoaffin-ity probe, whereas biologically inactive GA34did not compete. Polypeptides of 18 kDa and 68 kDa were also GA-photoaffinity labelled in plasma membrane from vegetative tissue of pea, sweet pea (Lathyrus odoratus) and Arabidopsis. The GA-binding pro-teins could not be detected on intracellular membranes and 2D-gel analysis demonstrated their low abundance15
. Preliminary N-termi-nal amino acid sequence from the 18-kDa polypeptide purified from Arabidopsis has yet to identify a potential function. Never-theless, the possibility that the polypeptides are involved in GA action is suggested by the observation that they are less intensely labelled in the sweet pea semi-dwarf GA-sensitivity mutant lb compared with wild-type Lb (Ref. 15). On non-reducing SDS gels, the 18 kDa and 68 kDa polypeptides are both resolved, indi-cating that they are not disulphide-linked as part of the same pro-tein (A. Lovegrove, unpublished). The fact that both polypeptides are labelled by the GA-photoaffinity probe suggests that the GA receptor might be a complex of at least two proteins (Box 1).
Heterotrimeric G proteins transduce GA signals
Evidence is emerging that functional counterparts of several com-ponents of the G protein-signalling pathway exist in plants16. Recent results from two independent lines of enquiry have revealed a possible role of heterotrimeric G proteins in GA signal transduction in wild oat and rice aleurone. The mastoparan ana-logue Mas7 stimulates GDP–GTP exchange by heterotrimeric G proteins, and is thought to mimic activated G protein-coupled receptors. When wild oat aleurone protoplasts are incubated with Mas7 they produce and secrete a-amylase in a dose-dependent manner and with an identical time course to GA (Ref. 17). As with GA, the effect is largely overcome by ABA. Mas7 induces a-amyl-ase mRNA and expression of a-Amy2/54:GUS. This raises the possibility that Mas7 is activating a heterotrimeric G protein in the GA-signalling pathway. Further evidence supporting this theory has come from studying the effects of hydrolysis-resistant guanine nucleotides on GA-induction of a-Amy2/54:GUS expression17. The hydrolysis-resistant guanine nucleotide analogues GTP-g-S and GDP-b-S bind to Gasubunits and hold them in either the acti-vated (GTP-g-S-bound) or inactiacti-vated (GDP-b-S-bound) form. GDP-b-S introduced into aleurone protoplasts during transfection with reporter gene constructs prevents GA induction of a-Amy2/54:GUS expression, whereas GTP-g-S stimulates expres-sion slightly. Taken together the Mas 7 and guanine nucleotide data strongly suggest that a heterotrimeric G protein(s) is involved at an early stage of the GA-signalling pathway in wild-oat aleu-rone. It predicts that aleurone cells should contain G protein sub-units, which was confirmed by PCR and northern analysis for a partial Ga subunit cDNA and two related Gb cDNAs (Ref. 17). ABA inhibition of GA-induction of a-amylase can partly be over-come by treating wild-oat aleurone protoplasts with cholera or per-tussis toxin (H.D. Jones and R. Hooley, unpublished), perhaps indicating G protein involvement in ABA signalling in aleurone.
Embryo-less half seed of the Dwarf 1 mutant of rice produce little a-amylase when treated with GA, and Dwarf 1 seedlings elongate only slightly in response to GA (Ref. 18). These obser-vations are consistent with Dwarf 1 being a GA-sensitivity mutant18
, although we await measurement of the endogenous GAs in Dwarf 1 to see if they are elevated, as would be expected for
such a mutant. Mutations giving rise to Dwarf 1 are now known to be in the Gasubunit GPA1 (Refs 19,20). Because these clearly affect GA sensitivity of rice aleurone, these observations add further support to the theory that a G protein is involved in GA signalling in aleurone.
Precedents from well characterized G protein-signalling path-ways21 make it tempting to speculate that GA, perceived by a receptor at the plasma membrane, signals through a heterotrimeric G protein. Although this hypothesis requires testing experimen-tally, it is clear that the theory that plant hormone signals might be transduced by G protein-signalling pathways is further supported by the discovery of GCR1 – the first plant G protein-coupled receptor homologue – and the evidence that it might be involved in cytokinin signal transduction22
(Box 2).
Ca21/CaM-dependent and -independent events
The earliest event following GA treatment of aleurone is an increase in cytoplasmic calcium concentration ([Ca21
]i). In barley aleurone protoplasts, it occurs after 4–6 h (Ref. 23). In intact wheat aleurone cells, a faster response (2–5 min) has been observed24
, which does not occur in response to the inactive GA8. Elevation of [Ca21
]i by GA is quicker in some cells than others 13,24
, perhaps because of heterogeneity in the sensitivity of individual aleurone cells and protoplasts25,26
. Provided GA is present continuously, it causes sustained elevations in [Ca21]
ithat, when averaged over the whole cell, amount to increases of 100–500 nM above a resting level of 100–250 nM. Localized [Ca21]ican be much higher. Com-bined temporal and spatial analysis of [Ca21
]i (S. Gilroy, pers. commun.; www.bio.psu.edu/faculty/gilroy/algal.html) coupled with a known dependency of these changes on extracellular Ca21
, has led to the suggestion that GA alters Ca21flux at the plasma membrane13,23,24
. The mechanism is unknown, although one hy-pothesis is that the GA receptor might, directly or indirectly, regu-late a plasma membrane Ca21
channel, possibly through a heterotrimeric G protein. Release of Ca21from intracellular stores is another possibility27
. However, it is unlikely to be mediated by IP3 (Ref. 28), even though there is some evidence that GA causes a transient elevation in IP3 in rice aleurone
27
. In barley, ABA pre-vents the GA-stimulated increases in [Ca21]
iand, in the absence of GA, high concentrations of ABA have been proposed to lower [Ca21]
iby activating a plasma membrane Ca
21-ATPase (Ref. 29). Box 1. Gibberellin and abscisic acid perception:
key points
• Gibberellin (GA) is perceived at the aleurone plasma membrane. • Plasma membrane GA-binding proteins (18 kDa and 68 kDa)
occur in aleurone and other tissues.
• Abscisic acid (ABA) inhibition of GA action is mediated by perception at the aleurone plasma membrane.
• ABA induction of Em:GUS expression is mediated by perception inside the cell.
• No ABA-binding proteins have been identified in aleurone.
Box 2. Heterotrimeric G proteins: key points
• G protein agonists and antagonists can mimic and block gibberellin (GA) action.
These alterations in [Ca21]
iindicate a potential signalling role, and the fact that calmodulin (CaM) protein levels are stimulated two-to fourfold by GA, and reduced by ABA (Ref. 30), suggest that a Ca21
/CaM system might operate. This, and earlier data5,6
, strongly implicate GA-induced changes in Ca21and CaM in the secretion of hydrolases that are induced by GA (Fig. 2). Likely targets of Ca21/CaM are:
• An ER Ca21
-ATPase that can be activated by CaM, and prob-ably supports the demand for endomembrane Ca21driven by secretion of the a-amylase metalloprotein31
. • A tonoplast Ca21transporter32.
• Ca21
-stimulated secretory vesicle fusion that is modulated by a G protein33, the identity of which is unknown, although it is probably monomeric.
• A storage vacuole membrane slow-vacuolar (SV)-type cation channel5
(Fig. 2).
The slow-vacuolar-type cation channel was originally thought to be involved in the transport of K1
out of vacuoles, but it is now considered more likely to be involved in Ca21-induced Ca21
release. Ca21
/CaM are thought to affect phosphorylation at two sites on the SV-type channel, regulating its activity in a complex way. CaM-affinity screening of an aleurone cDNA library has been used in an attempt to identify other targets of Ca21
/CaM (Ref. 34). This led to the iso-lation of a plasma membrane-located CaM-binding transporter that might poten-tially be regulated by cyclic nucleotide monophosphates such as cGMP.
To identify Ca21- and CaM-dependent and -independent signalling events in aleurone, the [Ca21]
iand CaM levels were modified by microinjecting CaCl2, caged Ca
21 , Ca21 chelators, CaM and CaM antagonists into barley aleurone protoplasts13
. Blocking the GA-induced increase in [Ca21]
i using the Ca21
buffer diazo-2 inhibits GA-induced a-amylase secretion but does not appreci-ably affect Amy:GUS expression. CaM antagonists also inhibit a-amylase secre-tion. Attempts to mimic GA-induced [Ca21
]i and CaM changes by means of microinjec-tion do not stimulate Amy:GUS expression. In addition, ABA induction of Em:GUS is independent of changes in [Ca21
]iand CaM (Ref. 13). These data therefore exclude changes in Ca21
/CaM from GA signalling, which leads to the induction of a-amylase gene expression and ABA induction of Em:GUS in barley aleurone13 (Fig. 2). However, a study with rice aleurone27
con-cludes that Ca21 and CaM are important intermediaries in the GA induction of gene expression. Furthermore, the fact that [Ca21] i is elevated within minutes after GA treatment of wheat aleurone cells, whereas hydrolase synthesis and secretion does not take place until several hours later, argues that changes in [Ca21
]i might be involved in events that precede hydrolase secretion.
Further complexity has been uncovered by the discovery that if the decrease in [Ca21
]icaused by ABA is blocked in GA-treated barley aleurone protoplasts, ABA is prevented from inhibiting GA-induced Amy:GUS expression and a-amylase secretion13. This presents a paradox; artificially reducing [Ca21] i in GA-treated protoplasts does not affect Amy:GUS expression, although the ABA-driven reduction in [Ca21]
i is essential for ABA-inhibition of GA-induced Amy:GUS expression (Fig. 3). The inhibitory effect of ABA on GA-induced Amy:GUS expres-sion and a-amylase secretion can also be overridden by microin-jecting CaM, although it is not clear what effect the CaM has on [Ca21
]i. These experiments highlight an additional level of poten-tial complexity that might be explained by temporal or spapoten-tial dynamics of the [Ca21
]isignature. A further glimpse of such com-plexity might be the observation that overexpression of a putative ER Ca21
-ATPase in rice aleurone leads to GA-independent Osamy-c:luciferase expression, possibly by disrupting spatial or temporal aspects of [Ca21
]isignals 27
.
Taken together these data suggest that for both GA and ABA there are specific Ca21
/CaM-dependent and -independent signalling events. In the case of ABA, these correlate with responses
Fig. 2. Principal components in Ca21and CaM signalling in aleurone. Gibberellin (GA),
perceived by a receptor (red) at the plasma membrane (green line), elevates [Ca21]
iand CaM
and this is prevented by abscisic acid (ABA) acting through a receptor (dark blue) at the plasma membrane. Ca21and CaM are necessary for hydrolase secretion. CaM stimulates an
endoplasmic reticulum (ER) Ca21ATPase, thus elevating ER [Ca21]. Ca21/CaM regulate the
activity of a slow vacuolar channel that probably contributes to [Ca21]
imaintenance. Ca 21
stimulates secretory vesicle fusion, probably via a monomeric G protein . ABA stimulation of
Em gene expression [via an intracellular ABA receptor (pale blue)], and GA stimulation
of a-amylase and CaM gene expression, are Ca21 and CaM-independent.
Trends in Plant Science
associated with extracellular and intracellular perception sites, and therefore further support the concept of dual pathways. For GA, the simplest interpretation is that signalling downstream from a plasma membrane receptor is bifurcated, and that ABA antagonism of GA-induced a-amylase gene expression involves more than just a simple reversal of the GA-signalling events. Nevertheless, the emerging complexity in Ca21
/CaM signalling in aleurone suggests that additional control factors impart specificity and integration, and the identification of these is a current challenge (Box 3).
cGMP – a GA second messenger
Radioimmunoassay has been used to measure the cGMP in barley aleurone layers35
. Controls and layers treated with 5 mMABA con-tained the same amounts of cGMP over a 6 h period. GA-treated layers showed an approximately threefold rise in cGMP 2 h after GA addition, which returned to control levels within a further 2 h. It is not possible to accurately estimate the timing of this rise in cGMP relative to the elevation of [Ca21]
ibecause the elevation of [Ca21
]iwas determined in barley aleurone protoplasts. However, based on measurements of [Ca21]
i in wheat aleurone layers, the alterations in cGMP probably occur after the rise in [Ca21
]i. Nevertheless, they certainly precede the increase in transcription of a-amylase genes. An inhibitor of guanylyl cyclase, LY 83583 (LY), reduces the increase in cGMP and inhibits the induction of a-amylase and GAMYB mRNAs, as well as a-amylase protein secretion. LY also prevents DNA degradation and cell death36. Diutyryl-cGMP can largely, and specifically, overcome the inhibitory effects of LY, although on its own it cannot elicit a GA response. Therefore it is likely that the transient rise in cGMP is an important component in the signalling necessary for GA-regu-lated gene expression, a-amylase secretion and programmed cell death35,36. These data provide further support for a prominent sig-nalling role of cGMP in plant cells, and raise the questions of how guanylyl cyclase and cGMP phosphodiesterase are regulated in aleurone, and the identity of downstream targets of cGMP.
Protein kinase cascades
Reversible protein phosphorylation is a universal signal-transduc-ing mechanism and, not unexpectedly, there is both molecular and pharmacological evidence for the involvement of protein kinase
cascades in GA and ABA action. ABA treatment of wheat and bar-ley aleurone induces the expression of a serine/threonine protein kinase, PKABA1 (Ref. 37). A possible role for this kinase in ABA inhibition of GA-regulated gene expression is suggested from experiments in which constitutive overexpression of PKABA1, strongly inhibits GA induction of a-Amy1, a-Amy2 and cysteine proteinase promoter:GUS constructs (Fig. 4). Overexpression of a null mutant of PKABA1, or of a different CDPK, has no effect on GA induction of the a-Amy2 promoter:GUS construct. Overex-pressing the kinase also has a minor effect on ABA induction of HVA1 gene expression37
. This could indicate a broader signalling role for PKABA1, or might be an artifact of overexpression.
In an ingenious assay, protein kinase substrate peptides have been microinjected into barley aleurone protoplasts in the expec-tation that they would compete with the endogenous protein phos-phorylation targets and block kinase cascades38. One peptide, syntide-2, selectively inhibits GA-induction of a-Amy2:GUS, a-amylase secretion and protoplast vacuolation. The peptide does not prevent GA-stimulation of [Ca21
]i, indicating that it is acting down-stream of this early event in GA-signalling. Syntide-2, is a specific substrate for mammalian CaM-kinase II. To date, there is no direct evidence for a CaM-kinase II in plants therefore the identity of the aleurone kinase, or kinases, blocked by syntide-2 is not clear. Nevertheless, on the basis of a series of in vitro phosphorylation and protein-labelling assays, a putative Ca21
-activated CaM-like domain protein kinase (CDPK) that interacts with syntide-2 has been identified in barley aleurone cytosol38
. At this stage it is not known if syntide-2 blocks one or more events in the GA-signalling
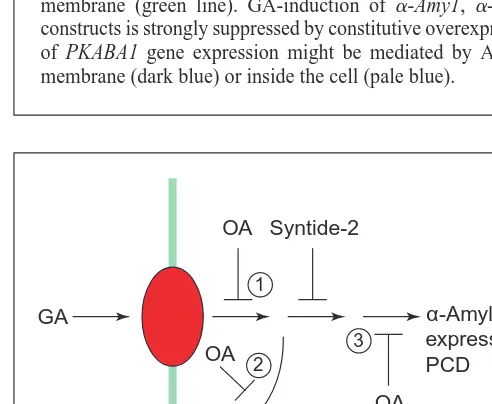
Fig. 3. Calcium signalling might be more complex than we presently understand. (a) Gibberellin (GA), perceived by a receptor (red) at the
plasma membrane (green line), stimulates an elevation in [Ca21]
iand expression of a-Amy:GUS. The Ca
21chelator diazo-2 will prevent the
elevation in [Ca21]
ibut does not inhibit a-Amy:GUS expression. (b) Abscisic acid (ABA), acting through a receptor (dark blue) at the plasma
membrane, prevents GA stimulation of both [Ca21]
iand a-Amy:GUS expression. However, microinjection of Ca
21or CaM restores a-Amy:GUS
expression. (c) Elevating [Ca21]
iand CaM in the absence of GA and ABA is not sufficient to stimulate a-Amy:GUS expression.
Trends in Plant Science
α α α
GA -Amy:GUS GA
[Ca2+]
i diazo-2 Ca2+ or CaM [Ca2+]i or CaM Ca2+/CaM [Ca2+]i/CaM
-Amy:GUS -Amy:GUS
(a) (b) (c)
ABA
Box 3. Calcium and calmodulin: key points
• Gibberellin (GA) stimulates [Ca21]
iand CaM, abscisic acid
(ABA) prevents this.
• Ca21/CaM maintain secretion in GA-treated protoplasts.
• Up-regulation of gene expression by GA and ABA is Ca21
/CaM-independent.
and response pathway (Fig. 5). Syntide-2 could potentially interfere with CDPK regulation of the SV-type cation channel5
although what effect this would have on signalling is not known. Syntide-2 does not affect ABA action, suggesting that the kinase acting on it does not crosstalk with ABA signalling.
Pharmacological studies have provided further evidence for kinases and phospha-tases in GA and ABA signalling. Okadaic acid (OA) inhibits GA-induced increases in [Ca21
]i, a-amylase mRNA, a-amylase secretion and aleurone cell death at con-centrations that inhibit the activity of serine/threonine protein phosphatases (PP) PP1 and PP2B (Ref. 39). The effect of OA on the elevation of [Ca21]
i caused by GA suggests that a reversible phosphorylation event is an essential part of this particular Ca21
signature, although not of the [Ca21 ]i elevation caused by hypoxia39. Although the target of this is not currently known it might prove useful to search for protein phosphorylation events that occur within minutes of GA treatment. Because OA also inhibits GA-induction of a-amylase gene expression and cell death – events indepen-dent of the elevation of [Ca21
]i– OA could be acting in several ways (Fig. 5).
In barley aleurone protoplasts, OA partly inhibits ABA-induced PHVA1 gene ex-pression39
, and tyrosine and serine/threo-nine phosphatase inhibitors prevent ABA-induced RAB gene expression, leading to tyrosine hyperphosphorylation of two 40 kDa polypeptides40
. A current hypothesis is that ABA regu-lation of rab16 gene expression in barley aleurone involves rapid stimulation of a putative mitogen-activated protein kinase (MAP kinase) via a tyrosine phosphatase41.
In summary, a range of molecular and pharmacological data supports the hypothesis that protein kinases and phosphatases are involved in GA and ABA signalling in aleurone. The identity of the enzymes, their targets and their precise relationships to GA-stimulated events are poorly defined. For ABA signalling, the apparent role of reversible protein phosphorylation in aleurone is supported by molecular genetic evidence that two OA-insensitive serine/threonine phosphatases are involved in ABA signalling in Arabidopsis3
. In the case of GA, investigations have highlighted that the role of [Ca21]
iin GA-regulation of gene expression is not fully understood (Box 4).
Putative serine/threonine-O-linked N-acetylglucosamine transferase is a negative regulator of GA signalling
Recessive mutations at the spindly (spy) locus of Arabidopsis confer a largely GA-independent phenotype characterized by a reduced GA requirement for germination, increased internode length and altered flowering time. All spy alleles tested partially suppress the phenotype of the GA-deficient mutant ga1-1. SPY is therefore thought to act as a negative regulator of GA signalling in Arabidopsis42. The barley homologue of SPY is expressed at a low level in aleurone cells. When it is over expressed behind the CaMV35S promoter in a tran-sient assay, SPY inhibits GA-induced expression of an a-amylase promoter-reporter construct43. Intriguingly, overexpression of SPY in aleurone stimulates the expression of an ABA-regulated dehydrin promoter. These data suggest that SPY has a conserved function as a negative regulator of GA responses in vegetative tissues and aleu-rone cells, and that it might be a positive regulator of ABA signalling in aleurone. Nevertheless, overexpression of signalling proteins might not reveal their true function in vivo, or accommodate sub-tleties of post-transcriptional regulation that might be essential to their action. Further studies will no doubt address such issues.
Fig. 4. The abscisic acid (ABA)-inducible protein kinase PKABA1 negatively regulates
gibberellin (GA)-inducible gene expression. GA is perceived by a receptor (red) at the plasma membrane (green line). GA-induction of a-Amy1, a-Amy2 or cysteine proteinase:GUS constructs is strongly suppressed by constitutive overexpression of PKABA1. ABA induction of PKABA1 gene expression might be mediated by ABA receptors at either the plasma membrane (dark blue) or inside the cell (pale blue).
Trends in Plant Science
α-Amy1/ α-Amy2:GUS
GA
ABA
ABA
PKABA1
Cysteine proteinase:GUS
PKABA1
?
Fig. 5. Scheme depicting possible protein phosphorylation events
revealed by okadaic acid (OA) and syntide-2. Gibberellin (GA) is perceived by a receptor (red) at the plasma membrane (green line). Because OA inhibits the GA stimulation of [Ca21
]i, a-amylase
gene expression and programmed cell death (PCD) it might be inhibiting PP1 or PP2B at a point in GA signalling upstream of these events (1), or at multiple sites downstream of this (2 and 3). Syntide-2 identifies a protein phosphorylation event downstream of the effect of GA on [Ca21
]ithat could be in the pathway to gene
expression or a-amylase secretion.
Trends in Plant Science
GA
α-Amylase secretion
[Ca2+] i
OA Syntide-2
Syntide-2 OA OA
1
2
3
α-Amylase gene
SPY has significant amino acid sequence identity to a family of serine and threonine-O-linked N-acetylglucosamine (O-GlcNAc) transferases (OGTs)42
that extends over both the characteristic tetratricopeptide repeat (TPR) and catalytic domains. Although OGT activity has yet to be demonstrated for SPY, it is likely that this signalling protein interacts with other proteins via the TPR domain and that O-GlcNAc modification affects their signalling potential42. However, there are numerous potential targets of OGTs: two possible targets of SPY O-GlcNAc modification are GAI and RGA.
GAI and RGA
To date there is no direct evidence for a role for GAI or RGA in GA signalling in aleurone cells. GAI and RGA are members of a small gene family in Arabidopsis, encoding nuclear-localized putative transcription factors44. GAI and RGA are GA-derepress-ible repressors of GA-mediated growth responses. The N-terminal region of these proteins contains a so-called DELLA region, which is thought to play a critical role in GA-derepression. It is now known that the maize dwarf-8 (d8) gene and two Reduced height loci of wheat, Rht-B1b (formerly Rht1) and Rht-D1b (formerly Rht2), are orthologs of GAI, which contain nucleotide substitutions resulting in the mutant genes encoding N-terminally truncated proteins45. The related Reduced height locus Rht-B1c of wheat is known to affect GA sensitivity in aleurone46
, raising the possibility that this class of proteins might have a role in GA signalling in aleurone (Box 5).
Regulation of gene transcription
The expression of a variety of aleurone hydrolase genes is induced or stimulated by GA, and this is overcome by ABA. Investigations have concentrated on the transcriptional regulation by GA and ABA of barley, wheat, rice and wild oat a-amylase gene promoters. Functional analysis of a-Amy1 and a-Amy2 gene promoters by transient expression and gel-retardation assays in combination with DNAse-1 footprinting, have led to the identification of several cis-elements and sites at which
nuclear proteins bind. In some cases, the trans-acting factors that bind to specific sequences have been cloned and character-ized. It appears that the regulation of a-Amy1 and a-Amy2 gene promoters involves the interplay of several classes of transcription factor (Fig. 6). As yet, relatively little is known about comparable elements in other GA-regulated genes.
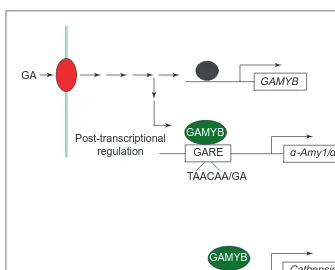
GA response element GAMYB and HRT
The GA response element (GARE) has been identified as a 21-nucleotide region of the barley Amy 1/6-4 promoter that, when present as six copies in a chimeric pro-moter driving a reporter gene, confers GA-responsive expression that could be overcome by ABA (Ref. 47). This region of the promoter in both a-Amy1 and a-Amy2 genes contains the sequence el-ement TAACAA/GA, which has been shown in functional studies to be an impor-tant component of the GARE (Refs 48,49). The GARE appears to bind GA-inducible nuclear proteins50. A potential similarity between the TAACAA/GA element and c-MYB and v-MYB recognition sequences
led to the isolation of a barley aleurone cDNA, encoding a MYB transcription factor that binds specifically to the TAACAA/GA element and is able to transactivate an a-Amy1 gene promoter in a transient assay51
. A rice GAMYB appears to have a similar function52(Fig. 7).
GAMYB expression is stimulated by GA in advance of a-amylase genes and, unlike a-amylase, is not dependent on de novo protein synthesis. These observations strongly suggest that GA signalling stimulates the expression of GAMYB, which in turn
Box 4. Protein kinase cascades: key points
• Protein kinase peptide substrate syntide-2 blocks some gibberellin (GA) responses.
• Ca21-activated CaM-like domain protein kinase might be
involved in GA-induction of a-amylase production. • GA stimulation of [Ca21
]iis inhibited by the protein phosphatase
inhibitor okadaic acid.
• Abscisic acid (ABA) suppression of GA-induced gene expression might involve protein kinase PKABA1.
• ABA-induced gene expression might involve a tyrosine-kinase and MAP-kinase.
Box 5. Arabidopsis gibberellin signalling genes: key points
• GA signalling genes identified in Arabidopsis might have functional counterparts in aleurone.
• SPY is a negative regulator of GA-induced a-amylase gene expression.
• SPY might be a positive regulator of ABA-induced dehydrin gene expression.
Fig. 6. Principal cis-elements in a-Amy1 and a-Amy2 promoters and factors that bind to them. GAMYB binds to the sequence TAACAA/GA in both a-Amy1 and a-Amy2 pro-moters. GAMYB functions within the GA-response complex (GARC) to transactivate
a-Amy1 and a-Amy2 promoters. The GA response element (GARE) of a-Amy1 promoters binds the repressor HRT. Box 2 is a binding site for the WRKY proteins ABF1 and ABF2 in a-Amy2 promoters, although the regulation of ABF1 and ABF2 is not understood.
Trends in Plant Science
α
α -Amy1
-Amy2
TAACAAA
TATCCAC GARE
Box 2
GARE Box 2
TAACAGA GARC
HRT GAMYB
GAMYB ABF1/2
GARC
induces the expression of a-Amy1 genes51
. Because GAMYB expression is super-induced by cycloheximide51one theory is that GA signalling might act on a rapidly turned-over repressor of GAMYB expression although the identity of this is unknown. Nevertheless, post-transcriptional regulation of GAMYB activity by phosphorylation, or interaction with other proteins, is also prob-ably based on models of how this class of transcription factors is regulated in other signalling systems53. Is GAMYB involved in stimulating the expression of other GA-regulated genes in aleu-rone? A recent study54has demonstrated that GAMYB can trans-activate the expression of GUS fused to promoter fragments of three other GA-regulated genes; an a-Amy2 gene, an EII(1-3,1-4)-b-glucanase gene and a cathepsin B-like protease gene. Analysis of these promoters reveals that the a-Amy2 gene contains a TAACAGA element, which is probably the GAMYB-binding site. However, neither the EII(1-3,1-4)-b-glucanase nor the cathepsin B-like protease promoters contain a TAACAA/GA element, and it is likely that GAMYB transactivates these promoters through closely related, although as yet not unambiguously defined, elements54.
GAMYB is not the only transcription factor that binds to the 21 nucleotide GARE (Ref. 47). Southwestern screens of a barley aleurone cDNA library using fragments of the Amy 1/6-4 moter have identified a novel, nuclear-located, zinc-finger pro-tein, HRT, which binds the 21 nucleotide GARE and can repress GA-induced expression from a-Amy1 and a-Amy2 promoters55. This HRT is therefore another control element acting on the GARE, although its regulation and relationships to GAMYB are not understood. The maize transcriptional activator VP1 can repress GA-induction of an a-Amy1 gene promoter56, although it
is not clear whether VP1 interacts directly with the a-amylase promoter or with other transcription factors bound to it.
GA-response complex
Although the GARE clearly plays a central role in GA activation of a-amylase gene expression, additional cis-elements are asso-ciated with the GARE and act as enhancers within a GA-response complex (GARC) that mediates GA- and ABA-responsive expres-sion in both high and low pI a-amylase pro-moters47,49,57–59
. In barley a-Amy1 genes, it is thought that the GARE and TATCCAT box comprise a GARC (Ref. 49). In a-Amy2 genes the GARC is thought to comprise the GARE and Box 2 (Ref. 59).
TATCCAC box
The TATCCAC box is present, and binds as yet uncloned nuclear protein, in both Amy1 and Amy2 genes51,59. Functional stud-ies suggest that this box49
, and sequence elements immediately downstream from it60
play an important role in driving GA-regulated expression.
Box 2 and WRKY proteins
Functional analysis has demonstrated that for a single copy of the GARE to function in a-Amy2 promoters, Box 2 must be pres-ent as a coupling elempres-ent57,58. Box 2 binds two members, ABF1 and ABF2, of the WRKY family of DNA-binding proteins61 although how these are regulated and whether or not they interact with GAMYB or HRT is not clear (Box 6).
Perspectives
Aleurone cells and protoplasts are an excellent system for study-ing GA regulation of gene expression, protein secretion, cell death and the antagonistic effect of ABA. The use of effector and reporter gene constructs, in combination with a range of pharma-cological agents, has helped to define elements of signalling path-ways that encompass events at the plasma membrane, in the cytoplasm and in the nucleus. Understanding the order and inte-gration of signalling events in aleurone is a challenge that should become accessible through the generation and use of mutants. Arabidopsis and cereal genomics are poised to identify a range of genes that might be involved – the function of these can be exam-ined in aleurone in the context of the role and regulation of this highly specialized secretory tissue.
Fig. 7. Model of gibberellin (GA) regulation of GAMYB. GA is perceived by a receptor
(red) at the plasma membrane (green line). GA stimulates the expression of GAMYB, possibly by acting on a rapidly turning-over repressor (black). GAMYB binds to the TAACAA/GA element in the GA response element (GARE) of a-Amy1 and a-Amy2 promoters, and to other sequences in cathepsin B-like protease and EII(1-3,1-4)-b-glucanase promoters. GA signalling might also act on GAMYB post-transcriptionally.
Trends in Plant Science GAMYB
GAMYB
GAMYB
GA
TAACAA/GA Post-transcriptional
regulation
GAMYB
-Amy1/ -Amy2
1-3,1-4 Glucanase Cathepsin B
GARE α
β
α
Box 6. Gene transcription: key points
• The gibberellin response element (GARE) of a-amylase promoters plays a central role in gibberellin (GA)- and abscisic acid (ABA)-regulated gene expression.
• GAMYB is induced by GA and binds to the TAACAA/GA element within the GARE.
• The repressor HRT also binds to elements within the GARE. • Other cis-elements and trans-acting factors are involved in
Acknowledgements
IACR receives grant-aided support from the Biotechnology and Biological Sciences Research Council of the UK. This work was supported by the BBSRC Intracellular Signalling Programme and EC Biotechnology FPIV. We apologize to colleagues whose research we were not able to cite because of space restrictions, and thank Drs John Lenton and Mike Holdsworth for useful comments on the manuscript.
References
1 Appleford, N.E.J. and Lenton, J.R. (1997) Hormonal regulation of a-amylase gene expression in germinating wheat (Triticum aestivum) grains. Physiol.
Plant. 100, 534–542
2 Hooley, R. (1994) Gibberellins: perception, transduction and responses.
Plant Mol. Biol. 26, 1529–1556
3 Giraudat, J. et al. (1994) Current advances in abscisic acid action and
signalling. Plant Mol. Biol. 26, 1557–1577
4 McCourt, P. (1999) Genetic analysis of hormone signaling. Annu. Rev. Plant
Physiol. Plant Mol. Biol. 50, 219–243
5 Bethke, P.C. et al. (1997) Hormonal signalling in cereal aleurone. J. Exp. Bot.
48, 1337–1356
6 Ritchie, S. and Gilroy, S. (1998) Tansley Review No 100. Gibberellins:
regulating genes and germination. New Phytol. 140, 363–383
7 Skadsen, R.W. (1998) Physiological and molecular genetic mechanisms regulating
hydrolytic enzyme gene expression in cereal grains. Physiol. Plant. 104, 486–502
8 Bethke, P.C. and Jones, R.L. (1998) Gibberellin signaling. Curr. Opin. Plant
Biol. 1, 440–446
9 Hooley, R. et al. (1991) Gibberellin perception at the plasma membrane of
Avena fatua aleurone protoplasts. Planta 183, 274–280
10 Gilroy, S. and Jones, R.L. (1994) Perception of gibberellin and abscisic acid at
the external face of the plasma membrane of barley (Hordeum vulgare L.) aleurone protoplasts. Plant Physiol. 104, 1185–1192
11 Beale, M.H. et al. (1992) A new approach to gibberellin perception in
aleurone: novel hydrophylic, membrane-impermeant, GA-sulphonic acid derivatives induce a-amylase formation. Physiol. Plant. 85, A136
12 Hooley, R. et al. (1992) Gibberellin perception and the Avena fatua aleurone:
do our molecular keys fit the correct locks? Biochem. Soc. Proc. 20, 85–89
13 Gilroy, S. (1996) Signal transduction in barley aleurone protoplasts is calcium
dependent and independent. Plant Cell 8, 2193–2209
14 Assmann, S.M. (1994) Ins and outs of guard cell ABA receptors. Plant Cell
6, 1187–1190
15 Lovegrove, A. et al. (1998) Gibberellin-photoaffinity labelling of two
polypeptides in plant plasma membrane. Plant J. 15, 311–320
16 Hooley, R. (1999) A role for G proteins in plant hormone signalling?
Plant Physiol. Biochem. 37, 393–402
17 Jones, H.D. et al. (1998) Heterotrimeric G proteins are implicated in
gibberellin induction of a-amylase gene expression in wild oat aleurone. Plant
Cell 10, 245–253
18 Mitsunaga, S. et al. (1994) Identification and characterization of
gibberellin-insensitive mutants selected from among dwarf mutants of rice. Theor. Appl.
Genet. 87, 705–712
19 Fujisawa, Y. et al. (1999) Suppression of the heterotrimeric G protein causes
abnormal morphology, including dwarfism, in rice. Proc. Natl. Acad. Sci.
U. S. A. 96, 7575–7580
20 Ashikari, M. et al. (1999) Rice gibberellin-insensitive dwarf mutant gene
Dwarf 1 encodes the a-subunit of GTP-binding protein. Proc. Natl. Acad. Sci.
U. S. A. 96, 10284–10289
21 Neer, E.J. (1995) Heterotrimeric G proteins: organizers of transmembrane
signals. Cell 80, 249–257
22 Plakidou-Dymock, S. et al. (1998) A higher plant seven-transmembrane
receptor that influences sensitivity to cytokinins. Curr. Biol. 8, 315–324
23 Gilroy, S. and Jones, R.L. (1992) Gibberellic acid and abscisic acid
coordinately regulate cytoplasmic calcium and secretory activity in barley aleurone protoplasts. Proc. Natl. Acad. Sci. U. S. A. 89, 3591–3595
24 Bush, D.S. (1996) Effects of gibberellic acid and environmental factors on
cytosolic calcium in wheat aleurone cells. Planta 199, 89–99
25 Hillmer, S. et al. (1992) Visualizing enzyme secretion from individual barley
(Hordeum vulgare) aleurone protoplasts. Plant Physiol. 102, 279–286
26 Ritchie, S. et al. (1999) The sensitivity of barley aleurone tissue to gibberellins
is heterogeneous and may be spatially determined. Plant Physiol. 120, 361–370
27 Chen, X. et al. (1997) Cloning of a Ca21-ATPase gene and the role of cytosolic Ca21in the gibberellin-dependent signaling pathway in aleurone cells. Plant J. 11, 363–371
28 Ritchie, S. and Gilroy, S. (1998) Abscisic acid signal transduction in the
barley aleurone is mediated by phospholipase D activity. Proc. Natl. Acad.
Sci. U. S. A. 95, 2697–2702
29 Wang, M. et al. (1991) Abscisic acid induces a cytosolic calcium decrease in
barley aleurone protoplasts. FEBS Lett. 278, 69–74
30 Schuurink, R.C. et al. (1996) Modulation of calmodulin mRNA and protein
levels in barley aleurone. Plant Physiol. 111, 371–380
31 Bush, D.S. (1995) Calcium regulation in plant cells and its role in signalling.
Annu. Rev. Plant Physiol. Plant Mol. Biol. 46, 95–122
32 Bush, D.S. and Wang, T. (1995) Diversity of calcium-efflux transporters in
wheat aleurone cells. Planta 197, 19–30
33 Homann, U. and Tester, M. (1997) Ca21-independent and Ca21/GTP-binding protein-controlled exocytosis in a plant cell. Proc. Natl. Acad. Sci. U. S. A. 94, 6565–6570
34 Schuurink, R.C. et al. (1998) Characterization of a calmodulin-binding
transporter from the plasma membrane of barley aleurone. Proc. Natl. Acad.
Sci. U. S. A. 95, 1944–1949
35 Penson, S.P. et al. (1996) cGMP is required for gibberellic acid-induced gene
expression in barley aleurone. Plant Cell 8, 2325–2333
36 Bethke, P.C. et al. (1999) Hormonally regulated programmed cell death in
barley aleurone cells. Plant Cell 11, 1033–1045
37 Gomez-Cadenas, A. et al. (1999) An abscisic acid-inducible protein kinase,
PKABA1, mediates abscisic acid-suppressed gene expression in barley aleurone layers. Proc. Natl. Acad. Sci. U. S. A. 96, 1767–1772
38 Ritchie, S. and Gilroy, S. (1998) Calcium-dependent protein phosphorylation may
mediate the gibberellic acid response in barley aleurone. Plant Physiol. 116, 765–776
39 Kuo, A. et al. (1996) Okadaic acid, a protein phosphatase inhibitor, blocks
calcium changes, gene expression, and cell death induced by gibberellin in wheat aleurone cells. Plant Cell 8, 259–269
40 Heimovaara-Dijkstra, S. et al. (1996) Abscisic acid-induced gene-expression
requires the activity of protein(s) sensitive to the protein-tyrosine phosphatase inhibitor phenylarsine oxide. Plant Growth Regul. 18, 115–123
41 Knetsch, M.L.W. et al. (1996) Abscisic acid induces mitogen-activated protein
kinase activation in barley aleurone protoplasts. Plant Cell 8, 1061–1067
42 Thornton, T.M. et al. (1999) Gibberellin signal transduction presents ...the
SPY who O-GlcNAc’d me. Trends Plant Sci. 4, 424–428
43 Robertson, M. et al. (1998) Identification of a negative regulator of gibberellin
action, HvSPY, in barley. Plant Cell 10, 995–1007
44 Harberd, N.P. et al. (1998) Gibberellin: inhibitor of an inhibitor of...?
BioEssays 20, 1001–1008
45 Peng, J. et al. (1999) ‘Green revolution’ genes encode mutant gibberellin
response modulators. Nature 400, 256–261
46 Flintham, J.E. and Gale, M.D. (1982) The Tom Thumb dwarfing gene, Rht3 in
wheat, I. Reduced pre-harvest damage to breadmaking quality. Theor. Appl.
Genet. 62, 121–126
47 Skriver, K. et al. (1991) cis-acting DNA elements responsive to gibberellin
and its antagonist abscisic acid. Proc. Natl. Acad. Sci. U. S. A. 88, 7266–7270
48 Huttly, A.K. et al. (1992) Localization of cis elements in the promoter of a
wheat a-Amy2 gene. Plant Mol. Biol. 19, 903–911
49 Gubler, F. and Jacobsen, J.V. (1992) Gibberellin-responsive elements in the
promoter of a barley high-pI a-amylase gene. Plant Cell 4, 1435–1441
50 Sutliff, T.D. et al. (1993) Gibberellin treatment stimulates nuclear factor
binding to the gibberellin response complex in a barley a-amylase promoter.
51 Gubler, F. et al. (1995) Gibberellin-regulated expression of a myb gene in
barley aleurone cells: evidence for Myb transactivation of a high-pI a-amylase gene promoter. Plant Cell 7, 1879–1891
52 Gubler, F. et al. (1997) Cloning of a rice cDNA encoding a transcription
factor homologous to barley GAMyb. Plant Cell Physiol. 38, 362–365
53 Weston, K. (1998) Myb proteins in life, death and differentiation. Curr. Opin.
Genet. Dev. 8, 76–81
54 Gubler, F. et al. (1999) Target genes and regulatory domains of the
GAMYB transcriptional activator in cereal aleurone. Plant Cell 17, 1–9
55 Raventos, D. et al. (1998) HRT, a novel zinc finger, transcriptional repressor
from barley. J. Biol. Chem. 273, 23313–23320
56 Hoecker, U. et al. (1995) Integrated control of seed maturation and
germination programs by activator and repressor functions of Viviparous-1 of maize. Genes Dev. 9, 2459–2469
57 Rogers, J.C. and Rogers, S.W. (1992) Definition and functional implications
of gibberellin and abscisic acid cis-acting hormone response complexes.
Plant Cell 4, 1443–1451
58 Rogers, J.C. et al. (1994) The cis-acting gibberellin response complex in
high-pI a-amylase gene promoters. Plant Physiol. 105, 151–158
59 Willmott, R.L. et al. (1998) DNase1 footprints suggest the involvement of at
least three types of transcription factors in the regulation of a-Amy2/A by gibberellin. Plant Mol. Biol. 38, 817–825
60 Tregear, J.W. et al. (1995) Functional analysis of linker insertions and point
mutations in the a-Amy2/54 GA-regulated promoter. Plant Mol. Biol. 29, 749–758
61 Rushton, P.J. et al. (1995) Members of a new family of DNA-binding proteins bind
to a conserved cis-element in the promoters of a-Amy2 genes. Plant Mol. Biol. 29, 691–702
P
ost-embryonic development in higher plants is character-ized by the reiterative formation of lateral organs from the flanks of apical meristems1. A shoot apical meristem (SAM) is initially formed during embryogenesis, and derivatives of this meristem give rise to the above-ground portion of the plant. The SAM contains a population of pluripotent stem cells, which serve three primary functions1–4
:
(1) Lateral organs, such as leaves, are produced from the peripheral regions of the SAM.
(2) The basal regions of the SAM contribute to the formation of the stem.
(3) The stem cells of the SAM must replenish those regions from which cells have been recruited and maintain the pool of stem cells required for further growth.
In general, we focus on SAMs in this review, although extrapo-lation of concepts to other shoot meristems, such as flower meristems, will be discussed when pertinent.
As a result of histological analyses the SAM has been subdi-vided in two different manners. First, three distinct zones of the SAM are defined by cytoplasmic densities and cell division rates: the peripheral zone, the central zone and the rib zone1–4
(Fig. 1). These three zones might represent a functional subdivision of the SAM although direct evidence for this is lacking. Lateral organs are produced from cells recruited from the peripheral zone
whereas stem tissue is derived from cells recruited from the rib zone. The central zone acts as a reservoir of stem cells, which replenish both the peripheral and rib zones, as well as maintaining the integrity of the central zone. It should be noted that these cells do not act as permanent initials, but rather their behavior is gov-erned in a position-dependent manner. Second, the SAM is also composed of clonally distinct layers of cells5
(Fig. 1). The fact that the peripheral and central zones, as well as the lateral organs pro-duced, contain cells from the three clonally distinct layers indi-cates that communication between cell layers is required to coordinate developmental processes5,6
. For example, leaves in most eudicot species are composed of derivatives from the epider-mal layer (L1), the subepiderepider-mal layer (L2) and corpus (L3)6
. One of the earliest markers of leaf initiation from the peripheral zone is the periclinal cell divisions in specific regions in the L2. Cells in the L1 and L3 adjust their growth accordingly, with the entire region acting coordinately to produce a leaf primordium.
In this review, we discuss some recent advances in our under-standing of three aspects of meristem functioning: the origin of the SAM during embryogenesis, the maintenance of the stem cell population in the central zone, and the relationships between lateral organ primordia and the meristems from which they are produced. Several excellent reviews cover broader views of the biology of the SAM (Refs 2–4).
Formation and maintenance
of the shoot apical meristem
John L. Bowman and Yuval Eshed
Development in higher plants is characterized by the reiterative formation of lateral organs from the flanks of shoot apical meristems. Because organs are produced continuously throughout the life cycle, the shoot apical meristem must maintain a pluripotent stem cell population. These two tasks are accomplished within separate functional domains of the apical meristem. These functional domains develop gradually during embryogenesis. Subsequently, communication among cells within the shoot apical meristem and between the shoot apical meristem and the incipient lateral organs is needed to maintain the functional domains within the shoot apical meristem.
Alison Lovegrove and Richard Hooley*are at IACR-Long Ashton
Research Station, Dept of Agricultural Sciences, University of Bristol, Long Ashton, Bristol, UK BS41 9AF.
*Author for correspondence (tel144 1275 392181;

![Fig. 2. Principal components in Ca2� and CaM signalling in aleurone. Gibberellin (GA), perceived by a receptor (red) at the plasma membrane (green line), elevates [Ca2�]i and CaMand this is prevented by abscisic acid (ABA) acting through a receptor (dark b](https://thumb-ap.123doks.com/thumbv2/123dok/1039186.929944/3.858.43.379.76.441/principal-components-signalling-aleurone-gibberellin-perceived-prevented-receptor.webp)