Expression of genes responsible for ethylene production and
wilting are differently regulated in carnation (
Dianthus caryophyllus
L.) petals
Yusuke Kosugi
a, Kenichi Shibuya
a, Nanako Tsuruno
a, Yujiro Iwazaki
a,
Atsushi Mochizuki
b, Toshihito Yoshioka
a, Teruyoshi Hashiba
a, Shigeru Satoh
a,*
aLaboratory of Bio-adaptation,Graduate School of Agricultural Science,Tohoku Uni6ersity,Tsutsumidori-amamiyamachi1-1,Aoba-ku, Sendai981-8555,Japan
bTohoku National Agricultural Experiment Station,Omagari,Akita014-0102,Japan
Received 7 February 2000; received in revised form 3 June 2000; accepted 7 June 2000
Abstract
Carnation petals exhibit autocatalytic ethylene production and wilting during senescence. The autocatalytic ethylene production is caused by the expression of 1-aminocyclopropane-1-carboxylate (ACC) synthase and ACC oxidase genes, whereas the wilting of petals is related to the expression of the cysteine proteinase (CPase) gene. So far, it has been believed that the ethylene production and wilting are regulated in concert in senescing carnation petals, since the two events occurred closely in parallel with time. In the present study, we investigated the expression of these genes in petals of a transgenic carnation harboring a sense ACC oxidase transgene and in petals of carnation flowers treated with 1,1-dimethyl-4-(phenylsulfonyl)semicarbazide (DPSS). In petals of the transgenic carnation flowers, treatment with exogenous ethylene caused accumulation of the transcript for CPase and in-rolling (wilting), whereas it caused no or little accumulation of the transcripts for ACC oxidase and ACC synthase and negligible ethylene production. In petals of the flowers treated with DPSS, the transcripts for ACC synthase and ACC oxidase were accumulated, but no significant change in the level of the transcript for CPase was observed. These results suggest that the expression of ACC synthase and ACC oxidase genes, which leads to ethylene production, is differentially regulated from the expression of CPase, which leads to wilting, in carnation petals. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords: Carnation flower senescence; 1,1-Dimethyl-4-(phenylsulfonyl)semicarbazide; Ethylene production; Gene expression; Petal wilting; Transgenic carnation
www.elsevier.com/locate/plantsci
1. Introduction
Ethylene is the primary plant hormone involved in the senescence of cut carnation flowers [1 – 3]. A large amount of ethylene is synthesized several days after full opening of the flowers [4 – 6], several
hours after compatible pollination [7 – 9] or soon after the treatment with exogenous ethylene [2,10]. Ethylene is produced first in the pistil during natural and pollination-induced senescence [11,12]. Then the produced ethylene, acting as a diffusible signal, is perceived by petals and induces autocata-lytic ethylene production in the petals, resulting in in-rolling of petal margins and wilting of the whole petals. A large portion of ethylene produced by senescing carnation flowers comes from the petals. On the other hand, exogenous ethylene applied to carnation flowers directly acts on the petals, and induces autocatalytic ethylene produc-tion and wilting of the petals (Shibuya et al., submitted).
Abbre6iations:ACC, 1-aminocyclopropane-1-carboxylate; AdoMet,
S-adenosyl-L-methionine; CPase, cysteine proteinase; CTAB, cetyltrimethyl-ammonium bromide; CaMV, cauliflower mosaic virus; DIG, digoxygenin; DPSS, 1,1-dimethyl-4-(phenylsulfonyl)semicar-bazide; GUS, b-glucuronidase; HPT, hygromycin phosphotrans-ferase; RT, reverse transcription..
* Corresponding author. Tel.: +81-22-717-8831; fax: + 22-717-8834.
E-mail address:[email protected] (S. Satoh).
Ethylene is synthesized in plant tissues through the following pathway:L-methionineAdoMet
ACCethylene. ACC synthase and ACC oxidase catalyze the last two reactions [13,14]. The increase in ethylene production in carnation petals is ac-companied by the expression of genes for both ACC synthase (DC-ACS1) [15] and ACC oxidase (DC-ACO1 corresponding to pSR120 cDNA) [16]. Petal wilting in flower senescence is caused by the decomposition of cell constituents by hy-drolytic enzymes such as protease and nuclease [17,18]. CPase is probably one of the enzymes responsible for hydrolytic degradation of cell com-ponents leading to cell death during senescence of petals [17,18]. A CPase gene is up-regulated during natural, pollination-induced and exogenous ethyl-ene-induced senescence of carnation petals [19]. So far, it has been believed that autocatalytic ethylene production and petal wilting are regulated in con-cert and can not be separated, since they occur closely in parallel.
Transgenic plants with suppressed ethylene pro-duction have been used as a powerful tool for understanding the regulation of the biosynthesis and action of ethylene in plant tissues. For in-stance, Theologies et al. [20], using a transgenic tomato with suppressed ethylene production, re-vealed that both ethylene-independent and ethyl-ene-dependent signal transduction pathways were responsible for ripening of tomato fruit.
In the present work, we generated a transgenic carnation plant harboring a sense ACC oxidase transgene, and examined the response of their petals to exogenous ethylene with regard to the induction of in-rolling, ethylene production and expression of genes involved in these events. Fur-thermore, we examined the changes in the levels of the transcripts for ACC synthase, ACC oxidase and CPase in petals of non-transformed carnation flowers after treatment with DPSS. We report that the expression of ACC synthase and ACC oxidase genes, which leads to ethylene production and that of CPase, which leads to wilting of petals, are differentially regulated in carnation petals.
2. Materials and methods
2.1. Plant materials
Carnation (Dianthus caryophyllus L. cvs. Nora
or Reiko) flowers at the full-opening stage (their outermost petals were at right angles to the stem of the flower) were obtained from a local grower. Ethylene production from flowers was monitored daily as described previously [21].
The shoots of carnation cv. Nora were cultured and maintained according to the method of Firoozabady et al. [22]. Shoot clusters were sub-cultured monthly for more than one year and used for transformation.
2.2. Cloning of cDNAs coding for ACC oxidase,
ACC synthase and CPase
cDNAs encoding ACC oxidase (corresponding to pSR120 [23]), ACC synthase (corresponding to
DC-ACS1, formerly CARACC3 [16]) and CPase [19] were obtained by RT-PCR cloning from total RNAs obtained from senescing carnation petals. RT-PCR was performed according to an ordinal procedure with necessary optimization. The up-stream and downup-stream primers for RT-PCR were: 5%
-CCCCTCTAGAATGGCAAACATTGT-CAACTT-3% and 5%
-GGGGTCTAGATCAAG-CAGTTGGAATGGGAC-3%, respectively, for the ACC oxidase cDNA; 5%
-CCCCACTAGTATGG-GTTC TTATAAGGGTGT-3% and 5% -GGGGAC-TAGTTTATGTTCTTGCTTTAACAA-3% for the
ACC synthase cDNA; and 5% -GCAAGCTTAT-CATCTTCAGTCGTGGTC-CGT-3% and 5%
-TT-GAATGAAAACCTTCACGATGATGTCTTC-3%
for the CPase cDNA. cDNA fragments were lig-ated into pBluescript II SK (+) (Stratagene), and the resultant plasmids were amplified in Es
reading frame (1554-bp) that shares 99.6% simi-larity with DC-ACS1 cDNA [16]. On the other hand, the cDNA for CPase was 1306 bp in size and contained an open reading frame that shared 99.2% similarity with the sequence re-ported previously [19].
2.3. Construction of a sense ACC oxidase transgene
For the transformation of carnation plant, we prepared a binary vector, pMLH2113-DCACO (OR+), by inserting the coding sequence of ACC oxidase cDNA in sense orientation into pMLH2113-GUS [24] at the BamHI/SacI site created by removing a GUS region. The pMLH2113-GUS vector was constructed by adding a HPT gene to an original vector pBE2113-GUS [25] that drives the GUS gene under the control of CaMV35S promoter with enhancer sequences El2 and V [24]. Correct ori-entation of the insert was confirmed by PCR. The plasmids, GUS and pMLH2113-DCACO (OR+), were introduced into Agrobac
-terium tumefaciens EHA101 by the freeze-thaw method [26].
2.4. Transformation and regeneration of transgenic carnation plants
Transformation by Agrobacterium-mediated gene transfer and regeneration of carnation plants were performed by the method of Firooz-abady et al. [22] with necessary modification. Briefly, leaf explants, pulled off from shoot clus-ters of the shoot culture, were mixed with bacte-ria in Minimal A medium [27] for 15 min, and cocultivated on BD medium [22] supplemented with 100 mM acetosyringone for a week at 24°C in the dark. For selection of transformants, we used hygromycin as a selection agent. Survived explants were subcultured repeatedly on Gerlite-based solid media supplemented with necessary nutrients and plant hormones until they formed normal shoots with distinct internodes. The shoots were rooted and they were transplanted to vermiculite in pots, then to soil. The plants were grown in a containment greenhouse until they flowered.
Carnation plants of a non-transformed control
line (cv. Nora), not through tissue culture, were also grown and flowered in the same greenhouse.
2.5. Treatment with exogenous ethylene of petals of the transgenic carnation flowers
Carnation flowers were harvested at their full opening stage (day 0). Calyxes were removed, and petals at the outermost and adjacent whorls were detached from receptacles. The petals de-tached from each flower were divided into two groups. Each petal was placed vertically in a 30-ml glass vial with the basal portion to the bot-tom, and 2-ml distilled water was added to the vial to avoid desiccation. Half of the vials with petals were enclosed in a 1-l glass jar and ethyl-ene was injected to a final concentration of 10 ml l−1 through a rubber septum on a lid. The other
half were enclosed in the same way but fresh air was injected instead of ethylene. The jars con-taining the vials with petals were left under white fluorescent light (12 mmol s−1 m−2) at
25°C for 18 h. Then, the vials were taken out from the jars and placed in open air for 1 h.
Ethylene production from the petals was mon-itored by enclosing the vials with petals in 450-ml glass containers for 1 h. A 1-450-ml gas sample was taken into a hypodermic syringe from the container through a rubber septum of a sam-pling port, and analyzed for ethylene with a gas-chromatograph (263-30, Hitachi) equipped with an alumina column and a flame ionization detec-tor.
Petals were detached from flowers after the as-say for ethylene production, immediately frozen in liquid N2 and stored at −80°C until isolation
of RNA.
2.6. Treatment with DPSS of flowers of non-transgenic carnation
Flowers of carnation cv. Reiko were used at the full-opening stage (day 0). Stems were trimmed to 5 cm. DPSS was administered to the flowers by immersing their cut ends in 0.1 mM DPSS solution for 24 h from day 0. Then, the flowers were left with their cut end placing in water under white fluorescent light (40 mmol m−2 s−1) at 23°C. Control flowers were treated
and adjacent whorls of the 3 randomly chosen flowers, combined and stored at −80°C until use.
2.7. RNA gel blot analysis
Total RNA was isolated by the SDS-phenol method [28] from carnation petals. mRNA was obtained from the total RNA using Oligotex-dT30 (Takara) according to the manufacturer’s instruction.
In the experiment with petals of the transgenic carnation treated with exogenous ethylene (Fig. 1), 10 mg of total RNA was denatured at 65°C for 15 min in 10 mM MOPS buffer at pH 7.0, containing 2.5 mM Na – acetate, 0.5 mM EDTA, 2.2 M form-aldehyde, and 50% (w/v) formamide. The dena-tured RNA was separated on a 1.0% agarose gel containing 2.2 M formaldehyde and transferred to a membrane filter (Hybond N+, Amersham Pharmacia Biotech). Blots were prehybridized at 50°C for 3 h in 10 mM Na – phosphate buffer at pH 6.5, containing 5×SSC (1×SSC is 0.15 M NaCl, 15 mM Na – citrate, pH 7.0), 10× Den-hardt’s solution, 0.5% SDS, 50% formamide and 0.1 mg l−1 denatured salmon sperm DNA,
fol-lowed by hybridization at 50°C for 16 h in the solution of the same composition but containing 600 ng ml−1of denatured DIG-labeled probes (see
below). Blots were washed twice, for 10 min each, at room temperature in the solution containing 2×SSC and 0.1% SDS, followed by two washes for 15 min each at 68°C in the solution containing 0.2×SSC and 0.1% SDS. Hybridization signals were detected with a DIG Luminescent Detection Kit (Hoffman-La Roche) and by exposure to X-ray film (RX-U, Fuji Photo Film). The duration of exposure was 4, 10 and 4 h for transcripts of ACC oxidase, ACC synthase and CPase, respectively. For DNA probes, we used the cDNAs coding for ACC oxidase, ACC synthase and CPase obtained from senescing carnation petals. The DNA probes were labeled with a PCR DIG Labeling Mix (Hoffmann-La Roche).
On the other hand, in the experiment with petals treated with DPSS (Fig. 2), two mg of mRNA was similarly treated and blots were hybridized in the solution containing 5×105 cpm ml−1 of 32
P-la-beled probes. The cDNA probes were laP-la-beled with
32P-dCTP by random priming using Multiprime
DNA labeling systems (Amersham Pharmacia Biotech) according to the manufacturer’s instruc-Fig. 1. Effects of exogenous ethylene treatment on in-rolling
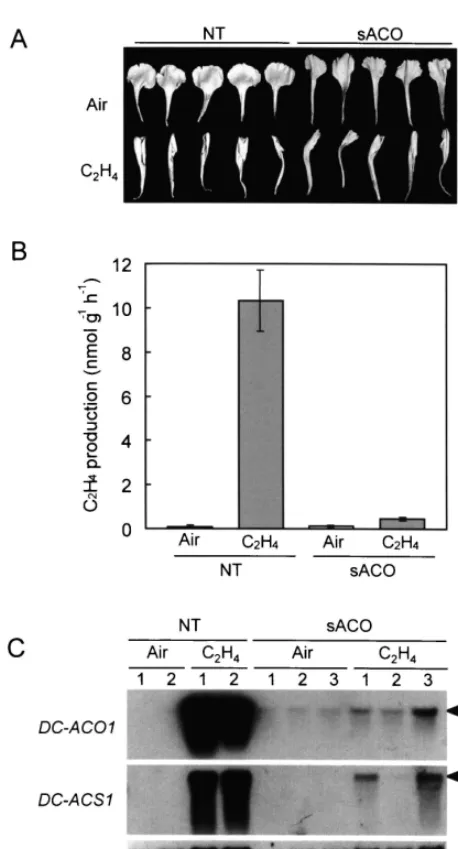
(wilting), ethylene production and levels of transcripts for ACC oxidase, ACC synthase and CPase in petals of the control and the transgenic carnations. Petals detached from flowers at full opening stage (day 0) were treated with or without 10 ml l−1 ethylene for 18 h. NT, the control line;
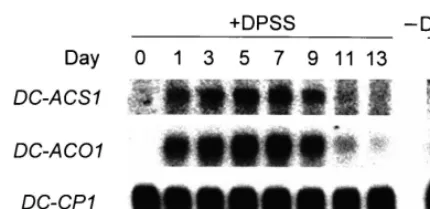
Fig. 2. Changes in the levels of transcripts for ACC oxidase, ACC synthase and CPase in petals of carnation flowers treated with DPSS. Carnation flowers at day 0 were treated with 0.1 mM DPSS for 24 h, then left for sampling of the petals at given time. Two micrograms of mRNAs isolated from petals was separated on an agarose gel and hybridized with32P-labeledDC-ACO1,DC-ACS1 or CPase probes. For
reference, the mRNAs isolated from petals of flowers, left to senesce naturally for 5 days, were treated similarly.
3.2. Two distinct responses to exogenous ethylene of the petals from the transgenic carnation
To investigate whether the petals of the trans-genic line with the sense ACC oxidase transgene respond to ethylene, we treated the petals ob-tained from the transgenic and control lines with 10 ml l−1 ethylene for 18 h at the full opening
stage (day 0), and compared their senescence be-havior. The petals of both lines exhibited in-rolling symptoms after the ethylene treatment (Fig. 1A). On the other hand, ethylene production from the petals after the ethylene treatment was quite different between the two lines (Fig. 1B). Petals of the transgenic line produced ethylene at 0.590.1 nmol h−1 g−1, whereas those of the
control line did so at 10.391.4 nmol h−1 g−1.
Ethylene production was negligible in the petals that had not been treated with ethylene in both lines.
Fig. 1C shows the levels of the transcripts for ACC oxidase, ACC synthase and CPase in the petals of the transgenic and control lines after the treatment with or without ethylene. A large amount of transcripts for both ACC oxidase and ACC synthase accumulated in the petals of the control line after ethylene treatment, whereas did only a little in the petals of the transgenic line. The large difference in the levels of the transcripts between the control and the transgenic lines corre-sponded with the difference in ethylene produc-tion between the two lines. Also, the difference in the transcript levels between the two lines after ethylene treatment was probably a reflection of the difference found in the flowers of the two lines, which underwent natural senescence as men-tioned above. On the other hand, the transcript for CPase accumulated at almost the same level in the petals of both the transgenic and control lines. The present finding that neither ACC synthase nor ACC oxidase transcripts accumulated in the petals after the ethylene treatment were different from those reported by Savin et al. [29] who showed a substantial accumulation of these tran-scripts in the petals of a carnation plant trans-formed with an antisense ACC oxidase transgene after ethylene treatment. However, their results might be caused by the use of a high concentra-tion of ethylene, 150 ml l−1, which might cause an
excess accumulation of transcripts. tions. Hybridization signals were detected with an
image analyzer (FLA2000, Fuji Photo Film).
3. Results
3.1. Characteristics of senescence in flowers of a transgenic carnation harboring a sense ACC oxidase transgene
3.3. Difference in the expression of genes for ACC synthase, ACC oxidase and CPase in carnation petals treated with DPSS
DPSS is an antisenescence preservative for cut carnation flowers [30]. DPSS prolongs the vase-life of carnation flowers by preventing ethylene pro-duction, but does not affect the ethylene-induced senescence in the flowers. Application of DPSS inhibits the increase in activities of ACC synthase and ACC oxidase, which occurred in non-treated control flowers during natural senescence [30,31]. DPSS does not inhibit in vitro activities of either ACC synthase or ACC oxidase obtained from senescing carnation petals [32]. Recently it was revealed that the inhibitory action of DPSS was specific to the ethylene production in carnation flowers undergoing natural senescence [31]. How-ever, the mechanism of DPSS action is not fully elucidated yet.
As shown in Fig. 2, the administration of DPSS to carnation flowers at day 0 induced the accumu-lation of transcripts for ACC synthase (DC
-ACS1) and ACC oxidase (DC-ACO1) from the next day on, but did not cause a significant change in the level of transcript for CPase. The increased level of transcripts for ACC synthase and ACC oxidase lasted until day 9. On the other hand, transcripts for all three genes were accumulated on petals, which underwent natural senescence for 5 days. The mechanism of the action of DPSS on the up-regulation of ACC synthase and ACC ox-idase genes and no increase in the activities of the enzymes in carnation petals is now under investi-gation. These findings clearly indicated that carna-tion petals can regulate the expression of genes for ACC synthase and ACC oxidase independent of that for CPase.
4. Discussion
Senescence of carnation petals is accompanied by autocatalytic ethylene production and wilting of the petals; the former is caused by the expres-sion of ACC synthase and ACC oxidase genes and the latter is related to the expression of CP gene. To our knowledge, there have been no reports that demonstrated separation of these two events in the petals of carnation flowers undergoing senescence. However, in the present study with the petals of a
transgenic carnation and the petals of non-trans-genic carnation treated with DPSS, we could show that the expression of genes for ACC synthase and ACC oxidase is regulated differently from that of the gene for CPase in carnation petals. In other words, carnation petals regulate the expression of ACC synthase and ACC oxidase genes indepen-dent of the CPase gene. During senescence of carnation flowers, exogenous ethylene, whether it comes from the pistil [15,12] or applied exoge-nously, is perceived by petals and induces autocat-alytic ethylene production and wilting in the petals. Thus, we propose that two ethylene-depen-dent pathways lead to the expression of genes in senescing carnation petals. One pathway leads to the expression of ACC synthase and ACC oxidase genes and autocatalytic ethylene production. The other leads to the expression of CPase gene and probably other genes for enzymes for hydrolytic degradation, and petal wilting. Thus two distinct ethylene signaling pathways may be functioning in senescing carnation petals, although an alternative interpretation is that these genes have rapid turnover rates in the absence of the signal and decrease more rapidly than the CPase mRNA.
Acknowledgements
We are grateful to Dr Y. Ohashi (National Institute of Agrobiological Resources) for supply-ing pMLH2113-GUS, and to Drs. T. Iwai and R. Honkura (Miyagi Prefectural Agricultural Center) for useful instructions for transformation and cul-ture of carnation plants.
References
[1] F.B. Abeles, P.W. Morgan, M.E. Saltveit Jr, Ethylene in Plant Biology, 2nd edn., Academic Press, San Diego, CA, 1992.
[2] A. Borochov, W.R. Woodson, Physiology and biochem-istry of flower petal senescence, Hort. Rev. 11 (1989) 15 – 43.
[3] M.S. Reid, M.-J. Wu, Ethylene and flower senescence, Plant Growth Regul. 11 (1992) 37 – 43.
[4] K. Manning, The ethylene forming enzyme system in carnation flowers, in: J.A. Roberts, G.A. Tucker (Eds.), Ethylene and Plant Development, Butterworths, Boston, MA, 1985, pp. 83 – 92.
[6] W.R. Woodson, K.Y. Park, D.A. Drory, P.B. Larsen, H. Wang, Expression of ethylene biosynthetic pathway transcripts in senescing carnation flowers, Plant Physiol. 99 (1992) 526 – 532.
[7] P.B. Larsen, E.N. Ashworth, M.L. Jones, W.R. Wood-son, Pollination-induced ethylene in carnation. Role of pollen tube growth and sexual compatibility, Plant Phys-iol. 108 (1995) 1405 – 1412.
[8] R. Nichols, Sites of ethylene production in the pollinated and unpollinated senescing carnation (Dianthus caryophyllus) inflorescence, Planta 135 (1977) 155 – 159. [9] R. Nichols, G. Bufler, Y. Mor, D.W. Fujino, M.S. Reid,
Changes in ethylene production and 1-aminocyclo-propane-1-carboxylic acid content of pollinated carna-tion flowers, J. Plant Growth Regul. 2 (1983) 1 – 8. [10] H. Wang, W.R. Woodson, Reversible inhibition of
eth-ylene action and interruption of petal senescence in carnation flowers by norbornadiene, Plant Physiol. 89 (1989) 434 – 438.
[11] M.L. Jones, W.R. Woodson, Interorgan signaling fol-lowing pollination in carnations, J. Am. Soc. Hort. Sci. 124 (1999) 598 – 604.
[12] A. ten Have, E.J. Woltering, Ethylene biosynthetic path-way genes are differentially expressed during carnation (Dianthus caryophyllusL.) flower senescence, Plant Mol. Biol. 34 (1997) 89 – 97.
[13] H. Kende, Ethylene biosynthesis, Ann. Rev. Plant Phys-iol. Plant Mol. BPhys-iol. 44 (1993) 283 – 307.
[14] S.F. Yang, N.E. Hoffman, Ethylene biosynthesis and its regulation in higher plants, Ann. Rev. Plant Physiol. 35 (1984) 155 – 189.
[15] M.L. Jones, W.R. Woodson, Differential expression of three members of the 1-aminocyclopropane-1-carboxy-late synthase gene family in carnation, Plant Physiol. 119 (1999) 755 – 764.
[16] K.Y. Park, A. Drory, W.R. Woodson, Molecular cloning of an 1-aminocyclopropane-1-carboxylate syn-thase from senescing carnation flower petals, Plant Mol. Biol. 18 (1992) 377 – 386.
[17] T. Panavas, P.D. Reid, R.B. Rubinstein, Programmed cell death of daylily petals: activities of wall-based en-zymes and effects of heat shock, Plant Physiol. Biochem. 36 (1998) 379 – 388.
[18] T. Panavas, A. Pikula, P.D. Reid, B. Rubinstein, E.L. Walker, Identification of senescence-associated genes from daylily petals, Plant Mol. Biol. 40 (1999) 237 – 248. [19] M.L. Jones, P.B. Larsen, W.R. Woodson, Ethylene-reg-ulated expression of a carnation cysteine proteinase dur-ing flower petal senescence, Plant Mol. Biol. 28 (1995) 505 – 512.
[20] A. Theologis, P.W. Oeller, L.-M. Wong, W.H. Rottman, D.M. Ganz, Use of a tomato mutant constructed with reverse genetics to study fruit ripening, a complex pro-cess, Dev. Genet. 14 (1993) 282 – 295.
[21] Y. Kosugi, N. Oyamada, S. Satoh, T. Yoshioka, E. Onodera, Y. Yamada, Inhibition by 1-aminocyclobu-tane-1-carboxylate of the activity of
1-aminocyclo-propane-1-carboxylate oxidase obtained from senescing petals of carnation (Dianthus caryophyllus L.) flowers, Plant Cell Physiol. 38 (1997) 312 – 318.
[22] E. Firoozabady, Y. Moy, W. Tucker, K. Robinson, N. Gutterson, Efficient transfomation and regeneration of carnation cultivars using Agrobacterium, Mol. Breed. 1 (1995) 283 – 293.
[23] H. Wang, W.R. Woodson, A flower senescence-related mRNA from carnation shares sequence similarity with fruit ripening-related mRNAs involved in ethylene biosynthesis, Plant Physiol. 96 (1991) 1000 – 1001. [24] I. Mitsuhara, M. Ugaki, H. Hirochika, M. Ohshima, T.
Murakami, Y. Gotoh, Y. Katayose, S. Nakamura, R. Honkura, S. Nishiyama, K. Ueno, A. Mochizuki, H. Tanimoto, H. Tsugawa, Y. Otsuki, Y. Ohashi, Efficient promoter cassettes for enhanced expression of foreign genes in dicotyledonous and monocotyledonous plants, Plant Cell Physiol. 37 (1996) 49 – 59.
[25] A. Mochizuki, Y. Nishizawa, H. Onodera, Y. Tabei, S. Toki, Y. Habu, M. Ugaki, Y. Ohashi, Transgenic rice plant expressing a trypsin inhibitor are resistant against rice stem borers, Chilo suppressalis, Ent. Exp. Appl. 93 (1999) 173 – 178.
[26] G. An, P.R. Ebert, A. Mitra, S.B. Ha, Binary vectors, in: S.B. Gelvin, R.A. Schilperoort (Eds.), Plant Molecu-lar Biology Manual, Kluwer Academic Publishers, Dor-drecht, Netherlands, 1988, pp. A3/1 – A3/19.
[27] K. Lech, R. Brent, Media preparation and bacteriologi-cal tools, in: F.M. Ausubel, R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, K. Struhl (Eds.), Short Protocols in Molecular Biology, 2nd edn., Green Publishing Associates and Wiley, New York, 1992, pp. 1 – 3.
[28] R.D. Palmiter, Magnesium precipitation of ribonucle-oprotein complexes: expedient techniques for the isola-tion of undegraded polysomes and messenger ribonucleic acid, Biochemistry 13 (1974) 3606.
[29] K.W. Savin, S.C. Baudinette, M.W. Graham, M.Z. Michael, G.D. Nugent, C.-Y. Lu, S.F. Chandler, E.D. Cornish, Antisense ACC oxidase RNA delays carnation petal senescence, HortScience 30 (1995) 970 – 972. [30] N. Midoh, Y. Saijou, K. Matsumoto, M. Iwata, Effects
of 1,1-dimethyl-4-(phenylsulfonyl)semicarbazide (DPSS) on carnation flower longevity, Plant Growth Regul. 20 (1996) 195 – 199.
[31] T. Onoue, M. Mikami, T. Yoshioka, T. Hashiba, S. Satoh, Characteristics of the inhibitory action of 1,1-dimethyl-4-(phenylsulfonyl)semicarbazude (DPSS) on ethylene production in carnation (Dianthus caryophyllus
L.) flowers, Plant Growth Regul. 30 (2000) 201 – 207. [32] S. Satoh, N. Oyamada, T. Yoshioka, N. Midoh,
1,1-Dimethyl-4-(phenylsulfonyl)semicarbazide (DPSS) does not inhibit the in vitro activities of 1-aminocyclo-propane-1-carboxylate (ACC) oxidase and ACC syn-thase obtained from senescing carnation (Dianthus caryophyllus L.) petals, Plant Growth Regul. 23 (1997) 191 – 193.