Summary Saplings of ozone-sensitive and ozone-tolerant birch (Betula pendula Roth.), clones B and C, respectively, were exposed to ozone concentrations that were 1.7-fold higher than ambient for one growing season under open-field condi-tions. Ambient air was used as the control treatment. In the ozone-sensitive clone B, there was an initial stimulation of leaf area growth in response to the ozone treatment, but further ozone exposure caused reductions in leaf and stem biomass growth, Rubisco and chlorophyll a contents, net photosynthe-sis, water use efficiency and chloroplast size. It also caused an alteration in chloroplast shape and injury to thylakoid mem-branes. In the ozone-tolerant clone C, ozone fumigation did not affect growth rate, and there were no consistent changes in chlorophyll content, photosynthesis or water use efficiency. There were also fewer ultrastructural abnormalities in the chloroplasts of clone C than of clone B. Based on the observed biochemical, physiological and structural changes in chloro-plasts of clone B in response to low concentrations of ozone, we conclude that the increasing concentration of tropospheric ozone represents a risk to natural birch populations.
Keywords: Betula pendula, growth ultrastructure, ozone, pho-tosynthetic machinery.
Introduction
Negative effects of ozone on growth have been reported in several tree species (e.g., Darrall 1989, Chappelka and Chevone 1992). However, the mechanisms responsible for these growth reductions are not completely understood. Im-paired photosynthesis, decreased activity and quantity of Ru-bisco, decreased chlorophyll content and decreased stomatal conductance are often reported in ozone-stressed plants (e.g., Reich 1983, Darrall 1989, Matyssek et al. 1991, Pell et al. 1992). In addition, many symptoms have been observed at the ultrastructural level, primarily in chloroplasts, in the early
phases of ozone injury (Holopainen et al. 1992, Pääkkönen et al. 1995).
During leaf aging and senescense, ozone has been reported to accelerate the normal decline in chlorophyll content and photosynthesis (e.g., Thomas and Stoddardt 1980, Reich 1983) and in the activity and quantity of Rubisco (Pell et al. 1992, Landry and Pell 1993). Decreased content of Rubisco protein may be caused by inhibition of protein synthesis or enhanced proteolysis, or both (Landry and Pell 1993, Eckardt and Pell 1994). Structural modification of the Rubisco molecule has been proposed as an explanation for the decreased activity of Rubisco preceding proteolytic degradation in ozone-stressed cuttings of hybrid poplar (Landry and Pell 1993). It has been suggested that, during ozone stress, free radicals are generated that result in increased oxidation of biological macromole-cules leading to structural modifications of Rubisco and chlo-rophyll (Pell and Dann 1991, Landry and Pell 1993).
The susceptibility of plants to ozone attack seems to depend on the developmental stage of the leaves (Pell et al. 1992, Eckardt and Pell 1994). In hybrid poplar, for example, the deleterious effects of ozone on Rubisco were most apparent at full leaf expansion, whereas during the early developmental stages, the leaf was capable of synthesizing new enzyme and therefore partially able to replace the ozone-damaged Rubisco (Pell et al. 1992). Similarly, in potato, exposure to ozone resulted in a significant decline in Rubisco of mature leaves, whereas immature leaves were unaffected (Eckardt and Pell 1994). Chronic ozone exposure throughout the life span of the leaves was necessary for continuous decline in Rubisco in hybrid poplar and radish (Pell et al. 1992) and potato (Eckardt and Pell 1994).
Physiological, anatomical and ultrastructural studies of five birch clones have revealed that clones differ in their sensitivity to ozone (Pääkkönen et al. 1993, 1995). In the most ozone-sen-sitive clone B, exposure to ozone resulted in decreased height growth and leaf biomass, leaf chlorosis and vein yellowing and increased numbers of necrotic flecks on the leaves. In the most
Growth responses and related biochemical and ultrastructural changes
of the photosynthetic apparatus in birch (
Betula pendula
) saplings
exposed to low concentrations of ozone
E. PÄÄKKÖNEN,
1J. VAHALA,
2T. HOLOPAINEN,
1R. KARJALAINEN
2and
L. KÄRENLAMPI
11
Department of Ecology and Environmental Science, University of Kuopio, P.O. Box 1627, 70211 Kuopio, Finland
2
Department of Plant Biology, Plant and Forest Pathology Section, University of Helsinki, 00014 Helsinki, Finland
Received July 25, 1995
ozone-tolerant clone C, exposure to ozone had no effect on height growth or the amount of visible foliar injury (Pääk-könen et al. 1993). However, ozone induced ultrastructural injuries in leaf mesophyll cells of both clones, including abnormal chloroplast morphology, dense stroma, curling and swelling of thylakoids, increased disintegration of the mito-chondrial matrix, a reduction of cristae, decreased amounts of tannin and increased amounts of cytoplasmic lipids (Pääk-könen et al. 1995). These changes were regarded as signs of accelerated senescence, because similar changes occurred more slowly during normal leaf aging.
This study was undertaken to determine whether ozone-in-duced responses in stem and leaf growth and ultrastructural changes in chloroplasts are related to the functioning of the photosynthetic apparatus in two birch clones (B and C) show-ing different susceptibilities to ozone. The experiment was carried out in an open-air fumigation system in an attempt to provide realistic information about the extent of ozone effects under field conditions.
Materials and methods
Plant material
Two-year-old saplings of clones KL-5-M (B) and KL-2-M (C) of European white birch (Betula pendula Roth.) were planted individually in 7.5-liter pots filled with fertilized sphagnum peat and garden soil (10/1, v/v). The saplings were watered as needed and fertilized weekly with 0.2% (19,5,20 N,K,P) nutri-ent solution.
Ozone fumigation
The saplings were transferred to the open-air exposure field at Kuopio Botanical Garden (62°13′ N, 27°13′ E) in central Finland on May 12, 1993. Ten saplings per clone per treatment were arranged in two blocks per treatment. The control sap-lings were grown in ambient air, whereas the ozone-treated saplings were fumigated continuously with ozone concentra-tions that were 1.7-fold higher than ambient air. The seedlings were exposed to ozone in the natural microclimate. The com-puter-controlled release of ozone, which was generated from pure oxygen, was from perforated tubes in up-wind positions. The ozone concentration was maintained continuously as a constant multiple of the ambient concentration, to mimic the natural hourly and seasonal variation. The fumigation system has been described in detail by Wulff et al. (1992) and Pääk-könen et al. (1993). The cumulative ozone exposures (ppm-h) for the periods between the sampling dates for Rubisco analy-sis are given in Table 1. The starting date for the biochemical and ultrastructural studies was June 28, when the leaves under investigation emerged.
Growth measurements
Immediately after planting, the height of each sapling was determined. All the seedlings were measured for height, num-ber of leaves, individual leaf size (mean) and foliage area (total leaf area) on July 12, July 27, August 9 and August 23. Foliage
area was estimated by multiplying the number of leaves by the mean outline area of the individual leaves, measured from 10 average-sized leaves per sapling. On September 13, dry weights for stem and roots were determined.
Electron microscopy studies
For the ultrastructural studies, leaf samples were collected on July 12, August 2, August 16 and August 30 from each sapling (10 saplings per clone per treatment). The samples were taken from among the leaves that emerged in the last week of June by cutting 5-mm strips each 1--2 cm in length from the leaf apex. These leaf strips were immediately placed in a fixative solution of 2.5% (v/v) glutaraldehyde in phosphate buffer (0.1 M, pH 7.0). In the laboratory, 1.5 mm2 square pieces were cut with a razor blade from the strips maintained under a drop of 2.5% glutaraldehyde fixative. Leaf pieces were postfixed in 1% buffered OsO4 solution, dehydrated in an ethanol series
and embedded in LX 112 epon. Thin sections were stained with uranyl acetate and lead citrate and were examined by means of a JEOL 1200 EX electron microscope operating at 80 kV.
Twenty randomly selected cross sections of chloroplast pali-sade and spongy mesophyll cells per clone and treatment were photographed. The length and width of chloroplasts, the num-ber of small (< 10 thylakoids) and large (> 10 thylakoids) grana, and the number and size of plastoglobuli were measured from the photographs. The proportion of the chloroplast area occupied by starch grains was calculated by a point analysis method, where a cross-hatched grid with random points was positioned over the photograph and the points within starch grains were counted to determine the relative area of starch in relation to chloroplast area. The changes in chloroplast shape, density of stroma and appearance of thylakoids and mitochon-drial matrix were determined by classifying the twenty photo-graphed chloroplasts and cells per tissue per sample into injury classes 0--3 (0 = not injured, 1 = slightly injured, 2 = clearly injured, 3 = severely injured). The amounts of tannin, cytoplas-mic lipids and cytoplas-microbodies were classified by visual observa-tion, and the electron density of plastoglobuli was determined.
Biochemical analyses
Leaves selected for the determination of Rubisco protein and chlorophyll were taken from the same position on the sapling as leaves used for the electron microscopy assays. Leaf sam-ples were collected on July 12, July 28, August 9 and Aug-ust 25 from five saplings per clone per treatment. After excision, the samples were weighed, and the outlines of the leaves drawn on plastic film in the field. The final leaf areas were determined from the plastic films with an LI-3000-A portable area meter (Li-Cor, Inc., Lincoln, NE).
was determined by scanning the gels and measuring the areas and intensities with the Pharmacia ImageMaster program (Ver-sion 1.0). For analysis of Rubisco small and large subunits, SDS-PAGE analyses (Laemmli 1970) were performed on each sample and the concentrations of large and small subunits were determined as for the PAGE gels. The concentrations of chlo-rophyll a and b were determined by Arnon’s (1949) method. Rubisco and the chlorophyll concentrations were calculated on a fresh weight basis.
Gas exchange measurements
Net photosynthesis and transpiration measurements were car-ried out with a closed-loop Li-Cor LI-6200 portable photosyn-thesis system. The fifth emergent leaf was selected for the measurements of gas exchange from 4--6 saplings per clone per treatment. Field measurements were made in saturating sunlight, over 900 µmol m−2 s−1 (PFD). Supplementary
halo-gen light (Sylvania professional, FTY/50W/8o) was used
whenever natural irradiance was below 900 µmol m−2 s−1.
Measurements were taken on the same leaves on six dates between August 3 and September 15.
Statistical methods
No significant block effects within the ozone and control treatments were revealed by ANOVA, and therefore data for these two blocks were pooled for further analysis. The Stu-dent’s t-test was used to test the differences in growth, para-metric ultrastructural characteristics, Rubisco and chlorophyll contents, photosynthesis, transpiration and WUE between ozone-treated and control saplings. Differences in the relative amounts of Rubisco subunits between treatments were tested by the nonparametric Mann-Whitney U-test. Differences were considered significant at P < 0.05.
Results
Growth responses
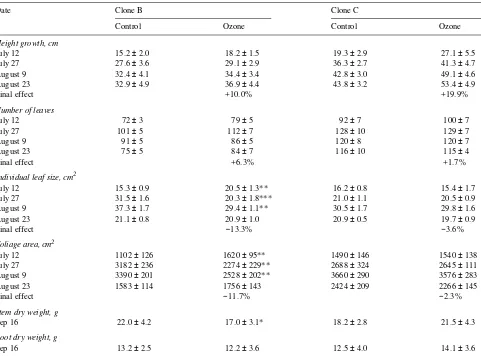
Ozone fumigation had no significant effect on any of the growth parameters measured in saplings of clone C (Table 2). For saplings of clone B, mean leaf size and foliage area were significantly larger in saplings exposed to ozone compared with control saplings on July 1, but on July 27 and August 9, mean leaf size and foliage area were significantly smaller in ozone-fumigated saplings than in control saplings (Table 2). Based on data pooled for all dates, the ozone-induced decrease in leaf area production was almost 10% greater in clone B than in clone C (Table 2). Ozone-fumigated saplings of clone B had significantly less (22.7%) stem dry weight than control sap-lings, whereas root dry weight was unaffected by the ozone treatment (Table 2).
Ultrastructural changes
The responses of palisade and spongy mesophyll cells to ozone were qualitatively similar, although some ozone-induced changes were more pronounced in spongy mesophyll cells. Ozone-induced ultrastructural changes were most evident in the sensitive clone B in late summer, but several ozone-induced structural injuries were observed in the ozone-tolerant clone C.
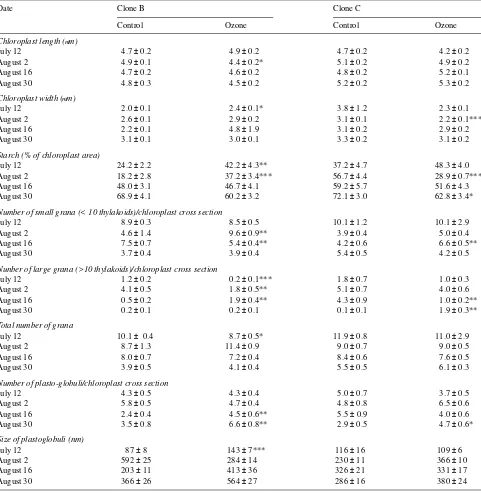
In clone B, the width of chloroplasts was significantly greater in ozone-exposed saplings than in control saplings on July 12, whereas the length of chloroplasts was significantly less in ozone-exposed saplings than in control saplings on August 2 (Table 3). Accompanying these ozone-induced changes in chloroplast size, the relative amount of starch was significantly increased. In contrast to clone B, ozone-fumi-gated saplings of clone C had significantly narrower chloro-plasts and less starch than control saplings on August 2 (Table 3). The number of large grana in ozone-treated saplings decreased significantly on July 12 and August 2 in clone B and Table 1. Cumulative ozone exposures (ppm-h) of the studied leaves. SUM00 accumulates all 1-h average concentrations and SUM40 accumulates those above the 40 ppb base-line.
Period Ambient ozone Elevated ozone
Nighttime Daytime Night + Day Nighttime Daytime Night + Day
SUM00
June 28--July 12 1.12 4.30 5.43 1.89 7.32 9.21
July 13--July 28 1.28 4.23 5.51 1.93 6.91 8.85
July 29--August 9 1.16 3.50 4.66 1.81 6.36 8.17
August 10--August 18 1.52 1.94 3.46 2.51 3.45 5.96
August 19--August 25 0.46 1.16 1.62 0.71 2.15 2.86
Total SUM00 20.68 35.05
SUM40
June28--July 12 0.00 0.00 0.00 0.00 0.44 0.44
July 13--July 28 0.00 0.00 0.00 0.00 0.20 0.20
July 29--August 9 0.00 0.00 0.00 0.07 0.61 0.68
August 10--August 18 0.00 0.00 0.00 0.28 0.12 0.40
August 19--August 25 0.00 0.00 0.00 0.00 0.00 0.00
on August 16 in clone C, followed by a significant increase on August 16 in clone B and on August 30 in clone C. Ozone fumigation significantly increased the number of small grana on August 2 in clone B and on August 16 in clone C, but later the ozone treatment caused a decreased in the number of small grana in both clones and the decrease was significant on August 16 in clone B. On July 12, the ozone treatment signifi-cantly reduced the total number of grana and signifisignifi-cantly increased the size of the plastoglobuli of saplings of clone B. Ozone-fumigated saplings had significantly more plasto-globuli than control saplings from August 16 onward in clone B and on August 30 in clone C.
In general, abnormal and spherically shaped chloroplasts were more common in ozone-exposed saplings than in controls in both clones. Ozone treatment resulted in approximately 10% more injured chloroplasts in clone B saplings than in clone C saplings during late summer (Table 4). An ozone-in-duced increase in the density of stroma was observed after August 2 in clone B, whereas clone C showed slight increases in stroma density at all sampling dates. Ozone fumigation of saplings resulted in increased swelling and curling of
thyla-koids in clone B after August 2, but not until August 30 in clone C. In both clones, disintegration of the mitochondrial matrix and a reduction of cristae of ozone-fumigated saplings were occasionally observed throughout the summer. In response to ozone fumigation, cytoplasmic lipids increased in clone B, whereas lipid was more abundant in early summer in clone C (Table 4). Ozone had no effect on the number of microbodies, the electron density of plastoglobuli or the amount of tannin in either clone.
Rubisco
Rubisco formed 42--57% of the total soluble protein in birch leaves and this proportion remained constant during the ex-periment. There were no statistically significant differences between the treatments in total Rubisco content in either clone. However, after July 12, the total amount of Rubisco in ozone-treated seedlings of clone B was consistently lower than in control saplings (Figure 1a). Ozone exposure changed the relative proportions of the small and large subunits of Rubisco in both clones; however, few of the changes were statistically significant (Table 5).
Table 2. Effects of ozone on growth parameters of 2-year-old seedlings of Betula pendula, clones B and C. Height growth indicates growth since date of planting on May 10. Values are means ± SE. Data are pooled for all measurement dates, when final ozone effects as a percent increase or decrease compared to control saplings are presented. Student’s t-test: ** = significant at the 1% level, *** = significant at the 0.1% level, n = 10.
Date Clone B Clone C
Control Ozone Control Ozone
Height growth, cm
July 12 15.2 ± 2.0 18.2 ± 1.5 19.3 ± 2.9 27.1 ± 5.5
July 27 27.6 ± 3.6 29.1 ± 2.9 36.3 ± 2.7 41.3 ± 4.7
August 9 32.4 ± 4.1 34.4 ± 3.4 42.8 ± 3.0 49.1 ± 4.6
August 23 32.9 ± 4.9 36.9 ± 4.4 43.8 ± 3.2 53.4 ± 4.9
Final effect +10.0% +19.9%
Number of leaves
July 12 72 ± 3 79 ± 5 92 ± 7 100 ± 7
July 27 101 ± 5 112 ± 7 128 ± 10 129 ± 7
August 9 91 ± 5 86 ± 5 120 ± 8 120 ± 7
August 23 75 ± 5 84 ± 7 116 ± 10 115 ± 4
Final effect +6.3% +1.7%
Individual leaf size, cm2
July 12 15.3 ± 0.9 20.5 ± 1.3** 16.2 ± 0.8 15.4 ± 1.7
July 27 31.5 ± 1.6 20.3 ± 1.8*** 21.0 ± 1.1 20.5 ± 0.9
August 9 37.3 ± 1.7 29.4 ± 1.1** 30.5 ± 1.7 29.8 ± 1.6
August 23 21.1 ± 0.8 20.9 ± 1.0 20.9 ± 0.5 19.7 ± 0.9
Final effect −13.3% −3.6%
Foliage area, cm2
July 12 1102 ± 126 1620 ± 95** 1490 ± 146 1540 ± 138
July 27 3182 ± 236 2274 ± 229** 2688 ± 324 2645 ± 111
August 9 3390 ± 201 2528 ± 202** 3660 ± 290 3576 ± 283
August 23 1583 ± 114 1756 ± 143 2424 ± 209 2266 ± 145
Final effect −11.7% −2.3%
Stem dry weight, g
Sep 16 22.0 ± 4.2 17.0 ± 3.1* 18.2 ± 2.8 21.5 ± 4.3
Root dry weight, g
Chlorophyll content and a/b ratio
After July 12, the total quantities of chlorophyll a and b and the chlorophyll a/b ratio were lower in ozone-treated saplings than in control saplings of clone B on most sampling dates (Figures 1b and 1c). In clone C, there were no consistent differences in chlorophyll a and b contents or chlorophyll a/b ratios between treatments (data not shown).
Net photosynthesis and transpiration
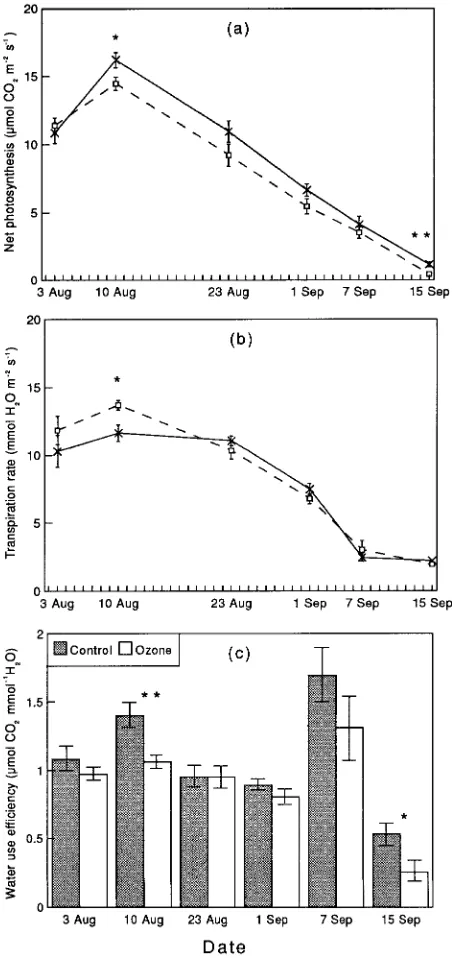
In clone B, net photosynthesis declined throughout most of the experiment and at all times it was lower in ozone-fumigated
saplings than in control saplings (Figure 2a). The average photosynthetic rate was 1.2 µmol CO2 m−2 s−1 lower in
ozone-treated saplings than in control saplings throughout the experi-mental period. The ozone treatment had little effect on transpiration rate of saplings of clone B except at the beginning of the measuring period (August 10) when transpiration rate of ozone-treated saplings was significantly greater than that of control saplings (Figure 2b).
In clone C, ozone exposure did not significantly reduce photosynthetic capacity and transpiration rate was also unaf-fected throughout the experiment (data not shown).
Table 3. Effects of ozone on the ultrastructure of spongy mesophyll cells of clones B and C of Betula pendula. Values are means ± SE. Student’s
t-test: * = significant at the 5% level, ** = significant at the 1% level, *** = significant at the 0.1% level, n = 20.
Date Clone B Clone C
Control Ozone Control Ozone
Chloroplast length (mm)
July 12 4.7 ± 0.2 4.9 ± 0.2 4.7 ± 0.2 4.2 ± 0.2
August 2 4.9 ± 0.1 4.4 ± 0.2* 5.1 ± 0.2 4.9 ± 0.2
August 16 4.7 ± 0.2 4.6 ± 0.2 4.8 ± 0.2 5.2 ± 0.1
August 30 4.8 ± 0.3 4.5 ± 0.2 5.2 ± 0.2 5.3 ± 0.2
Chloroplast width (mm)
July 12 2.0 ± 0.1 2.4 ± 0.1* 3.8 ± 1.2 2.3 ± 0.1
August 2 2.6 ± 0.1 2.9 ± 0.2 3.1 ± 0.1 2.2 ± 0.1***
August 16 2.2 ± 0.1 4.8 ± 1.9 3.1 ± 0.2 2.9 ± 0.2
August 30 3.1 ± 0.1 3.0 ± 0.1 3.3 ± 0.2 3.1 ± 0.2
Starch (% of chloroplast area)
July 12 24.2 ± 2.2 42.2 ± 4.3** 37.2 ± 4.7 48.3 ± 4.0
August 2 18.2 ± 2.8 37.2 ± 3.4*** 56.7 ± 4.4 28.9 ± 0.7***
August 16 48.0 ± 3.1 46.7 ± 4.1 59.2 ± 5.7 51.6 ± 4.3
August 30 68.9 ± 4.1 60.2 ± 3.2 72.1 ± 3.0 62.8 ± 3.4*
Number of small grana (< 10 thylakoids)/chloroplast cross section
July 12 8.9 ± 0.3 8.5 ± 0.5 10.1 ± 1.2 10.1 ± 2.9
August 2 4.6 ± 1.4 9.6 ± 0.9** 3.9 ± 0.4 5.0 ± 0.4
August 16 7.5 ± 0.7 5.4 ± 0.4** 4.2 ± 0.6 6.6 ± 0.5**
August 30 3.7 ± 0.4 3.9 ± 0.4 5.4 ± 0.5 4.2 ± 0.5
Nunber of large grana (>10 thylakoids)/chloroplast cross section
July 12 1.2 ± 0.2 0.2 ± 0.1*** 1.8 ± 0.7 1.0 ± 0.3
August 2 4.1 ± 0.5 1.8 ± 0.5** 5.1 ± 0.7 4.0 ± 0.6
August 16 0.5 ± 0.2 1.9 ± 0.4** 4.3 ± 0.9 1.0 ± 0.2**
August 30 0.2 ± 0.1 0.2 ± 0.1 0.1 ± 0.1 1.9 ± 0.3**
Total number of grana
July 12 10.1 ± 0.4 8.7 ± 0.5* 11.9 ± 0.8 11.0 ± 2.9
August 2 8.7 ± 1.3 11.4 ± 0.9 9.0 ± 0.7 9.0 ± 0.5
August 16 8.0 ± 0.7 7.2 ± 0.4 8.4 ± 0.6 7.6 ± 0.5
August 30 3.9 ± 0.5 4.1 ± 0.4 5.5 ± 0.5 6.1 ± 0.3
Number of plasto-globuli/chloroplast cross section
July 12 4.3 ± 0.5 4.3 ± 0.4 5.0 ± 0.7 3.7 ± 0.5
August 2 5.8 ± 0.5 4.7 ± 0.4 4.8 ± 0.8 6.5 ± 0.6
August 16 2.4 ± 0.4 4.5 ± 0.6** 5.5 ± 0.9 4.0 ± 0.6
August 30 3.5 ± 0.8 6.6 ± 0.8** 2.9 ± 0.5 4.7 ± 0.6*
Size of plastoglobuli (nm)
July 12 87 ± 8 143 ± 7*** 116 ± 16 109 ± 6
August 2 592 ± 25 284 ± 14 230 ± 11 366 ± 10
August 16 203 ± 11 413 ± 36 326 ± 21 331 ± 17
Water use efficiency (WUE)
There were significant differences in water use efficiency (WUE) between ozone-fumigated saplings and control sap-lings (Figure 2c). In clone B, ozone caused a reduction in WUE throughout the experiment and the decreases were significant on August 10 and September 15. In clone C, significantly higher WUE was observed in ozone-treated saplings than in control saplings on August 11, but on other dates WUE was higher in control saplings than in ozone-treated saplings (data not shown).
Discussion
Exposure to low ozone concentrations over one growing sea-son resulted in many structural and physiological changes in the ozone-sensitive clone B. The changes led to enhanced senescence and reductions in leaf and stem growth that ap-peared to be related to biochemical and structural changes of the cellular photosynthetic machinery.
The negative effects of ozone on final stem biomass produc-tion in clone B were in accordance with earlier results with this clone (Pääkkönen et al. 1993), but the initial stimulation of leaf growth by ozone was not observed in the previous study. The size of the grana was influenced by fumigation in both clones.
Initially, ozone exposure increased the proportion of small grana, whereas in the late summer a decrease was observed, possibly indicating enhanced stacking of thylakoids in re-sponse to long-term ozone stress.
There were large differences in ozone-induced ultrastructu-ral changes between the clones (Table 3), which supports earlier findings (Swanson et al. 1973, Pääkkönen et al. 1993, 1995). Changes in amount of starch and structure of grana occurred within two weeks of the start of ozone fumigation. Enhanced stacking of grana under ozone stress occurred two weeks earlier in clone B than in clone C, and may be associated with more rapid leaf development and aging processes in clone B compared with clone C (Pääkkönen et al. 1993, 1995).
Generally, the ozone-induced changes in chloroplast struc-ture paralleled the ozone-induced changes in Rubisco and chlorophyll contents and net photosynthesis. Concomitant with the transient stimulation of growth in the ozone-treated clone B, increases were observed in chloroplast diameter, and in contents of starch, Rubisco and chlorophyll. Later, in con-junction with the reduced rate of shoot growth of clone B, we observed chloroplasts that were smaller in diameter, and also reductions in Rubisco and chlorophyll contents and net photo-synthetic rates. However, in clone C, ozone fumigation had no effect on biomass production, but resulted in smaller diameter chloroplasts and reduced amounts of starch and the small subunit of Rubisco. Ozone induced ultrastructural changes, including abnormal and spherically shaped chloroplasts, swel-ling and curswel-ling of thylakoid membranes, and increased den-sity of stroma were observed in both clones, although the changes were more evident in the ozone-sensitive clone B than in the ozone-tolerant clone C.
Lehnherr et al. (1987) and Pell et al. (1992) reported that the decline in net photosynthesis in ozone-treated wheat and hy-brid poplar was correlated with decreased activity and quantity of Rubisco. Concomitant with lower photosynthesis, lower Rubisco content and reduced WUE, decreases particularly in chlorophyll a were observed in clone B. Previously, reduced WUE in saplings exposed to 2--4-fold higher ozone concentra-Table 4. Percentage increase or decrease (−) in ultrastructural symptoms in spongy mesophyll cells of clones B and C of Betula pendula in response to ozone treatment compared with control saplings (n = 20).
Symptoms Clone B Clone C
July 12 August 2 August 16 August 30 July 12 August 2 August 16 August 30
Chloroplast
mitochondrial matrix 17.5 42.6 18.6 4.9 1.2 19.8 24.7 −0.5
Amount of cytoplasmic lipids 3.3 39.0 25.0 25.7 9.2 6.5 3.8 3.6
Table 5. Effects of ozone on relative amounts (%) of Rubisco small subunit as a proportion of total Rubisco content in clones B and C of
Betula pendula. Values are means ± SE. Mann-Whitney’s U-test: * = significant at the 5% level, n = 10.
Date Clone B Clone C
Control Ozone Control Ozone
tions than in our experiment has been reported, e.g., in birch by Matyssek et al. (1991) and in hybrid poplar by Reich and Lassoie (1984). Reduced content of chlorophyll a has been observed earlier, e.g., in hybrid poplar (Reich 1983), in red spruce (Rebbeck et al. 1992) and in Norway spruce (Robinson and Wellburn 1991) after exposure to 2--5 fold higher ozone concentrations than in our experiment. This suggests that the reduction in net photosynthesis of the ozone-sensitive clone of birch could be explained by changes in stomatal action
result-ing in reduced WUE, and Rubisco and chlorophyll a contents of ozone-treated saplings.
The temporal pattern in the quantity of Rubisco protein was in accordance with the temporal pattern of growth rate of leaves in the ozone-sensitive clone B. Stimulation of leaf growth at the beginning of the experiment was accompanied by increased Rubisco, followed by reduced growth rate and Figure 1. Time course of effects of elevated ozone concentrations on
Rubisco content (a); chlorophyll content (b); and chlorophyll a/b ratio (c) of the ozone-sensitive clone B of Betula pendula (continuous line = control plants; broken line = ozone-fumigated plants). Each point represents the mean ± SE of the third leaf of the emerged shoot collected from five plants per treatment. Student’s t-test, P < 0.05.
lower Rubisco content. The ozone-induced reduction of the Rubisco small subunit is in agreement with the recent studies on potato by Reddy et al. (1993) and by Eckardt and Pell (1994), where ozone exposure reduced the amount of the Rubisco small subunit more than that of the large subunit. Pell et al. (1994) considered that reduced amounts of mRNA for the small subunit of Rubisco reduced the potential for synthesis of the Rubisco protein. In hybrid poplar, ozone was found to increase the loss of large subunits and to result in accumulation of Rubisco large subunit aggregates (Landry and Pell 1993). The early decline in Rubisco mRNA immediately after ozone exposure (Reddy et al. 1993) indicates that ozone may be capable of directly affecting synthesis of Rubisco. In addition, Mehta et al. (1992) showed that Rubisco protein is highly sensitive to oxidative stress in vivo, which affects its transloca-tion and degradatransloca-tion as well as cross-linking of the large subunit.
We conclude that ozone impaired the photosynthetic capac-ity of the ozone-sensitive birch clone as a result of multiple injuries. Chloroplast injuries and reductions in Rubisco con-tent were the main cause of reduced rates of photosynthesis and leaf and stem growth and accelerated rates of senescence-related changes in the ozone-sensitive clone. The low ozone stress treatment had little effect on the structure, physiology and biochemistry of the chloroplast of the ozone-tolerant birch clone, which exhibited normal growth and leaf senescence.
The cumulative ozone exposures during the periods between observations were low (range of SUM40 was 0.00--0.68). The UN-ECE workshop in Bern concluded that a SUM40 of 10 ppm-h was the critical ozone exposure for trees (Fuhrer and Achermann, unpublished observations). We conclude that this ozone exposure threshold is too high, because our evidence suggests that ozone exposures between 0.0 and 0.68 ppm-h have more harmful impacts on ozone-sensitive individual trees and species in northern Europe than in more southerly latitudes (Beck and Grennfelt 1994).
Acknowledgments
This study was supported by the Maj and Tor Nessling Foundation. We thank Timo Oksanen for valuable assistance in ozone data collection.
References
Arnon, D.I. 1949. Copper enzymes in isolated chloroplasts. Polyphe-noloxidases in Beta vulgaris. Plant Physiol. 24:1--15.
Beck, J.P. and P. Grennfelt. 1994. Estimate of ozone production and destruction over Northwestern Europe. Atmos. Environ. 28:129--140.
Chappelka, A.H. and B.I. Chevone. 1992. Tree responses to ozone. In
Surface Level Ozone Exposures and Their Effects on Vegetation. Ed. A.S. Lefohn. Chelsea, Lewis Publishers, Inc., pp 271--323. Darrall, N.M. 1989. The effect of air pollutants on physiological
processes in plants. Plant Cell Environ. 12:1--30.
Eckardt, N.A. and E.J. Pell. 1994. O3-Induced degradation of Rubisco
protein and loss of Rubisco mRNA in relation to leaf age in So-lanum tuberosum L. New Phytol. 127:741--748.
Gezelius, K. and M. Hallen. 1980. Seasonal variation in ribulose bisphosphate carboxylase activity in Pinus sylvestris. Physiol. Plant. 48:88--98.
Holopainen, T., S. Anttonen, A. Wulff, V. Palomäki and L. Kärenlampi. 1992. Comparative evaluation of the effects of gaseous pollutants, acidic deposition and mineral deficiences: structural changes in the cells of forest plants. Agric. Ecosyst. Environ. 42:365--398. Laemmli, U.K. 1970. Cleavage of structural proteins during the
as-sembly of the head of bacteriophage T4. Nature 277:680--685. Landry, L.G. and E.J. Pell. 1993. Modification of Rubisco and altered
proteolytic activity in O3-stressed hybrid poplar (Populus
maxi-mowizii × trichocarpa). Plant Physiol. 101:1355--1362.
Lehnherr, B., A. Grandjean, F. Mächler and J. Führer. 1987. The effect of ozone in ambient air on ribulose bisphosphate carboxylase/oxy-genase activity decreases photosynthesis and grain yield in wheat. J. Plant Physiol. 130:189--200.
Matyssek, R., M.S. Günthardt-Goerg, T. Keller and C. Scheidegger. 1991. Impairment of gas exchange and structure in birch leaves (Betula pendula) caused by low ozone concentrations. Trees 5:5--13.
Mehta, R.A., T.W. Fawcette, D. Porath and A.K. Mattoo. 1992. Oxi-dative stress causes rapid membrane translocation and in vivo deg-radation of ribulose-1,5-bisphosphate carboxylase/oxygenase. J. Biol. Chem. 267:2810--2816.
Pell, E.J. and M.S. Dann. 1991. Multiple stress-induced foliar senes-cence and implications for whole-plant longevity. In Responses of Plants to Multiple Stresses. Eds. H.A. Mooney, W.E. Winner and E.J. Pell. Academic Press, San Diego, pp 189--205.
Pell, E.J., N.A. Eckardt and A.J. Enyedi. 1992. Timing of ozone stress and resulting status of ribulose bisphosphate carboxylase/oxy-genase and associated net photosynthesis. New Phytol. 120:397--405.
Pell, E.J., N.A. Eckardt and R.E. Glick. 1994. Biochemical and mo-lecular basis for impairment of photosynthetic potential. Photo-synth. Res. 39:453--462.
Pääkkönen, E., S. Paasisalo, T. Holopainen and L. Kärenlampi. 1993. Growth and stomatal responses of birch (Betula pendula Roth.) clones to ozone in open-air and field fumigations. New Phytol. 125:615--623.
Pääkkönen, E., T. Holopainen and L. Kärenlampi. 1995. Ageing-re-lated anatomical and ultrastructural changes in leaves of birch (Betula pendula Roth.) clones as affected by low ozone exposure. Ann. Bot. 75:285--294.
Rebbeck, J., K.J. Jensen and M.S. Greenwood. 1992. Ozone effects on grafted mature and juveline red spruce: photosynthesis, stomatal conductance, and chlorophyll concentration. Can. J. For. Res. 23:450--456.
Reddy, G.N., R.N. Arteca, Y.-R. Dai, H.E. Flores, F.B. Nerm and E.J. Pell. 1993. Changes in ethylene and polyamines in relation to mRNA levels of the large and small subunits of ribulose bispho-phate carboxylase/oxygenase in one-stressed potato foliage. Plant Cell Environ. 16:819--826.
Reich, P.B. 1983. Effects of low concentration of O3 on net
photosyn-thesis, dark respiration, and chlorophyll contents in aging hybrid poplar leaves. Plant Physiol. 73:291--296.
Reich, P.B. and J.P. Lassoie. 1984. Effects of low level O3 exposure on
leaf diffusive conductance and water-use efficiency in hybrid pop-lar. Plant Cell Environ. 7:661--668.
Robinson, D.C. and A.R. Wellburn. 1991. Seasonal changes in the pigments of Norway spruce, Picea abies (L.) Karst, and the influ-ence of summer ozone exposures. New Phytol. 119:251--259. Swanson, E.S., W.W. Thomson and J.B. Mudd. 1973. The effect of
ozone on leaf cell membranes. Can. J. Bot. 51:1213--1219.
Thomas, H. and J.L. Stoddardt. 1980. Leaf senescence. Annu. Rev. Plant Physiol. 31:83--111.