Immunoanalysis of isoflavonoids in
Pisum sati
6
um
and
Vigna
radiata
Oldrˇich Lapcˇı´k
a,*, Martin Hill
a, Ivan C
&
erny´
b, Jaromı´r Lachman
c,
Nawaf Al-Maharik
d, Herman Adlercreutz
e, Richard Hampl
aaInstitute of Endocrinology,Na´rodnı´ trˇı´da8,116 94Praha1,Czech Republic bInstitute of Organic Chemistry and Biochemistry,Praha,Czech Republic
cCzech Uni6ersity of Agriculture,Praha,Czech Republic dDepartment of Chemistry,Uni6ersity of Helsinki,Helsinki,Finland eDepartment of Clinical Chemistry,Uni6ersity of Helsinki,Helsinki,Finland
Received 19 January 1999; received in revised form 25 June 1999; accepted 25 June 1999
Abstract
Radioimmunoassays (RIAs) combined with liquid chromatography were used for study of isoflavonoids in seeds of peaPisum sati6umand mung beanVigna radiata. Radioimmunoassays with these specifities were used: (1) daidzein and its 4%-derivatives (e.g. formononetin); (2) daidzein and its 7-derivatives (e.g. daidzin, isoformononetin); (3) genistein and its 4%-derivatives (e.g. biochanin
A); and (4) genistein and its 7-derivatives (e.g. genistin, prunetin). Dormant or germinating seeds were extracted with 80% ethanol. Immunoreactivities were measured either in crude extracts or after chromatographic fractionation by HPLC (reversed phase, octadecylsilica). Chromatographic mobilities of immunoreactive fractions were compared to those of daidzein, daidzin, for-mononetin, isoforfor-mononetin, genistein, genistin biochanin A and prunetin standards. Extracts from Vigna radinfa contained daidzein, genistein and their 7-O glucosides, daidzin and genistin, respectively. No immunoreactivity was recorded in HPLC fractions corresponding to glycosides in extracts from P. sati6um, but the methods sensitive to 7-derivatives of daidzein and genistein showed peaks with chromatographic mobilities identical to those of the 7-methoxyderivatives, isoformononetin and prunetin, respectively. In additional experiments, the pea extracts were fractionated either by thin layer chromatography (TLC) on silica or by ion-exchange TLC on aminosilica. Identity of the daidzein-7 and genistein-7 immunoreactive entities with isoformononetin and prunetin, respectively, was confirmed by the identical chromatographic behavior in all these different chromatographic systems. © 1999 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Vigna radiata;Pisum sati6um; Isoflavonoid; 7-methoxy isoflavonoid; Isoformononetin; Prunetin; Immunoassay
www.elsevier.com/locate/plantsci
1. Introduction
Isoflavonoids are found characteristically in the
Viciaceae sub-family of the Fabaceae. They were found in different parts of leguminous plants (roots, leaves, seeds). Isoflavonoids are typically stored as 7-O glycosides, whilst secretion of agly-cones is elicited by different physiologic or patho-logic situations [1]. In addition to their presence in legumes, isoflavonoids were found in genera Iris
(Iridaceae),Prunus(Rosaceae),Podocarpus (Podo
-carpaceae), Maclura (Moraceae) and Iresine
(Amaranthaceae) [2,3]. Low concentrations of cer-tain isoflavonoids were found recently in numer-ous ‘non-classical’ plants, e.g. barley, wheat, sunflower seeds or tea [4,5].
Isoflavonoids, present in food of plant origin, were shown to influence the endocrine system in animals by interaction with hormone receptors and/or steroid metabolizing enzymes [5]. Recently, we have developed radioimmunoassays (RIA) for measurement of daidzein and genistein in human body fluids and in beer [6 – 9] (Fig. 1). These
* Corresponding author. Tel.: +420-2-24905-275; fax:+ 420-2-24905-325.
E-mail address:[email protected] (O. Lapcˇı´k)
methods are now being used for screening of isoflavonoids in dormant and germinating seeds. Here we present evidence of daidzein, for-mononetin, isoformononetin and prunetin in P.
sati6um and daidzin, daidzein, genistin and genis-tein in V. radiata.
2. Experimental
2.1. Chemicals
Daidzin was a generous gift of Dr Takaaki Yasuda from Tohoku College of Pharmacy, Miyagi, Japan. Equol, O-desmethylangolensin, dihydrodaidzein and carboxymethyl derivatives of daidzein and genistein were synthesized as de-scribed elsewhere [6,7].
Diethyl ether was from Synthesia (Pardubice, Czech Republic), all other organic solvents were from Merck (Darmstadt, Germany). Daidzein, genistein, genistin, luteolin, quercetin, and api-genin were from Sigma (St. Louis, Missouri, USA), dextran T-70 from Pharmacia (Uppsala, Sweden). All other substances were of analytical grade, from Lachema (Brno, Czech Republic).
2.2. NMR
1H NMR spectra were taken on a
Varian-UNITY-200 (200 MHz) and Varian UNITY-500 (500 MHz) spectrometers at 23°C in hex-adeuteroacetone and hexadeuterodimethyl sulfox-ide, being referenced to acetone (d 2.20 ppm)
and dimethyl sulfoxide (d 2.50 ppm), respec-tively.
2.3. Mass spectrometry
Mass spectrum of prunetin preparation was recorded on a VG Analytical ZAB-EQ spectrom-eter (energy of ionizing electrons 70 eV, ion source temperature 170°C).
2.4. Synthesis and characterization of isoformononetin and prunetin
Isoformononetin was synthesized from daidzein as described by Ingham [10], using dia-zomethane as a methylation agent. Prunetin was prepared from genistein in the same way. Ethe-real diazomethane (0.5 ml, approximately 250
mmol) was added to an ice cooled solution of the
parent isoflavone (approximately 50 mmol) in
dimethyl sulfoxide (0.1 ml) and methanol (0.5 ml). The reaction vessel was stopped, the cooling was removed, and the mixture was stirred for 25 min. at room temperature. Then acetic acid was added (1 drop), the solvents were removed in vacuum, and the residue was coevaporated with toluene (2×1 ml). The product was separated from unreacted isoflavone by column chromatog-raphy on silica gel (5 ml) in a mixture of chloro-form-methanol (50:1). Crystallization of the main fraction from hot methanol gave the correspond-ing 7-O-methyl isoflavone in the yield of about 50%.
7-O-methyl daidzein (isoformononetin), m.p. 224 – 225°C (Ref. [11] gives 224 – 225°C). 1H
NMR spectrum in d6-acetone was in accord with
results in the literature [10]. 7-O-methylgenistein (prunetin), m.p. 238 – 239°C (Ref. [12] gives 233 – 235°C from ethanol). 1H NMR spectrum (500
MHz, d6-dimethylsulfoxide): 12.96 s, 1 H
(5-OH), 9.60 s, 1 H (4%-OH), 8.42 s, 1 H (H-2), 6.83 d, 2 H (J=8.5, H-3% and H-5%), 7.39 d, 2 H (J=8.5, H-2% and H-6%), 6.67 d, 1 H (J=2.3, H-8), 6.42 d, 1 H (J=2.3, H-6), 3.87 s, 3 H (OCH3). Mass spectrum m/z (%) 284 (100, M+).
In addition, both preparations displayed im-munochemical and chromatographic behavior ex-pected for 7-methoxy derivatives, namely: high crossreactivity in the 7-position based im-munoassays and low crossreactivity in the 4% -po-sition based ones, different chromatographic
Fig. 1. Analytes: Daidzein, R1=R2=R3=H; Daidzin, R1= developing of the immunoassays: (1) Daidzein 4% -car-boxymethylether, R1=H, R2=CH2COOH, R3=H; (2) Daidzein 7-carboxymethylether, R1=CH
mobility on aminosilica. Fluorescence of the TLC spots in UV, typical for daidzein and formononetin, was absent in isoformononetin preparation.
2.5. Plant material
Mung beans V. radiata and pea P. sati6um var. Kapucin were purchased in Country Life (Hostivice, Czech Republic). Pea P. sati6um var. Lantra was obtained in Czech University of Agriculture, Prague, Czech Republic.
Seeds were placed into a polypropylene beaker on a cellulose sheet wet with tap water (1 ml/1 g of seeds) and the top was covered partly with a glass plate. One beaker was harvested each day, the others were wet with several additional drops of water. Seeds were disintegrated by an Eta 1042 mixer ( Electro-Praga, Hlinsko, Czech Re-public) in 80% ethanol (10 ml/g of dry weight) and left to extract at 4°C for at least 72 h with several shakings every day. Finally, the debris was left to settle and the supernatant was cen-trifuged to complete the sedimentation of solid particles. Aliquots of the extracts were analyzed by radioimmunoassays either directly, or after chromatographic fractionation. Before chro-matography the extracts were evaporated on a speedvac and reconstituted in water.
Lipids were extracted with petroleum ether, and the water phase evaporated again The dry residue was then dissolved in 80% ethanol (a quarter of the original volume). Typically 20 m1
of this solution (corresponding to an extract from 8 mg of seeds) were injected to the HPLC, 5 ml of this solution were applied to a TLC
plate.
2.6. Hydrolysis
For some experiments, the water-ethanol ex-tracts (2.0 ml) were evaporated, reconstituted in 1.0 ml of water, and non polar substances were extracted to light petroleum ether. The lipids de-pleted water phase was then hydrolyzed by adding hydrochloric acid (final volume 2 ml, final concentration 1.0 M, 90°C, 2 h). After hy-drolysis, the mixture was cooled, and the agly-cones were extracted by 2 ml of diethyl ether. The dry ether extract was dissolved in original volume of ethanol again. These conditions were
previously shown to hydrolyze glycosides of daidzein and genistein, whilst the aglycones, in-cluding the methoxy derivatives, were preserved [4].
2.7. Radioimmunoassay
RIA methods were used, based on polyclonal rabbit antibodies to bovine serum albumin con-jugates with daidzein-4%-carboxymethyl ether (Dai-4%-), daidzein-7-carboxymethyl ether (Dai-7), genistein-4%-carboxymethyl ether (Gen-4%-) and genistein-7-carboxymethyl ether (Gen-7). Ho-mologous conjugates of respective isoflavone with [125I] tyrosine methyl ester were used as
ra-dioligands. Parameters of the assays are summa-rized in Table 1 [6 – 8].
2.8. TLC
Direct phase TLC: Alugram Nano-Sil G/UV254
aluminium sheets Art. 818 142 from Macherey Nagel (Duren, Germany) were developed in dichloromethane — isopropanol 95:5. Ion-ex-change TLC: TLC aluminium sheets NH2F254S
Art. 1.05533 from Merck (Darmstad, Germany) were developed twice in the same system.
After developing the plates were cut with scis-sors to 30 strips (6 mm of width), and each strip was eluted with 2 ml of ethanol. Aliquots (200
ml) of the eluate were pipetted to glass tubes,
evaporated on a speedvac, dissolved in the assay buffer and analyzed by the appropriate RIA.
2.9. HPLC
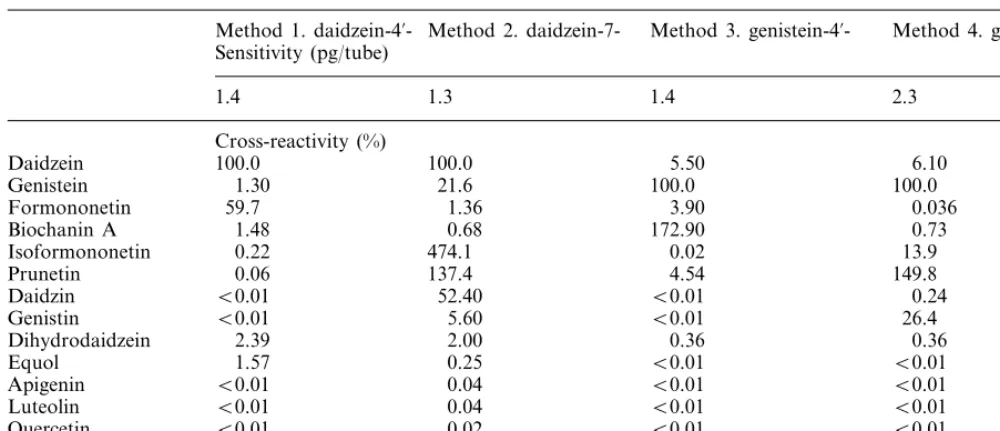
Table 1
Parameters of the radioimmunoassays used in this study. The sensitivities were expressed as the lowest levels of daidzein (methods 1, 2) and genistein (methods 3, 4), distinguishable from zero with 95% probability. The sensitivity to other compounds detected by the above methods may differ in a similar manner as do the cross-reactivities. The cross-reactivities were expressed as ratios of 50% intercept of the main analytes to 50% intercepts of the cross-reactants. The cross-reactivities of very weak cross-reactants (below 0.5%) were calculated as a ratio of the signal elicited by 10 ng of the respective cross-reactant to the analyte
Method 2. daidzein-7- Method 3. genistein-4%- Method 4.
genistein-7-Method 1. daidzein-4%
-Sensitivity (pg/tube)
1.3 1.4
1.4 2.3
Cross-reactivity (%)
Daidzein 100.0 100.0 5.50 6.10
21.6 100.0
1.30 100.0
Genistein
59.7
Formononetin 1.36 3.90 0.036
0.68 172.90
Biochanin A 1.48 0.73
474.1 0.02
0.22 13.9
Isoformononetin
0.06
Prunetin 137.4 4.54 149.8
B0.01
Daidzin 52.40 B0.01 0.24
5.60 B0.01
B0.01 26.4
Genistin
2.39
Dihydrodaidzein 2.00 0.36 0.36
0.25 B0.01
Equol 1.57 B0.01
0.04 B0.01
B0.01 B0.01
Apigenin
B0.01
Luteolin 0.04 B0.01 B0.01
0.02 B0.01 B0.01
Quercetin B0.01
B0.01 0 01 B0. 01 B0.01
O -desmethylango-lensin
3. Results
3.1. Isofla6onoid immunoreacti6ity in crude extracts
Daidzein- and genistein- like immunoreactivi-ties were found by RIAs in non-fractionated
wa-ter-ethanolic extracts from dormant seeds of P.
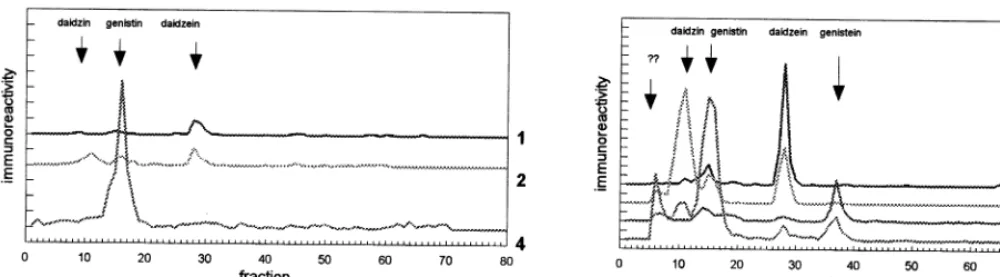
sati6um and Vigna radiata, and the signal in-creased during germination. In both species, the immunoassays sensitive to 7-derivatives of daidzein and genistein measured mostly higher levels than the 4%-derivatives sensitive ones (Fig. 2).
Fig. 2. Isoflavonoid immunoreactivities in ethanolic extracts from P.sati6um and V. radiata: (1) daidzein-4%-specific RIA; (2)
Fig. 3. Immunoreactivity in HPLC fractions of ethanolic extracts from dormant and germinating seeds ofV.radiata. Extract from 8 mg of seeds was chromatographed, the fractions were divided to ten aliquots and analyzed by RIA. The number assigns the type of immunoassay used in the same set of samples. Daidzein (1, 2) and genistein (3, 4) were used for construction of calibration curves, respectively. Arrows indicate position of standards on a control chromatogram. One segment on the Y-axis corresponds to the signal of 10 pg of the respective standard.
3.2. HPLC of V. radiata extracts
Immunoassay of individual fractions after HPLC on octadecyl silica showed analogous pat-terns in extracts from dormant seeds and after 3 days of germination. Germinating seeds con-tained higher amounts of all detected immunore-activities. According to the chromatographic behavior of the immunoreactive peaks, V. radi
-ata contained daidzin, daidzein, genistin and genistein (Fig. 3). No signal was detected in that part of chromatogram, where methoxy deriva-tives of daidzein and genistein would occur. The genistein-7 sensitive method showed two addi-tional peaks, more polar than genistin, in
ex-tracts from germinating V. radiata. It is known, that isoflavone glycosides may occur malonylated or acetylated at the glucose moiety [13]. The ad-ditional genistein-7 immunoreactive peaks in the ‘glucoside part’ of chromatogram most probably reflect formation of conjugates of this type in germinating V. radiata.
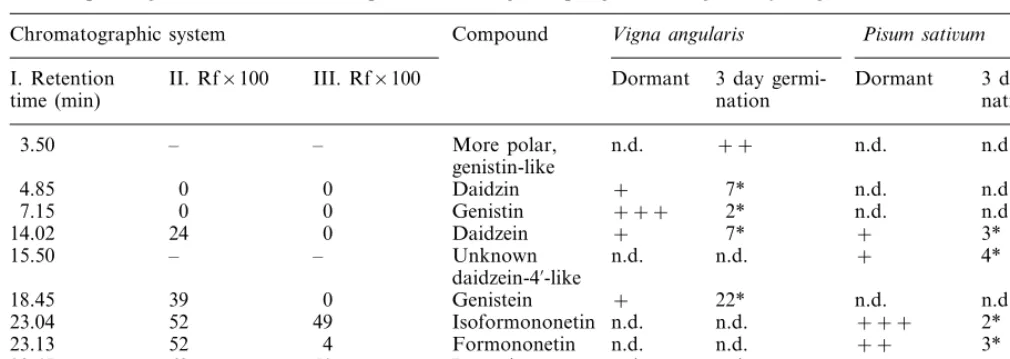
3.3. HPLC of P. sati6um extracts
The extracts from P. sati6um contained im-munoreactivities in the positions on chro-matogram corresponding to daidzein, formononetin, isoformononetin, and prunetin (Fig. 4). The immunoreactivity corresponding to
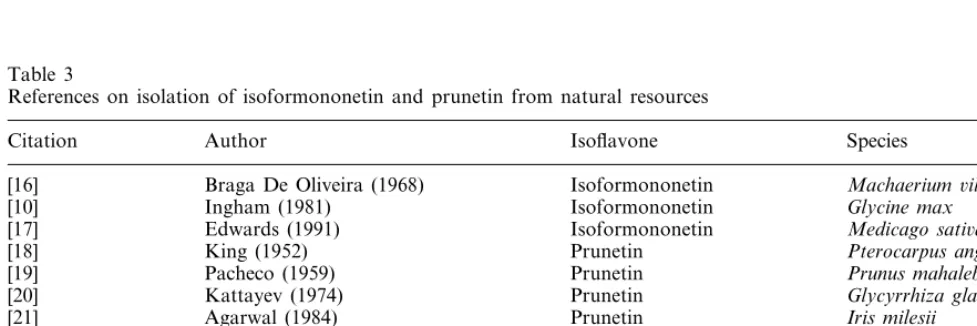
Table 2
Isoflavonoids detected in chromatographic fractions by radioimmunoassays. The individual levels were estimated from the analysis of corresponding HPLC fractions and expressed in nanograms per gram of original dry weight of seedsa
Vigna angularis Pisum sati6um
Chromatographic system Compound
II. Rf×100
I. Retention III. Rf×100 Dormant 3 day germi- Dormant 3 day
germi-nation nation
time (min)
– More polar,
–
3.50 n.d. ++ n.d. n.d.
genistin-like 0
4.85 0 Daidzin + 7* n.d. n.d
0 Genistin +++
0 2*
7.15 n.d. n.d.
24
14.02 0 Daidzein + 7* + 3*
–
15.50 – Unknown n.d. n.d. + 4*
daidzein-4%-like
0 Genistein +
18.45 39 22* n.d. n.d.
23.04 52 49 Isoformononetin n.d. n.d. +++ 2*
4 Formononetin n.d.
52 n.d.
23.13 ++ 3*
68
29.47 50 Prunetin n.d. n.d. ++ ++
3 Biochanin A
31.61 68 n.d. n.d. Detected occasionally
aDormant seeds, n.d.: not detected;+: 10–50 ng/g; ++: 50–200 ng/g; +++: 200–500 ng/g. 3 day germination: n.d.: not detected; N*: increase of the signal during germination N-fold. Chromatographic systems: I. C18 RP HPLC, II. TLC on silica, III. TLC on aminosilica.
biochanin A was detected only in some samples, as the concentration was low. The daidzein-4% sensitive method always detected one additional peak, not detected by the complementary daidzein-7 sensitive method, moving between daidzein and genis-tein on the RP HPLC. The signal of this unknown cross-reactant was ap-proximately equal to that of daidzein, regardless of the strain of pea or germination status.
No immunoreactivity was found in that part of chromatogram, where glycosides would appear. Genistein was not clearly detected either.
3.4. TLC of P. sati6um extracts
Chromatographic mobilities of all detected im-munoreactivities were identical to those of the authentic standards in direct phase TLC on sil-ica. Chromatographic mobilities of the daidzein-7 and genistein-7 immunoreactivities were identical to those of isoformononetin and prunetin standards, respectively, in ion exchange TLC on aminosilica. TLC on aminosilica sepa-rates both 7-methoxy isoflavones from the other forms (7-glucoside, 4%-methoxy and free) due to absence of the 7-OH group.
3.5. Hydrolysis of P. sati6um extracts
All immunoreactivities found in P. sati6um al-most completely resisted hydrolysis in hydrochlo-ric acid (1 M, 2 h, 90°C) and were extractable with diethyl ether.
3.6. Conclusion
In conclusion, by combination of different types of liquid chromatography and radioim-munoassay we have identified several isoflavone derivatives in dormant and germinating seeds of
P. sati6um and V. radiata as summarized in Table 2.
4. Discussion
for construction of the immunogen. Some other undesired cross-reactivities or matrix effects may occur as well. New application of a method always requires its validation for the type of samples under study. A simple finding of immunoreactivity in certain material indicates only a possibility of occurrence of the molecule(s) of interest, whilst a negative finding would indicate the absence of whole group of related compounds. Combination of immunoassay with powerful separation meth-ods increases the specificity by orders of magni-tude and diminishes the possibility of error close to zero.
Despite none of the RIA methods used in this study were specific enough to be used for estima-tion of only one analyse directly in crude pea extracts, the data presented here could serve as a basis for development of very sensitive and specific methods, combining immunoassays with appropri-ate pre-separation of the samples. Simple one step liquid-liquid extraction or solid phase extraction could probably be efficient enough in many appli-cations, as the solubility of glycosides and agly-cones differs notably in numerous solvents. Furthermore, the chromatographic behavior of 7-methoxy-, 7-hydroxy- and 7 glucosyl isoflavones on aminosilica allows a good separation. It is also possible that for some purposes (e.g. in study of plant — microbe interactions) the whole im-munoreactivity measured by some of the above mentioned immunoassays could serve as a useful parameter, reflecting certain physiological pro-cesses. Considerable differences in isoflavonoid im-munoreactivities were observed in P. sati6um,
depending on variety, locality and year. Epicoccum purpurascens elicitors increased isoflavonoid con-tent in pea (Lachman et al., manuscript in prepa-ration). The inconvenience, and sometimes a negative public attitude, connected with use of radiolabelled indicators, will be overcome in near future by using alternative non-isotopic im-munoassays [14,15].
Prunetin and isoformononetin were found to-gether with daidzein and formononetin in P. sa
-ti6um. Prunetin was previously found in six different genera, isoformononetin was so far re-ported in heartwood extracts of Machaerium6illo -sum, in leaves from Glycine max and cell cultures from Medicago sati6a (Table 3). A preferential methylation of the 7-hydroxyl group of daidzein by purified isoflavone O-methyl transferase from
Medicago sati6awas observed by He et al., but the authors questioned, if the same reaction occurs also in vivo [28]. The sequence analysis of
Medicago sati6a isoflavone O-methyl transferase cDNA revealed a 50.5% amino acid identity of this enzyme to the 6-alpha hydroxy
maackiain-3-O-methyl transferase from P. sati6um [29]. In a recent study, we have found isoformononetin and prunetin in Medicago sati6a using the same HPLC-RIA methodology. The attempt to confirm these findings by capillary electrophoresis — diode array detection is in progress (unpublished data). The daidzein-4%-specific RIA detected one immunoreactive peak in HPLC fractions of ex-tracts from P. sati6um, moving between daidzein and formononetin. Our previous experience with specificity of hapten immunoassays tempted us to
Table 3
References on isolation of isoformononetin and prunetin from natural resources
Citation Author Isoflavone Species
Braga De Oliveira (1968) Isoformononetin Machaerium6illosum
[16]
Ingham (1981)
[10] Isoformononetin Glycine max
Edwards (1991)
[17] Isoformononetin Medicago sati6a
[18] King (1952) Prunetin Pterocarpus angolensis
Prunus mahaleb
Prunetin Pacheco (1959)
[19]
Kattayev (1974)
[20] Prunetin Glycyrrhiza glabra.
[21] Agarwal (1984) Prunetin Iris milesii
Bezuidenhoudt (1987)
[22] Prunetin Pterocarpus soyouxii.
Bandara (1990)
[23] Prunetin Butea monosperma
[24] Fukai (1990) Prunetin Glycyrrhiza pallidiflora.
Prunetin
[25] Gebiel (1991) Prunus ceraseus
Lin (1991)
[26] Prunetin Derris laxiflora
Hayashi (1996)
hypothesize, that this daidzein-4%-cross-reactive compound might be closely related structurally to daidzein or formononetin, differing only slightly at the B ring. Calycosin (3%-hydroxy formononetin), the first intermediate on the metabolic pathway from formononetin to pisatin, could fulfil these criteria. However, only comparing this compound with corresponding standards may bring proof.
The isoflavonoid concentrations detected in P.
sati6um and V. radiata in our study were in a ng/gmg/g range. Thus, the detected compounds
represent minor constituents of the isoflavonoid complex in the above species, being probably in-termediates or by-products in metabolic pathways leading to more typical compounds. Pterocarpan phytoalexins ofP. sati6um, maackiain and pisatin, are synthesised from isoflavones. The established biosynthetic pathway leads from daidzein to for-mononetin (methylation at 4%), calycosin (3%-OH formononetin), c-baptigenin and several further intermediates to 6a-hydroxymaackiain, which is finally methylated to pisatin [30]. There are only few reports about 7-methoxy isoflavonoids in the literature. Despite their doubts about occurrence of 7-methylation of daidzein inMedicago sativa in vivo, He et al. have hypothesised, that isofor-mononetin could define a new branch of the isoflavone pathway [29]. They mentioned a single report of afalone, an isoflavone methylated at both 4%and 7-positions in alfalfa cell cultures [31]. Methylation of 6a-hydroxymaackiain (at the posi-tion corresponding to 7-hydroxy group of the isoflavone precursor) is the final step of pisatin synthesis inP. sati6um. It is tempting to speculate: if the enzymes on the pathway from daidzein to 6a-hydroxy maackiain were able to metabolize also the analogues coming from isoformononetin (i.e. 7 methoxy daidzein), it would be a new alter-native pathway from daidzein to pisatin.
To our best knowledge, this is the first report on isoformononetin and prunetin in P. sati6um.
Acknowledgements
This study was supported by Grant 311-97-468 from the Grant Agency of the Czech Republic. The synthesis of carboxymethyl isoflavones, used for preparation of immunogenes and radioindica-tors, and standards equol, O-desmethylangolensin and dihydrodaidzein was supported by NIH
(USA) grant no. 2 RO1 CA56289-04. We are grateful to acting ass. Prof. Kristiina Wa¨ha¨la¨, Ph.D. for providing us with equol, O-desmethy-langolensin and dihydrodaidzein standards and for supervision of Nawaf Al Maharik during synthesis of isoflavonoid carboxy derivatives. The skillful technical assistance of Ms Antonie Kaasova is acknowledged.
References
[1] F.D. Dakora, D.A. Phillips, Diverse functions of isoflavonoids in legumes transcend anti-microbial defini-tions of phytoalexins, Physiol. Mol. Plant Pathol. 49 (1996) 1 – 20.
[2] W.D. Ollis, The Isoflavonoids, in: T.A. Geissman (Ed.), The Chemistry of Flavonoid Compounds, Pergamon Press LTD, Oxford, 1962, pp. 353 – 399.
[3] J.B. Harborne, Comparative Biochemistry of the Flavonoids, Academic Press INC., London, 1967, pp 166 – 170.
[4] W. Mazur, T. Fotsis, K.T. Wa¨ha¨la¨, S. Ojala, A. Salakka, H. Adlercreutz, Isotope dilution gas chromatographic-mass spectrometric method for the determination of isoflavonoids, coumestrol, and lignans in food samples, Anal. Biochem. 233 (1996) 169 – 180.
[5] H. Adlercreutz, W. Mazur, Phytoestrogens and western diseases, Annals Med. 29 (1997) 95 – 120.
[6] O. Lapcˇı´k, R. Hampl, N. Al-Maharik, A. Salakka, K. Wa¨ha¨la¨, H. Adlercreutz, A novel radioimmunoassay for daidzein, Steroids 62 (1997) 315 – 320.
[7] O. Lapcˇı´k, M. Hill, R. Hampl, K. Wa¨ha¨la¨, N. Al-Ma-harik, H. Adlercreutz, Radioimmunoassay of free genis-tein in human serum, J. Steroid Biochem. Molec. Biol. 64 (1998) 261 – 268.
[8] R. Hampl, O. Lapcˇı´k, L. Starka, K. Wa¨ha¨la¨, H. Adler-creutz, Radioimunoanaly´za fytoestrogenu˚ isoflavonoidove´ rˇady, Chemicke´ listy 92 (1997) 44 – 50. [9] O. Lapcˇı´k, M. Hill, R. Hampl, K. Wa¨ha¨la¨, H.
Adler-creutz, Identification of isoflavonoids in beer, Steroids 63 (1998) 14 – 20.
[10] J.L. Ingham, N.T. Keen, L.J. Mulheirn, R.L. Lyne, Inducibly-formed isoflavonoids from leaves of soybean, Phytochemistry 20 (1981) 795 – 798.
[11] A.C. Jain, J. Singh, Synthesis of corylin methyl ether — structures of corylin & neobavaisoflavone, Indian J. Chem. 13 (1975) 789 – 790.
[12] H. Sekizaki, R. Yokosawa, Ch. Chinen, H. Adachi, Y. Yamane, Studies on zoospore attracting activity. II. Syn-thesis of isoflavones and their attracting activity to Aphanomyces enteiches zoospore, Biol. Pharm. Bull. 16 (1993) 698 – 701.
[13] P.A. Murphy, T. Song, G. Buseman, K. Barua, Isoflavones in soy-based infant formulas, J. Agric. Food Chem. 45 (1997) 4635 – 4638.
assess-ment of dietary soya exposure, J. Steroid Biochem. Molec. Biol. 64 (1998) 217 – 222.
[15] H. Adlercreutz, O. Lapcı´k, R. Hampl, K. Wa¨ha¨la¨, N. Al-Maharik, G.J. Wang, H. Mikola, Immunoassay of phytoestrogens in human plasma, in: Proceedings of Symposium on Phytoestrogen Research Methods, Sep-tember 21 – 24, Tucson, Arizona, 1997.
[16] A. Braga De Oliveira, O.R. Gottlieb, W.D. Ollis, Brazilian leguminosae (XV), An. Acad. Bras. Cienc. 40 (1968) 147. [17] R. Edwards, R. Dixon, Isoflavone O-methyltransferase activities in elicitor treated cell suspension cultures of medicago sativa, Phytochemistry 30 (1991) 2597 – 2606. [18] F.E. King, L. Jurd, The chemistry of extractives from
hardwoods. Part VIII.* The isolation of 5:4%
-dihydroxy-7-methoxyisoflavone (Prunetin) from the heartwood ofPte
-rocarpus angolensis and a synthesis of 7:4%
-dihydroxy-5-methoxyisoflavone hitherto known as prunusetin, J. Chem. Soc. (1952) 3211 – 3215.
[19] H. Pacheco, Compose´s flavoniques des extraits de bois. VI.-Sur les compose´s flavoniques du bois de Prunus mahaleb, Bull. Soc. Chim. Biol. 41 (1959) 111 – 117. [20] N.S. Kattayev, G.K. Nikonov, Flavonoidy Glycyrrhiza
glabra, Chimija prirodnych sojedinenij 10 (1974) 94. [21] V.K. Agarwal, R.K. Thappa, S.G. Agarwal, K.L. Dhar,
Phenolic constituents ofIris milesiirhizomes, Phytochem-istry 23 (1984) 1342 – 1343.
[22] B.B. Bezuidenhoudt, E.V. Brandt, D. Ferreira, Flavonoid analogues from Pterocarpus species, Phytochemistry 26 (1987) 531 – 535.
[23] B.M.R. Bandara, N.S. Kumar, K.M.S. Wimalasiri, Con-stituents of the stem bark fromButea monosperma (Legu-minosae), J. Natl. Sci. Counc. Sri Lanka 18 (1990) 97 – 103.
[24] T. Fukai, Q. Wang, R. Inami, T. Nomura, Structures of prenylated dihydrochalcone, Gancaonin J and ho-moisoflavanone, Gancaonin K from Glycyrrhiza pallid
-iflora, Heterocycles 31 (1990) 643 – 650.
[25] M. Geibel, W. Feucht, Flavonoid 5-glucosides from
Prunus cerasusbark and their characteristic weak glyco-sidic bonding, Phytochemistry 30 (1991) 1519 – 1522. [26] Y.L. Lin, Y.L. Chen, Y.H. Kuo, Three new flavonoids,
3%-methoxylupin, laxifolin, and isolaxifolin from the
roots ofDerris laxiflora Benth, Chem. Pharm. Bull. 39 (1991) 3132 – 3135.
[27] H. Hayashi, M. Yasuma, N. Miraoka, Y. Ikeshiro, H. Yamamoto, E. Yesilada, E. Sezik, G. Honda, M. Tabata, Flavonoid variation in the leaves ofGlycyrrhiza glabra, Phytochemistry 42 (1996) 701 – 704.
[28] X.Z. He, R.A. Dixon, Affinity chromatography, sub-strate/product specificity and amino acid sequence analy-sis of an isoflavone O-methyl transferase from alfalfa (Medicago sati6a L.), Arch. Biochem. Biophys. 336
(1996) 121 – 129.
[29] X.Z. He, J.T. Reddy, R.A. Dixon, Stress response in alfalfa (Medicago sati6aL.). XXII. CDNA cloning and
characterization of an elicitor inducible isoflavone 7-O -methyltransferase, Plant. Mol. Biol. 36 (1998) 43 – 54. [30] D.A. Smith, S.W. Banks, Biosynthesis, elicitation and
biological activity of isoflavonoid phytoalexins, Phyto-chemistry 25 (1986) 979 – 995.
[31] A. Kobayashi, S Yata, K. Kawazu, A O -hydroxychal-cone and flavonoids from alfalfa callus stimulated by a fungal naftochinone, Agric. Biol. Chem. 52 (1988) 3223 – 3227.