Summary We compared the physiological and morphologi-cal responses of rooted cuttings of Populus trichocarpa Torr. & Gray and P. trichocarpa ×P. deltoides Bartr. ex Marsh. grown in either near-ambient solar ultraviolet-B (UV-B; 280--320 nm) radiation (cellulose diacetate film) or subambient UV-B radiation (polyester film) for one growing season. Mid-day biologically effective UV-B radiation was 120.6 and 1.6 mJ m−2 s−1 under the cellulose diacetate and polyester films, respectively. Gas exchange, leaf chlorophyll, light harvesting efficiency of photosystem II, and foliar UV-B radiation-absorb-ing compounds (i.e., flavonoid derivatives) were measured in expanding (leaf plastochron index (LPI) 5), nearly expanded (LPI 10), and fully expanded mature (LPI 15) leaves of intact plants of plastochron index 30 to 35. Plants were then harvested and height, diameter, biomass allocation and leaf anatomical attributes determined.
Net photosynthesis, transpiration, and stomatal conductance were significantly greater in mature leaves exposed to subam-bient UV-B radiation than in mature leaves exposed to near-ambient UV-B radiation. Concentrations of UV-B radia-tion-absorbing compounds (measured as absorbance of metha-nol-extracts at 300 nm) were significantly greater in mature leaves exposed to near-ambient UV-B radiation than in mature leaves exposed to subambient UV-B radiation. The UV-B ra-diation treatments had no effects on chlorophyll content or intrinsic light harvesting efficiency of photosystem II.
Height, diameter, and biomass were not significantly af-fected by UV-B radiation regime in either clone. Leaf anatomi-cal development was unaffected by UV-B radiation treatment in P. trichocarpa × P. deltoides. For P. trichocarpa, leaf anatomical development was complete by LPI 10 in the near-ambient UV-B radiation treatment, but continued through to LPI 15 in the subambient UV-B radiation treatment. Mature leaves of P. trichocarpa were thicker in the subambient UV-B radiation treatment than in the near-ambient UV-B radiation treament as a result of greater development of palisade paren-chyma tissue.
We conclude that exposure to near-ambient UV-B radiation for one growing season caused shifts in carbon allocation from leaf development to other pools, probably including but not limited to, UV-B absorbing compounds. This reallocation cur-tailed leaf development and reduced photosynthetic capacity of the plants compared with those in the subambient UV-B radiation treatment and may affect growth over longer periods of exposure.
Keywords: biomass accumulation, chlorophyll content, dark respiration, deciduous trees, diameter, height, leaf anatomical development, light harvesting, net photosynthesis, photosys-tem II, Populus trichocarpa, Populus trichocarpa × P. del-toides, stomatal conductance, transpiration, UV-B radia-tion-absorbing compounds.
Introduction
Depletion of stratospheric ozone (O3) over northern temperate
latitudes of North America and Europe is causing increased solar ultraviolet-B (UV-B; 280--320 nm) radiation at the ground in these regions (Madronich 1992, Kerr and McElroy 1993, Jokela et al. 1995, Zerefos et al. 1995). Because UV-B radiation can degrade and cause conformational changes in proteins, nucleic acids, and other macromolecules (Caldwell 1981), enhanced UV-B radiation resulting from stratospheric O3 depletion is a potentially important factor in terrestrial and
marine ecosystems.
Crop and herbaceous species that are sensitive to solar UV-B radiation often exhibit decreased carbon assimilation (Van et al. 1976, Brandle et al. 1977, Sisson and Caldwell 1977, Tevini and Iwanzik 1983, Sisson 1986, Bornman 1989), and stomatal conductance (Bennett 1981, Negash and Björn 1986, Tevini and Teramura 1989), accentuated mesophyll resistance (Brandle et al. 1977, Teramura 1983) and disrupted electron transport (Caldwell et al. 1982, Iwanzik et al. 1983, Kulan-daivelu and Noorudeen 1983, Renger et al. 1989) compared to species that are not sensitive to solar UV-B radiation. In
addi-Growth, leaf anatomy, and physiology of
Populus
clones in response to
solar ultraviolet-B radiation
MICHAEL A. SCHUMAKER,
1JOHN H. BASSMAN,
2,4RONALD ROBBERECHT
3and GARY
K. RADAMAKER
21 Plant Gene Expression Center, University of California, Berkeley/U.S. Department of Agriculture, 800 Buchanan Street, Albany, CA 94710, USA 2
Department of Natural Resource Sciences, Washington State University, Pullman, WA 99164-6410, USA
3 Department of Range Resources, University of Idaho, Moscow, ID 83844, USA 4 Author to whom correspondence should be addressed
Received August 9, 1995
tion, UV-B radiation induces production of UV-B-absorbing compounds (Biggs et al. 1981, Caldwell et al. 1983b, Warner and Caldwell 1983, Flint et al. 1985, Schnabl et al. 1986), and damage to photosynthetic pigments (Vu et al. 1982a, Teramura 1983, Day and Vogelmann 1995). The main site for damage appears to be photosystem II (PS II) (Melis et al. 1992, van Leewen and van Gorkom 1992). Photosynthetic effects vary with leaf ontogeny (Teramura and Caldwell 1981); younger leaves or those of maximum photosynthetic activity are more sensitive to UV-B radiation than older leaves. Solar UV-B radiation can also increase dark respiration (Sisson and Caldwell 1976), reduce transpiration (Tevini and Teramura 1989) and induce the closure of stomata (Negash and Björn 1986). Ultraviolet-B radiation reduces plant height and biomass (Tevini and Teramura 1989, Teramura and Sullivan 1994), leaf area, and can retard leaf maturation in many sensi-tive species (Tevini and Teramura 1989). High UV-B irradi-ance increases leaf weight:leaf area ratios (Teramura 1983) reflecting increased carbon allocation to leaves. Root:shoot ratios can also fluctuate with UV-B dose, although to a smaller degree than leaf weight:leaf area ratios (Teramura 1980a, Biggs et al. 1981).
Although trees, unlike most crop and herbaceous plants, are subject to long-term, accumulated effects of environmental stress, the responses of trees and other perennial plants to UV-B radiation are not well understood. Substantial interspe-cific growth responses to enhanced UV radiation have been demonstrated in some conifers (Cline and Salisbury 1966, Kaufmann 1978, Kossuth and Biggs 1981, Sullivan and Tera-mura 1988, Stewart and Hodinott 1993, Sullivan and TeraTera-mura 1994). In loblolly pine (Pinus taeda L.), enhanced UV-B radia-tion had minimal effects on photosynthesis, dark respiraradia-tion, stomatal conductance, chlorophyll concentrations and appar-ent quantum efficiency (PS II) (Sullivan and Teramura 1989, 1992, Naidu et al. 1993); however it caused reductions in needle elongation, total leaf area and biomass over a 3-year period suggesting cumulative effects with additive growth reductions.
Recently, several studies have focused on the effects of UV-B radiation on angiosperm tree species (Lovelock et al. 1992, Sullivan et al. 1992, DeLucia et al. 1994, Searles et al. 1995). Angiosperm trees may be more sensitive to UV-B radiation than conifers because they may have a less effective UV-B screen (Day et al. 1992, 1993). In angiosperms, UV-B-absorbing compounds occur primarily in vacuoles of epider-mal cells (Weissenböck et al. 1984, Schnabl et al. 1986, Weissenböck et al. 1986, Schmelzer et al. 1988), whereas in conifers these compounds occur both in vacuoles and are conjugated in cell walls (Strack et al. 1988, 1989). Angio-sperms allow considerable penetration of UV-B through anti-clinal epidermal cell walls to sensitive mesophyll tissue below, whereas in conifers the epidermis attenuates a large proportion of incoming UV-B radiation (Day et al. 1993).
Solar UV-B radiation may affect anatomical development in leaves (Caldwell 1981, Barnes et al. 1990). It may also result in protein degradation (Jordan et al. 1992) and reductions in ribulose 1,5-bisphosphate carboxylase-oxygenase (Rubisco)
and PEP carboxylase activities (Vu et al. 1982a, 1982b, 1984). Thus, exposure to UV-B radiation may inhibit development of photosynthetic capacity in expanding leaves and increase the rate of senescence in mature leaves. As a result, the contribu-tions of both young and old leaves to the carbon balance of trees may be severely reduced.
We have evaluated the effects of solar UV-B radiation on a fast-growing angiosperm tree species (Populus) by comparing responses under near-ambient UV-B radiation to those where UV-B was excluded from natural sunlight. Specific objectives were to quantify effects of UV-B radiation regime on: (1) growth and carbon allocation; (2) gas exchange and internal leaf CO2 concentrations; (3) leaf chlorophyll contents and light
harvesting efficiency of photosystem II; (4) accumulation of UV-B radiation-absorbing compounds; and (5) leaf anatomy. We also determined how these responses varied with leaf ontogeny.
Materials and methods
Plant material
Ramets from trees of Populus trichocarpa Torr. & Gray (Clone 1A) and Populus trichocarpa×P. deltoides Bartr. ex Marsh. (Hybrid 5 [11-5]; hereafter Clone HY5) were studied. Clone 1A is native black cottonwood originating in the Palouse River drainage of eastern Washington (45°50′ N, 117°05′ W). Clone HY5 is a cross between western Washington state P. tricho-carpa and a variety of eastern cottonwood (P. deltoides) devel-oped at Stoneville, MS (Stettler 1968). This clone demonstrates considerable heterosis, having much larger leaves, greater growth and biomass accumulation than either parent.
Plant culture
Twenty-four dormant hardwood cuttings of each clone were removed from cold storage, soaked in water for 72 h at room temperature, and then planted in 11-liter pots containing stan-dard nursery potting medium. After 24 h in a greenhouse, pots containing planted cuttings were placed in a second pot to prevent root-binding with substrate outside the pots. The entire unit was set in the ground (to maintain root temperatures near normal) inside a 3.0 × 1.2 × 2.0-m gable-end plastic-covered house oriented with the long axis east and west at the E. H. Steffen Center in Pullman, WA (46°44′ N, 117°10′ W). Each house contained two trees of each clone that were randomly arranged in a single row to minimize shading. Plants were grown from late June until mid-August 1994. The ground area within the houses was covered with tan bark to minimize soil temperature variations.
appreciable solarization of the cellulose diacetate film was detected after 28 days of use in the field.
Ultraviolet-B radiation treatment
Midday spectral irradiance under cloudless conditions was measured in mid-August under the cellulose diacetate and polyester films with a spectroradiometer (Optronic Laborato-ries 754, Optronic LaboratoLaborato-ries, Orlando, FL). Unweighted UV-B irradiance was 2.33 W m−2 nm−1 under the cellulose diacetate and 0.14 W m−2 nm−1 under the polyester film. The absolute spectral irradiance, weighted using the generalized plant response function (Caldwell 1971) normalized to 300 nm, was equivalent to 200.1 and 0.08 mW m−2 biologi-cally effective UV-B radiation (UV-BBE) under the cellulose
diacetate and polyester films, respectively. Midday solar spec-tral irradiance under cloudless conditions was calculated ac-cording to Björn and Murphy (1985).
Environmental conditions were similar in the cellulose diacetate and polyester houses. Photosynthetic photon flux density (PPFD; 400--700 nm) measured around solar noon at the top of the plant canopies ranged from 150 µmol m−2 s−1 on
cloudy days to 2050 µmol m−2 s−1 on sunny days. The PPFD
under the plastic films ranged between 90 and 95% of ambient. Noon temperatures at 1.0 m above the ground within the houses ranged from 29 to 40 °C and were typically 1 to 3 °C higher than temperatures recorded outside the houses. Houses were vented by leaving the lower 0.3 m uncovered at the base and 48 cm2 uncovered at the peak of the gable ends.
Plants were watered to maintain soil water near field capac-ity and fertilized at regular intervals with N,P,K (20,20,20) commercial liquid fertilizer containing micronutrients. To minimize herbivory by insects, plants were periodically sprayed with Xclude PT 1600A (Whitmire Research Labora-tories, Inc., St. Louis, MO).
Height and diameter
Height and diameter (10 mm above the intersection with the cutting) were measured weekly during the growing period. The plastochron index (PI) and leaf plastochron index (LPI) (Lar-son and Isebrands 1971) were used as the morphological time scales. A reference length of 40 mm for the lamina of the index leaf was used. Thus at PI 30, the index leaf (LPI 0), the 30th leaf from the base, would be exactly 40 mm long. When plants reached a plastochron index (PI) of 30 to 35 (7 to 9 weeks for Clone 1A; 9 to 10 weeks for Clone HY5), one plant of each clone was randomly selected from each house for final growth measurements.
Chlorophyll fluorescence
Chlorophyll fluorescence measurements were made following illumination of each plant with a sodium halide lamp. Minimal fluorescence (Fo) and maximum fluorescence (Fm) were
deter-mined for a vertical series of leaves consisting of LPI 5 (devel-oping leaf), LPI 10 (nearly expanded leaf), and LPI 15 (fully expanded leaf) for each plant with a modulated fluorometer (Opti-Sciences OS-500, Opti-Sciences, Inc., Tewksbury, MA). Leaves were placed in the dark for 10 min before
measure-ment. Variable to maximal fluorescence ratios (Fv/Fm) were
calculated with variable fluorescence (Fv) equal to Fm − Fo.
Gas exchange
Immediately following chlorophyll fluorescence measure-ments, gas exchange was measured on the same leaves with an open, compensating gas exchange system (Bingham Inter-space BI-7, Bingham InterInter-space Co., Logan, UT) connected to an infrared gas analyzer (225 MK, Analytical Development Company, Ltd., Hoddesdon, U.K.). Portions of intact leaves were enclosed in a 300 cm3 stainless steel and glass cuvette. Because of absorption of UV-B radiation by the cuvette glass, gas exchange measurements represent cumulative rather than instantaneous effects of UV-B radiation. Temperature within the cuvette was controlled thermoelectrically, and air was circulated by three fans. Fluctuations in water vapor concen-tration within the cuvette were monitored with a dewpoint sensor (General Eastern Instruments Corp., Watertown, MA), and leaf temperature was measured by a small copper-constan-tan thermocouple appressed to the abaxial leaf surface. Irradi-ance was supplied by a metal halide lamp and photo-synthetically active radiation (PAR) was measured at the leaf surface with a Li-Cor 190-SB quantum sensor (Li-Cor, Inc., Lincoln, NE). During measurements, radiation was filtered through a water bath to reduce infrared heating within the cuvette. Foliage outside the cuvette was covered with cheese-cloth to prevent heat damage from the lamps. All measure-ments were made at saturating PAR (≥ 1100 µmol m−2 s−1), optimal leaf temperature (25.0 °C), and minimal leaf-to-air vapor density difference (VDD ≤ 6.0 g cm−3). Net
photosyn-thesis, dark respiration, transpiration, water-use efficiency, sto-matal conductance, and leaf internal CO2 concentrations were
calculated according to Ball (1987). Leaf area enclosed in the cuvette was measured with a leaf area meter (Delta-T Devices, Cambridge, U.K.).
Chlorophyll analysis
Three leaf discs (1.0 cm2) were cut from each leaf immediately after gas exchange measurements and placed in a light-proof vial containing 5.0 ml N,N-dimethylformamide (DMF). The vials were stored in the dark for 24 h at 4 °C. Absorbance of the chlorophyll extract was determined at 647 and 664.5 nm (Bausch and Lomb Spectronic 21, Bausch and Lomb, Woburn, MA). Total chlorophyll (total Chl), chlorophyll a (Chl a), chlorophyll b (Chl b), and the ratio of chlorophyll a to b (Chl a/b) were calculated as described by Inskeep and Bloom (1985).
Ultraviolet-B radiation-absorbing compounds
Three leaf discs (1.0 cm2) were excised from each leaf used in the gas exchange measurements, dried at 95 °C for at least 24 h, and then ground in a mortar with a pestle in 10 ml of methanol/H2O/HCl solution (70/29/1 v/v). The extract was
300 nm was used as an index of the relative content of UV-B radiation-absorbing pigments. Values were calculated on a leaf area basis (Robberecht and Caldwell 1986).
Leaf anatomy
Transverse sections of fresh leaf tissue, taken at a point ap-proximately one-third of the lamina length from the tip of leaves used for the physiological measurements, were fixed in 2.5% (v/v) glutaraldehyde, and stored at 4 °C. Fixed specimens were washed three times with a 0.5 M phosphate buffer (pH 7.2) and dehydrated in a graded ethanol series. Samples were then embedded in LR White resin (1/3, 1/2, 1/2, 2/1, resin to ethanol, pure resin three times, 18 to 24 h each). The resin was polymerized at 60 °C for 20 to 24 h within gelatin cap-sules. Sections 1.0 µm thick were cut from each sample with a glass knife (Sorvall MT2-B ultra-microtome, DuPont Co., Newtown, CT), mounted on gelatin-coated slides and stained with either 0.5% aqueous Stevenel’s blue or 1% aqueous Safranin O. Total leaf thickness and thickness of the upper and lower epidermal, palisade parenchyma, and spongy mesophyll layers of each section were quantified by means of an image analysis program (NIH Image 1.56, National Institute of Health, Washington, DC), high-resolution video camera (Pul-nix America TM-7CN, Pul(Pul-nix America, Inc., Sunnyvale, CA) and light microscope (Olympus BH-2, Olympus Corp., Lake Success, NY).
Biomass
Following physiological determinations and collection of ana-tomical samples, plants were harvested and partitioned into leaf (main stem leaves, lateral branch leaves and petioles), stem (main stem and lateral branches), and root components. All biomass components were dried separately at 70 °C to constant mass, and weighed. Shoot (sum of stem and leaf dry weights) to root ratio was calculated for each plant. For each leaf used in the physiological and anatomical studies, leaf area was measured with a leaf area meter (Delta-T Devices), leaf length was measured to the nearest mm, and leaf dry weight per unit of leaf area (g m−2) was calculated.
Experimental design
The experiment was a split-plot design with UV-B radiation treatments as a whole-plot factor and Populus clones as a sub-plot factor. Data were subjected to analysis of variance (ANOVA) using the SYSTAT statistical package (SYSTAT, Inc., Evanston, IL). Whole-plot factor was analyzed as a com-pletely randomized design. Sub-plot means were pooled and compared by least significant difference (LSD) tests when no interaction was detected. Differences were considered signifi-cant at P ≤ 0.05 or 0.10.
Results
The UV-B radiation treatments had no effect on the physical appearance of the Populus trichocarpa and P. trichocarpa × P. deltoides plants during the growing period. There were no clonal or UV-B radiation treatments effects on plant growth
rates, which were linear and added approximately 4--5 plasto-chrons weekly. At harvest, plants were between 0.85 to 1.07 m tall with diameters of 11 to 15 mm. Total dry weight and shoot:root ratio of plants of Clone 1A were about 50 g and 2.74, respectively. Total dry weight of plants of Clone HY5 was 160 to 180 g and shoot:root ratios were 1.59 in the near-ambient UV-B radiation treatment and 1.39 in the subam-bient UV-B radiation treatment. There were no statistically significant differences between UV-B radiation treatments for either clone in any of these variables.
Gas exchange, chlorophyll fluorescence, and chlorophyll pigments
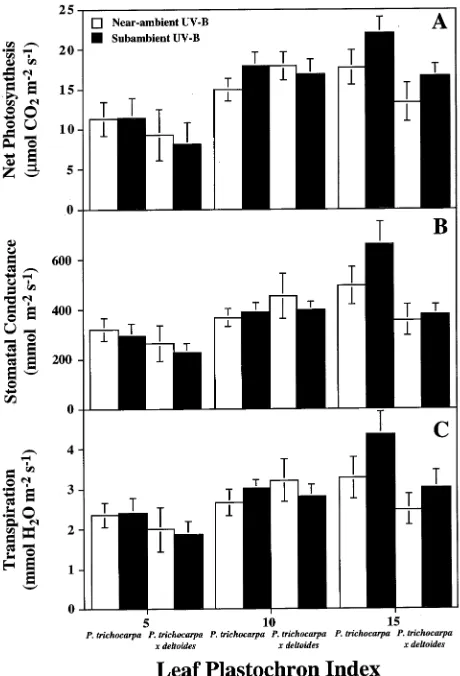
Net photosynthesis ranged from about 10 µmol CO2 m−2 s−1
for immature leaves (LPI 5) to > 22 µmol CO2 m−2 s−1 for fully
expanded mature leaves (LPI 15). The UV-B radiation treat-ments had no statistically significant effect on gas exchange in expanding leaves (LPI 5 and 10) of either clone (Figure 1). Although the near-ambient UV-B radiation treatment de-creased net photosynthesis, stomatal conductance and
ration more in mature leaves (LPI 15) of plants of Clone 1A than in mature leaves of plants of Clone HY, neither the clonal nor treatment effects were statistically significant. However, when data for both clones were pooled, net photosynthesis, stomatal conductance, and transpiration were decreased by 24% (P ≤ 0.05), 20% (P ≤ 0.10) and 28% (P ≤ 0.05), respec-tively, in plants in the near-ambient UV-B radiation treatment compared with plants in the subambient UV-B radiation treat-ment. Rates of dark respiration decreased with leaf age in both clones from about 3 µmol CO2 m−2 s−1 for immature leaves
(LPI 5) to < 2 µmol CO2 m−2 s−1 for fully expanded mature
leaves (LPI 15) with no effects by UV-B radiation regime (data not shown). Leaf internal CO2 concentrations were
consis-tently between 275 and 283 µl l−1 irrespective of leaf age, clone and UV-B treatment. In both clones and UV-B radiation re-gimes, water-use efficiency (WUE) was about 4.2 in immature leaves and 5.5 in mature leaves (data not shown). The UV-B radiation regime had no effect on the maximum intrinsic light harvesting efficiency of photosystem II. Chlorophyll fluores-cence (Fv/Fm) was about 0.75 in both clones and for both UV-B
treatments. Chlorophyll a content ranged from about 6 mg l−1 cm−2 in immature leaves to 10.6 mg l−1 cm−2 in mature leaves, and the corresponding values for chlorophyll b were 1.75 and 3.7 mg l−1 cm−2, respectively. There were no effects of UV-B radiation regime on chlorophyll a and b or total chlorophyll content, or on chlorophyll a/b ratios (data not shown).
Ultraviolet-B radiation-absorbing compounds
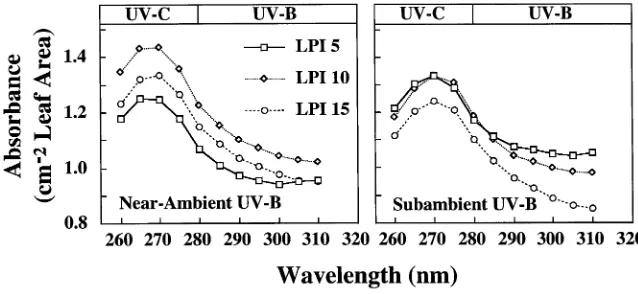
Leaf extracts from plants in both treatments showed a peak of absorbance at 270 nm (Figure 2). At all LPIs, absorbances of leaf extracts from plants in the near-ambient UV-B treatment were somewhat higher than those of leaf extracts from plants in the subambient treatment over the range 260--290 nm. At wavelengths greater than 290 nm, absorbances of extracts from LPI 15 leaves were significantly greater (P ≤ 0.10) for plants grown in near-ambient UV-B radiation than for plants grown in subambient UV-B radiation (Figure 2). For LPI 15 leaves, absorbances at 300 and 310 nm were slightly greater in Clone 1A than in Clone HY5, and for both clones they were higher in the near-ambient UV-B radiation treatment than in the subambient UV-B radiation treatment (Figure 3).
Leaf morphology and anatomy
Clones 1A and HY5 differed in rates of leaf expansion, leaf lengths, leaf weight:leaf area ratios, and leaf area; however, there were no statistically significant treatment differences at any leaf developmental stage (Table 1). Both clones exhibited similar leaf anatomical organization.
Exposure to UV-B radiation did not affect leaf anatomical development in Clone HY5 (Tables 1 and 2). In Clone 1A, the near-ambient UV-B radiation treatment resulted in leaves that were 11 and 10% (P ≤ 0.10) thicker at LPIs 10 and 15, respectively, than equivalent leaves in the subambient UV-B radiation treatment (Table 1). Leaves attained maximum thick-ness at LPI 10 in the near-ambient UV-B regime, whereas leaf thickness continued to increase through to LPI 15 in the subambient UV-B radiation regime (Table 1), resulting in LPI 15 leaves that were significantly thicker in the subambient UV-B radiation treatment than in the near-ambient UV-B ra-diation treatment (Table 1).
Differences in palisade parenchyma layer thickness ac-counted for most of the treatment differences in overall leaf thickness for Clone 1A (Table 2). Palisade parenchyma thick-ness was 17% greater (P ≤ 0.05) at LPI 5 and 10% greater (P ≤ 0.10) at LPI 10 for plants grown in near-ambient UV-B radiation compared with plants grown in subambient UV-B radiation. At LPI 15, palisade parenchyma thickness was 15% greater (P ≤ 0.05) for plants grown in subambient UV-B radia-tion than for plants grown in near-ambient UV-B radiaradia-tion (Table 2). Generally, there were no differences in other leaf tissues between the UV-B treatment regimes with the excep-tion of lower epidermal thickness in LPI 5 leaves (Table 2).
Discussion
The UV-B radiation treatments had no significant effects on height, diameter, total biomass and biomass allocation of plants of either clone. In contrast, Bogenrieder and Klein (1982) found that biomass was significantly greater for Frax-inus excelsior L., Carpinus betulus L., Fagus sylvatica L., Acer platanoides L., and Acer pseudoplatanus L. trees grown with exclusion of ambient solar UV-B radiation than with ambient UV-B radiation. However, in the study of Bogenrieder and
Figure 3. Relationship between ab-sorbance at 300 and 310 nm of leaf extracts in Populus grown in near-ambient UV-B radiation or subambi-ent UV-B radiation. Vertical lines represent ± SE of the mean. The data represent the mean of n = 6 for LPI 15.
Table 1. Relationships between leaf development and total leaf thickness and leaf age (LPI) in Populus trichocarpa (1A) and P. trichocarpa × P. deltoides (HY5) plants grown in near-ambient UV-B radiation or subambient UV-B radiation. Values are mean ± 1 SE of n = 6 for LPI 5 and 15; n = 5 for LPI 10. Asterisks indicate significant differences at P ≤ 0.10 LSD (*).
LPI Clone UV-B Treatment Leaf length Leaf area Leaf weight: Leaf thickness
(cm) (cm2) leaf area (g m−2) (µm)
5 1A Near-ambient 11.3 ± 0.6 35.3 ± 3.9 78.0 ± 5.4 257.7 ± 11.4
Subambient 11.4 ± 0.5 34.8 ± 3.5 78.6 ± 1.9 227.0 ± 8.8 HY5 Near-ambient 12.4 ± 0.9 71.6 ± 13.6 92.8 ± 4.1 205.7 ± 16.7
Subambient 12.1 ± 1.1 67.2 ± 11.7 83.8 ± 4.2 208.7 ± 9.8
10 1A Near-ambient 13.2 ± 0.4 54.7 ± 2.2 93.2 ± 7.8 305.3 ± 7.4
Subambient 14.3 ± 0.7 61.5 ± 4.8 90.2 ± 2.6 272.6 ± 19.2* HY5 Near-ambient 13.5 ± 0.5 93.4 ± 4.7 102.5 ± 6.5 247.5 ± 7.4
Subambient 15.4 ± 1.2 122.3 ± 20.4 94.2 ± 3.7 250.2 ± 3.6
15 1A Near-ambient 13.9 ± 0.4 56.8 ± 3.9 93.9 ± 7.2 305.3 ± 9.0
Subambient 14.9 ± 0.5 66.1 ± 3.2 100.9 ± 2.0 330.9 ± 6.8* HY5 Near-ambient 18.0 ± 0.6 150.9 ± 9.8 106.0 ± 3.3 278.0 ± 10.3 Subambient 18.6 ± 0.6 157.1 ± 6.8 106.8 ± 1.8 289.4 ± 5.9
Table 2. Relationships between leaf anatomical development and leaf age (LPI) in Populus trichocarpa (1A) and P. trichocarpa ×P. deltoides (HY5) plants grown in near-ambient UV-B radiation or subambient UV-B radiation. Values represent mean ± 1 SE of n = 6 for LPI 5 and 15; n = 5 for LPI 10. Asterisks indicate significant differences at P ≤ 0.05 LSD (**) or at P ≤ 0.10 LSD (*).
LPI Clone UV-B Treatment Leaf tissue thickness (µm)
Palisade Spongy Upper Lower
parenchyma mesophyll epidermis epidermis
5 1A Near-ambient 105.3 ± 5.0 126.3 ± 5.7 14.2 ± 1.0 14.0 ± 0.6
Subambient 87.0 ± 2.8** 118.0 ± 6.6 13.2 ± 0.7 11.8 ± 0.6* HY5 Near-ambient 72.9 ± 5.6 107.7 ± 9.3 13.2 ± 0.9 11.6 ± 0.9
Subambient 73.4 ± 3.5 112.9 ± 6.3 12.3 ± 0.3 10.3 ± 0.2
10 1A Near-ambient 121.9 ± 1.5 154.3 ± 6.2 16.6 ± 1.1 13.9 ± 0.5
Subambient 109.8 ± 7.8* 140.5 ± 11.7 14.0 ± 0.8 12.2 ± 1.1 HY5 Near-ambient 95.5 ± 4.1 130.3 ± 5.8 11.0 ± 2.5 10.7 ± 0.4 Subambient 95.5 ± 2.9 130.8 ± 1.0 12.9 ± 0.5 12.2 ± 0.7
15 1A Near-ambient 127.3 ± 5.7 147.0 ± 6.7 16.3 ± 1.5 14.7 ± 1.1
Klein (1982), the Plexiglas (transmittance down to 380 nm) used to exclude UV-B also absorbed a large percentage of ambient UV-A radiation, which is known to moderate the effects of UV-B radiation (Middleton and Teramura 1993). In the temperate angiosperm Liquidambar styraciflua L. (sweet-gum), Sullivan et al. (1994) found that supplemental UV-B applied for two years to seedlings grown in the field had no effect on total plant biomass; however, small changes were observed in leaf physiology, carbon allocation and growth. DeLucia et al. (1994) reported that supplemental UV-B radia-tion had no effect on growth of Amelanchier arborea (Michx. f.), Betula papyrifera Marsh., Nyssa sylvatica Marsh., and Robinia pseudoacacia L., whereas total biomass, plant height and leaf area were significantly reduced in Cercis ca-nadensis L. and Morus alba L. Searles et al. (1995) reported that exclusion of UV-B radiation improved height growth of the tropical trees Tetragastris panamensis Kuntze and Calo-phyllum longifolium Willd., but had no effect on height of Cecropia obtusifolia Bertol., and Swietenia macrophylla King. Total plant biomass was reduced only in Cecropia.
We found that the shoot:root ratio was unaffected by UV-B radiation regime. Similar findings have been reported for other angiosperm trees (DeLucia et al. 1994, Searles et al. 1995) and for some crop plants (Teramura 1980a, Biggs et al. 1981, Teramura et al. 1991). It is possible that effects of near-ambient UV-B radiation on height and biomass of Populus were too small to detect over the 7 to 10-week growing period. Sullivan and Teramura (1992) found that sensitivity of loblolly pine (Pinus taeda) seedlings from several seed sources to enhanced UV-B radiation varied considerably after one field season but growth reductions of 12--20% were observed for all seed sources after three field seasons. They concluded that assess-ment of UV-B radiation damage over one growing season was not adequate to determine tolerance to UV-B radiation.
Leaf growth and anatomical development appeared to vary between treatments in Clone 1A but not in Clone HY5. In Clone 1A, although palisade parenchyma tissue was slower to develop in the subambient UV-B radiation treatment than in the near-ambient UV-B radiation treatment, the additional time over which development proceeded resulted in significantly more palisade parenchyma tissue (Table 2), which in turn contributed to greater overall leaf thickness of plants in the subambient UV-B radiation treatment compared with plants in the near-ambient UV-B radiation teatment (Table 1). Because palisade parenchyma cells are responsible for a major fraction of carbon fixation within the leaf, the increased rates of photo-synthesis observed in the subambient UV-B radiation treat-ment (Figure 1A) may be the result of increased palisade parenchyma tissue.
Duration of leaf expansion and palisade tissue development in the near-ambient UV-B radiation treatment could have been curtailed because of demands from competing sinks for car-bon, in particular, enhanced production of UV-B-absorbing compounds (Figure 3). Alternatively, UV-B radiation could cause greater turnover of proteins involved in cell wall expan-sion thereby resulting in earlier termination of palisade paren-chyma cell elongation. Damage to proteins by UV-B radiation
has been well documented (Caldwell 1971), including the protein xyloglucan endotransglycosylase (XET), which plays a major role in cell expansion (McCann and Roberts 1994, Tomos and Pritchard 1994). Another possible explanation is that auxin mediated the inhibition of palisade parenchyma cell elongation, because auxin concentrations in plant tissue can be reduced by UV-B radiation (Witztum et al. 1978).
Leaf expansion and anatomical development are altered by UV-B in other agronomic and tree species. For example, Staxen and Bornman (1994) reported that Petunia × hybrida plants grown without UV-B irradiance had thicker leaves than UV-B-irradiated plants, whereas enhanced UV-B radiation caused increased leaf thickness in Brassica napus L. (Cen and Bornman 1993). Supplemental UV-B applied to sweetgum (Liquidambar styraciflua) seedlings had no effect on final leaf size but rates of leaf elongation and accumulation of leaf area was slower in leaves exposed to daily UV-B supplementation rates of 3 kJ UV-B (Dillenburg et al. 1995). In field studies of loblolly pine (Pinus taeda), enhanced UV-B radiation caused reductions in needle lengths ranging from 4 to 25% (Naidu et al. 1993).
Ontogenetic patterns of gas exchange paralleled those of leaf development in Clone 1A (Figure 1A). In response to near-ambient UV-B radiation, net photosynthesis reached maximum rates at LPI 10 and then remained constant with further leaf development, whereas photosynthetic rates contin-ued to increase through LPI 15 in the subambient UV-B re-gime. Because the UV-B radiation treatment had no significant effects on either chlorophyll content or photosystem II (as determined by chlorophyll fluorescence), we postulate that the increase in net photosynthesis in response to the subambient UV-B radiation treatment was partly a consequence of in-creased photosynthetic capacity resulting from inin-creased amounts of palisade parenchyma tissue.
Increased net photosynthetic rates in response to the subam-bient UV-B radiation treatment may also be partly the result of increased flux of CO2 to the leaf mesophyll (Figure 1B),
The near-ambient UV-B radiation either stimulated the pro-duction of, or retarded the degradation of, UV-B radiation-ab-sorbing compounds, typically flavonoids or their derivatives (Figures 2 and 3), but in both treatments absorbance decreased with leaf age (Figure 2). Leaves exposed to enhanced UV-B radiation frequently show increased production of flavonoids and related compounds that reduce UV-B radiation penetration to sensitive mesophyll tissues thereby mitigating the damaging effects of UV-B radiation (Sisson 1981, Robberecht and Caldwell 1983, Flint et al. 1985, Lois and Buchanan 1994). Despite the significant increase in UV-B radiation-absorbing compounds, net photosynthesis was significantly reduced in leaves at LPI 15 grown in near-ambient UV-B radiation com-pared to leaves grown in subambient UV-B radiation (cf. Fig-ures 1A, 2 and 3).
The two clones differed in their response to the exclusion of UV-B radiation, implying some degree of genetic adaptation to ambient UV-B radiation. The environment where Clone 1A originated (eastern Washington state) is characterized by com-paratively clear skies during the growing season with concomi-tantly high solar radiation. In contrast, Clone HY5 derives from a cross between western Washington Populus tricho-carpa and a Mississippi source of Populus deltoides. Native climates for both parents have considerably more cloudy peri-ods during the growing season. Moreover, clear days are fre-quently accompanied by considerable water vapor and other particulates in the air that may attenuate or scatter incoming solar UV-B radiation. Thus, Clone 1A is from a location with comparatively higher ambient UV-B radiation compared with Clone HY5. Therefore, the exclusion of UV-B radiation might be expected to have a larger effect on Clone 1A than on Clone HY5. Such an effect was evident for leaf development and gas exchange, although not for many of the other parameters evaluated. Clone 1A tended to have slightly higher concentra-tions of UV-B-absorbing compounds than Clone HY5, which is consistent for a genotype evolving under conditions of high UV-B radiation (Robberecht et al. 1980).
The Populus clones responded to near-ambient solar UV-B radiation primarily through small alterations in leaf anatomy and morphology rather than large reductions in growth or biomass accumulation (cf. Barnes et al. 1988, Caldwell and Flint 1994, Sullivan et al. 1994, Searles et al. 1995). The ontogenetic effects support our hypothesis that UV-B radiation inhibits development of photosynthetic capacity in expanding leaves.
Acknowledgments
The authors thank Dr. R.A. Black and Dr. G.E. Edwards for early, critical review of the manuscript. This material is based on work supported by the Cooperative State Research Service, U.S. Depart-ment of Agriculture, under agreeDepart-ment 94-37100-0312. Research was also supported by the Agriculture Research Center, College of Agri-culture and Home Economics, Washington State University under Project 0113 and by the Northwest Scientific Association. Any opin-ions, findings, conclusopin-ions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the U.S. Department of Agriculture.
References
Ball, J.T. 1987. Calculations related to gas exchange. In Stomatal Function. Eds. E. Zeiger, G.D. Farquhar and I.R. Cowan. Stanford University Press, Stanford, pp 445--476.
Barnes, P.W., P.W. Jordan, W.G. Gold, S.D. Flint and M.M. Caldwell. 1988. Competition, morphology and canopy structure in wheat (Triticum aestivum L.) and wild oat (Avena fatua L.) exposed to enhanced ultraviolet-B radiation. Funct. Ecol. 2:319--330. Barnes, P.W., S.D. Flint and M.M. Caldwell. 1990. Morphological
responses of crop and weed species of different growth forms to ultraviolet-B radiation. Am. J. Bot. 77:1354--1360.
Bennett, J.H. 1981. Photosynthesis and gas diffusion in leaves of selected crop plants exposed to ultraviolet-B radiation. J. Environ. Qual. 10:271--275.
Biggs, R.H., S.V. Kossuth and A.H. Teramura. 1981. Response of 19 cultivars of soybeans to ultraviolet-B irradiance. Physiol. Plant. 53:19--26.
Björn, L.O. and T.M. Murphy. 1985. Computer calculation of solar ultraviolet radiation at ground level. Physiol. Veg. 23:555--561. Bogenrieder, A. and R. Klein. 1982. Does solar UV influence the
competitive relationship in higher plants? In The Role of Solar Ultraviolet Radiation in Marine Ecosystems. Ed. J. Calkins. Plenum Press, New York, pp 641--649.
Bornman, J.F. 1989. Target sites of UV-B radiation in photosynthesis of higher plants. J. Photochem. Photobiol. B Biol. 4:145--158. Brandle, J.R., W.F. Campbell, W.B. Sisson and M.M. Caldwell. 1977.
Net photosynthesis, electron transport capacity, and ultrastructure of Pisum sativum L. exposed to ultraviolet-B radiation. Plant Physiol. 60:165--169.
Caldwell, M.M. 1971. Solar UV irradiation and the growth and devel-opment of higher plants. In Photophysiology, Vol. 6. Ed. A.C. Giese. Academic Press, New York, pp 131--177.
Caldwell, M.M. 1981. Plant responses to solar ultraviolet radiation. In Responses to the Physical Environment, Physiological Plant Ecol-ogy I. Encyclopedia of Plant PhysiolEcol-ogy, New Series, Vol. 12A. Eds. O.L. Lange, P.S. Nobel, C.B. Osmond and H. Ziegler. Sprin-ger-Verlag, Berlin, pp 170--197.
Caldwell, M.M. and S.D. Flint. 1994. Stratospheric ozone reduction, solar UV-B radiation and terrestrial ecosystems. Clim. Change 28:375--394.
Caldwell, M.M., R. Robberecht and R.S. Nowak. 1982. Differential photosynthetic inhibition by ultraviolet radiation in species from the arctic-alpine life zone. Arct. Alp. Res. 14:195--202.
Caldwell, M.M., W.G. Gold, G. Harris and C.W. Ashurst. 1983a. A modulated lamp system for solar UV-B (280--320 nm) supplemen-tation studies in the field. Photochem. Photobiol. 37:479--485. Caldwell, M.M., R. Robberecht and S.D. Flint. 1983b. Internal filters:
Prospects for UV-acclimation in higher plants. Physiol. Plant. 58:445--450.
Caldwell, M.M., S.D. Flint and P.S. Searles. 1994. Spectral balance and UV-B sensitivity of soybean: A field experiment. Plant Cell Environ. 17:267--276.
Cen, Y. and J.F. Bornman. 1993. The effect of exposure to enhanced UV-B radiation on the penetration of monochromatic and polychro-matic UV-B radiation in leaves of Brassica napus. Physiol. Plant. 87:249--255.
Cline, M.G. and F.B. Salisbury. 1966. Effects of ultraviolet radiation on the leaves of higher plants. Radiat. Bot. 6:151--163.
Day, T.A., T.C. Vogelmann and E.H. DeLucia. 1992. Are some plant life forms more effective than others in screening out ultraviolet-B radiation? Oecologia 83:513--519.
Day, T.A., G. Martin and T.C. Volgelmann. 1993. Penetration of UV-B radiation in foliage: evidence that the epidermis behaves as a non-uniform filter. Plant Cell Environ. 16:735--741.
DeLucia, E.H., S.L. Naidu and J. Chen. 1994. Effects of simulated stratospheric ozone depletion on seedling growth of several species of hardwood trees. Erigenia 13:132--140.
Dillenburg, L.R., J.H. Sullivan and A.H. Teramura. 1995. Leaf expan-sion and development of photosynthetic capacity and pigments in Liquidambar styraciflua (Hamamelicaceae)----effects of UV-B ra-diation. Am. J. Bot. 82:878--885.
Flint, S.D., P.W. Jordan and M.M. Caldwell. 1985. Plant protective response to enhanced UV-B radiation under field conditions: Leaf optical properties and photosynthesis. Photochem. Photobiol. 41:95--99.
Inskeep, W.P. and P.R. Bloom. 1985. Extinction coefficients of chloro-phyll a and b in N,N-dimethylformamide and 80% acetone. Plant Physiol. 77:483--485.
Iwanzik, W., M. Tevini, M. Voss, W. Weiss, P. Graber and G. Renger. 1983. Action of UV-B radiation on photosynthetic primary reac-tions in spinach chloroplasts. Physiol. Plant. 58:401--407. Jokela, K., K. Leszcynski, R. Visuri and L. Yliantila. 1995. Increased
UV exposure in Finland in 1993. Photochem. Photobiol. 62:101--107.
Jordan, B.R., J. He, W.S. Chow and J.M. Andersen. 1992. Changes in mRNA levels and polypeptide subunits of ribulose 1,5-bisphos-phate carboxylase in response to supplementary ultraviolet-B radia-tion. Plant Cell Environ. 15:91--98.
Kaufmann, M.R. 1978. The effect of ultraviolet (UV-B) radiation on Engelmann spruce and lodgepole pine seedlings. UV-B Biological and Climatic Effects Research, EPA-IAG-D6-0168, EPA, Washing-ton, DC, pp 1--17.
Kerr, J.B. and C.T. McElroy. 1993. Evidence for large upward trends of ultraviolet-B radiation linked to ozone depletion. Science 262:1032--1034.
Kossuth, S.V. and R.H. Biggs. 1981. Ultraviolet-B radiation effects on early seedling growth of Pinaceae species. Can. J. For. Res. 11:243--248.
Kulandaivelu, G. and A.M. Noorudeen. 1983. Comparative study of the action of ultraviolet-C and ultraviolet-B radiation on photosyn-thetic electron transport. Physiol. Plant. 58:389--394.
Larson, P.R. and J.G. Isebrands. 1971. The plastochron index as applied to developmental studies of cottonwood. Can. J. For. Res. 1:1--11.
Lois, R. and B.B. Buchanan. 1994. Severe sensitivity to ultraviolet radiation in an Arabidopsis mutant deficient in flavonoid accumu-lation. II. Mechanisms of UV-resistance in Arabidopsis. Planta 194:504--509.
Lovelock, C.E., B.F. Clough and I.E. Woodrow. 1992. Distribution and accumulation of ultraviolet-radiation absorbing compounds in leaves of tropical mangroves. Planta 188:143--154.
Madronich, S. 1992. Implications of recent total atmospheric ozone measurements for biologically active ultraviolet radiation reaching the earth’s surface. Geophys. Res. Lett. 19:37--40.
McCann, M.C. and K. Roberts. 1994. Changes in cell wall architecture during cell elongation. J. Exp. Bot. 45:1683--1691.
Melis, A., J.A. Nemson and M.A. Harrison. 1992. Damage to func-tional components and partial degredation of photosystem II reac-tion center proteins upon chloroplast exposure to ultraviolet-B radiation. Biochim. Biophys. Acta 1100:312--320.
Middleton, E.M. and A.H. Teramura. 1993. The role of flavonol glycosides and carotenoids in protecting soybean from ultraviolet-B damage. Plant Physiol. 103:741--752.
Musil, C.F. and J.E. Wand. 1993. Responses of sclerophyllous Eri-caceae to enhanced levels of ultraviolet-B radiation. Environ. Exp. Bot. 33:233--242.
Naidu, S.L., J.H. Sullivan, A.H. Teramura and E.H. DeLucia. 1993. The effects of ultraviolet-B radiation on photosynthesis of different aged needles in field-grown loblolly pine. Tree Physiol. 12:151--162.
Negash, L. and L.O. Björn. 1986. Stomatal closure by ultraviolet radiation. Physiol. Plant. 66:360--364.
Renger, G.M., M. Volker, H.J. Eckert, R. Fromme, S. Hohm-Veit and P. Graber. 1989. On the mechanism of photosystem II deterioration by UV-B irradiation. Photochem. Photobiol. 49:97--105.
Robberecht, R. and M.M. Caldwell. 1983. Protective mechanisms and acclimation to solar ultraviolet-B radiation in Oenothera stricta. Plant Cell Environ. 6:477--485.
Robberecht, R. and M.M. Caldwell. 1986. Leaf optical properties of Rumex patientia L. and Rumex obtusifolius L. in regard to a protec-tive mechanism against solar UV-B radiation injury. In Strato-spheric Ozone Reduction, Solar Ultraviolet Radiation and Plant Life. Eds. R.C. Worrest and M.M. Caldwell. Springer-Verlag, Ber-lin, pp 251--260.
Robberecht, R., M.M. Caldwell and W.D. Billings. 1980. Leaf ultra-violet optical properties along a latitudinal gradient in the arctic-al-pine life zone. Ecology 61:612--619.
Schmelzer, E., W. Jahnen and K. Hahlbrock. 1988. In situ localization of light-induced chalcone-synthase mRNA, chalcone synthase, and flavonoid end products in epidermal cells of parsley leaves. Proc. Nat. Acad. Sci. 85:2989--2993.
Schnabl, H., G. Weissenböck and H. Scharf. 1986. In vivo microspec-trophotometric characterization of flavonol glycosides in Vicea faba guard and epidermal cells. J. Exp. Bot. 37:61--72.
Searles, P.S., M.M. Caldwell and K.W. Winter. 1995. The response of five tropical dicotyledon species to solar ultraviolet-B radiation. Am. J. Bot. 82:445--453.
Sisson, W.B. 1981. Photosynthesis, growth, and ultraviolet irradiance absorbance of Cucurbita pepo L. leaves exposed to ultraviolet-B radiation (280--315 nm). Plant Physiol. 67:120--124.
Sisson, W.B. 1986. Effects of UV-B radiation on photosynthesis. In Stratospheric Ozone Reduction, Solar Ultraviolet Radiation and Plant Life. Eds. R.C. Worrest and M.M. Caldwell. Springer-Verlag, Berlin, pp 161--169.
Sisson, W.B. and M.M. Caldwell. 1976. Photosynthesis, dark respira-tion, and growth of Rumex patientia L. exposed to ultraviolet irradiance (288 to 315 nanometers) simulating a reduced atmos-pheric ozone column. Plant Physiol. 58:563--568.
Sisson, W.B. and M.M. Caldwell. 1977. Atmospheric ozone depletion: Reduction of photosynthesis and growth of a sensitive higher plant exposed to enhanced UV-B radiation. J. Exp. Bot. 28:691--705. Staxen, I. and J.F. Bornman. 1994. A morphological and cytological
study of Petunia hybrida exposed to UV-B radiation. Physiol. Plant. 91:735--740.
Stettler, R.F. 1968. Irradiated mentor pollen: its use in remote hybridi-zation of black cottonwood. Nature 219:746--747.
Stewart, J.D. and J. Hodinott. 1993. Photosynthetic acclimation to elevated atmospheric carbon dioxide and UV irradiation in Pinus banksiana. Physiol. Plant. 88:493--500.
Strack, D., J. Heilemann, V. Wray and H. Dirks. 1989. Structures and accumulation patterns of soluble and insoluble phenolics from Norway spruce needles. Phytochemistry 28:2071--2078.
Sullivan, J.H. and A.H. Teramura. 1988. Effects of ultraviolet-B irra-diation on seedling growth in the Pinaceae. Am. J. Bot. 75:225--230. Sullivan, J.H. and A.H. Teramura. 1989. The effects of ultraviolet-B radiation on loblolly pine. I. Growth, photosynthesis and pigment production in greenhouse-grown seedlings. Physiol. Plant. 77:202--207.
Sullivan, J.H. and A.H. Teramura. 1992. The effects of ultraviolet-B radiation on loblolly pine. 2. Growth of field-grown seedlings. Trees 6:115--120.
Sullivan, J.H. and A.H. Teramura. 1994. The effects of UV-B radiation on loblolly pine. 3. Interaction with CO2 enhancement. Plant Cell
Environ. 17:311--317.
Sullivan, J.H., A.H. Teramura and L.R. Dillenburg. 1994. Growth and photosynthetic responses of field-grown sweetgum (Liquidambar styraciflua; Hamamelidaceae) seedlings to UV-B radiation. Am. J. Bot. 81:826--832.
Sullivan, J.H., A.H. Teramura and L.H. Ziska. 1992. Variation in UV-B sensitivity in plants from a 3,000-m elevational gradient in Hawaii. Am. J. Bot. 79:737--743.
Teramura, A.H. 1980a. Effects of ultraviolet-B irradiances on soy-bean. I. Importance of photosynthetically active radiation in evalu-ating ultraviolet-B irradiance effects on soybean and wheat growth. Physiol. Plant. 48:333--339.
Teramura, A.H. 1980b. Effects of ultraviolet-B irradiances on soy-bean. II. Interaction between ultraviolet-B and photosynthetically active radiation on net photosynthesis, dark respiration, and transpi-ration. Plant Physiol. 65:483--488.
Teramura, A.H. 1983. Effects of ultraviolet-B radiation on the growth and yield of crop plants. Physiol. Plant. 58:415--427.
Teramura, A.H. and M.M. Caldwell. 1981. Effects of ultraviolet-B irradiances on soybean. IV. Leaf ontogeny as a factor in evaluating ultraviolet-B irradiance effects on net photosynthesis. Am. J. Bot. 68:934--941.
Teramura, A.H. and J.H. Sullivan. 1994. Effects of UV-B radiation on photosynthesis and growth of terrestrial plants. Photosynth. Res. 39:463--473.
Teramura, A.H., L.H. Ziska and A.E. Stein. 1991. Changes in growth and photosynthetic capacity of rice with increased UV-B radiation. Physiol. Plant. 83:373--380.
Tevini, M. and W. Iwanzik. 1983. Inhibition of photosynthetic activity by UV-B radiation in radish seedlings. Physiol. Plant. 58:395--400. Tevini, M. and A.H. Teramura. 1989. UV-B effects on terrestrial
plants. Photochem. Photobiol. 50:479--487.
Tevini, M., P. Grusemann and G. Fieser. 1988. Assessment of UV-B stress by chlorophyll fluorescence analysis. In Applications of Chlorophyll Fluorescence. Ed. H.K. Lichtenthaler. Kluwer Aca-demic, Dordrecht, pp 229--238.
Tomos, D. and J. Pritchard. 1994. Biophysical and biochemical control of gene expression in roots and leaves. J. Exp. Bot. 45:1721--1731. Van, T.K., L.A. Garrard and S.H. West. 1976. Effects of UV-B radia-tion on net photosynthesis of some crop plants. Crop Sci. 16:715--718.
van Leewen, P.J. and H.J. van Gorkom. 1992. Analysis of UV absorp-tion changes with periodicity four in oxygen-evolving photosystem II membranes. J. Photochem. Photobiol. B Biol. 15:33--43. Vu, C.V., L.H. Allen, Jr. and L.A. Garrard. 1982a. Effects of UV-B
radiation (280--320 nm) on photosynthetic constituents and proc-esses in expanding leaves of soybean (Glycine max (L.) Merr.). Environ. Exp. Bot. 22:465--473.
Vu, C.V., L.H. Allen, Jr. and L.A. Garrard. 1982b. Effects of supple-mental UV-B radiation on primary photosynthetic carboxylating enzymes and soluble proteins in leaves of C3 and C4 crop plants.
Physiol. Plant. 55:11--16.
Vu, C.V., L.H. Allen, Jr. and L.A. Garrard. 1984. Effects of enhanced UVB radiation (280--320 nm) on ribulose-1,5-bisphosphate car-boxylase in pea and soybean. Environ. Exp. Bot. 24:131--143. Warner, C.W. and M.M. Caldwell. 1983. Influence of photon flux
density in the 400--700 nm wavelength on inhibition of photosyn-thesis by UV-B (280--320 nm) irradiance in soybean leaves: Sepa-ration of indirect and immediate effects. Photochem. Photobiol. 38:341--346.
Weissenböck, G., H. Schnabl, G. Sachs, C. Elbert and F.O. Heller. 1984. Flavonol content in guard cell and mesophyll cell protoplasts isolated from Vicia faba leaves. Physiol. Plant. 62:356--362. Weissenböck, G., R. Hedrich and G. Sachs. 1986. Secondary products
in isolated guard cell, epidermal cell and mesophyll cell protoplasts from pea (Pisum sativum L.) leaves: distribution and determination. Protoplasma 134:141--148.
Witztum, A., O. Keren and Z. Even-Chen. 1978. The effect of ultravio-let-radiation and sucrose on IAA levels in Spirodela oligorhiza. Ann. Bot. 42:595--598.
Zerefos, C.S., A.F. Bais, C. Meleti and I.C. Ziomas. 1995. A note on the recent increase of solar UV-B radiation over northern middle latitudes. Geophys. Res. Lett. 22:1245--1247.