Soil application of insecticides influences microorganisms
and plant nutrients
A.C. Das
∗, D. Mukherjee
Department of Agricultural Chemistry and Soil Science, Bidhan Chandra Krishi Viswavidyalaya, Mohanpur-741252, India
Received 9 March 1999; received in revised form 16 September 1999; accepted 20 September 1999
Abstract
An experiment was conducted under laboratory conditions to investigate the effect of four insecticides, viz. HCH, phorate,
carbofuran and fenvalerate at their field application rates (7.5, 1.5, 1.0 and 0.35 kg a.i. ha−1, respectively), on the growth and
development of bacteria, actinomycetes and fungi as well as their role in the transformations and availability of some plant nutrients in laterite soil (Typic Orchragualf). All the insecticides in general, and HCH and phorate in particular, significantly increased the population of microorganisms in soil. The most predominant genera of microorganisms, such as Bacillus,
Micrococcus and Aspergillus were not affected by most of the insecticides. However, some of the insecticides stimulated the
growth and development of Bacillus, Proteus, Corynebacterium, Streptomyces, Fusarium, Trichoderma and Rhizopus. On the other hand, some insecticide exerted deleterious effect on the proportions of Pseudomonas, Staphylococcus, Nocardia,
Micromonospora, Aspergillus and Rhizopus. Incorporation of insecticides also significantly stimulated the mineralization and
availability of organic C, N and P in soil. Among the tested substances, the stimulations were more pronounced with HCH followed by phorate and fenvalerate. ©2000 Elsevier Science B.V. All rights reserved.
Keywords: Insecticides; Microbial population; Plant nutrients; Laterite soil
1. Introduction
Soil is the repository of all types of chemical in-puts including insecticides applied to combat insect pests of crops. As organic substance of any kind can-not escape the onslaught of microbial degradation, insecticides are no exception. Insecticidal residues are generally degraded and degradation products as-similated by soil microorganisms (El-Shahaat et al., 1987; Rache and Coats, 1988; Bhuyan et al., 1993) resulting in increased population sizes and activities of microorganisms which in turn influences the
trans-∗Corresponding author. Tel.:+91-03473-22068;
fax:+91-03473-22290.
E-mail address: [email protected] (A.C. Das).
formations of plant nutrient elements in soil (Das and Mukherjee, 1994; Jana et al., 1998). On the other hand, there are some insecticides which are not uti-lizable by soil microorganisms and these types of insecticides are degraded in soil by microorganisms through cometabolism (Bollag and Liu, 1990). Other insecticides exert deleterious effects on microorgan-isms (Moorman, 1989; Martinez-Toledo et al., 1992). Therefore, no definite conclusion can be made on the effect of insecticides on microorganisms and their associated transformations of nutrients in soil, since different groups of insecticides exhibit manifold variations in toxicity (Matsumura and Boush, 1971; Simon-Sylvestre and Fournier, 1979). It is interesting to note that insecticides frequently applied in modern agriculture mostly belong to the chlorinated
carbon, organophosphate, carbamate and synthetic pyrethroid groups, but their comparative residual ef-fects on microorganisms under a particular soil con-ditions have rarely been reported (El-Shahaat et al., 1987; Singh and Prasad, 1991). Based on the above reports, an experiment was conducted to investigate the effects of HCH (hexachlorocyclohexane), phorate (O,O-diethyl-S-ethylthiomethyl dithiophosphate), car-bofuran (2,2-dimethyl-2,3-dihydrobenzofuran-7-yl-N-methyl carbamate) and fenvalerate [(RS)-cyano (3-phenoxyphenyl) (RS) methyl-4-chloro-a
(1-methylethyl) benzene acetate], representing the chlo-rinated hydrocarbon, organophosphate, carbamate and synthetic pyrethroid groups of insecticides, respec-tively, at their recommended field application rates, on the growth and distribution of representative soil microorganisms and the mineralization of C, N and P.
2. Materials and methods
2.1. Experimental methods
A laterite soil (Typic Orchragualf) having the gen-eral characteristics as presented in Table 1 was col-lected from Midnapore district in West Bengal, In-dia by taking several thin slices from the surface soil layer (0–10 cm) by means of a spade as outlined by Jackson (1973). The composite soils were air-dried (at 30–35◦C) in shade and passed through a 2 mm
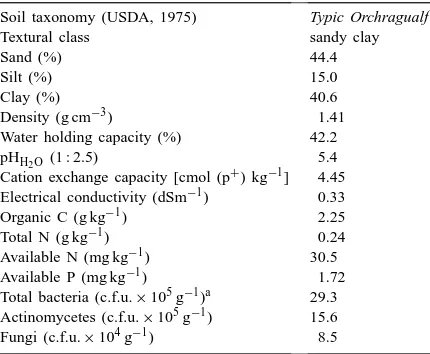
Table 1
General characteristics of the soil used in the experiment
Soil taxonomy (USDA, 1975) Typic Orchragualf
Textural class sandy clay
Sand (%) 44.4
Silt (%) 15.0
Clay (%) 40.6
Density (g cm−3) 1.41
Water holding capacity (%) 42.2
pHH2O(1 : 2.5) 5.4
Cation exchange capacity [cmol (p+) kg−1] 4.45
Electrical conductivity (dSm−1) 0.33
Organic C (g kg−1) 2.25
(4–8 mesh cm−1) sieve. The processed soils were then stored in screw-cap jars and were used for the ex-periment. Four insecticides, HCH (50% active ingre-dient (a.i.) in wettable powder formulation; Rhone Poulenc), phorate (10% a.i. in granular formulation; Pesticide India), carbofuran (3% a.i. in granular for-mulation; Bayer India) and fenvalerate (20% a.i. in emulsifiable concentrate formulation; Rallis India) at rates of 7.5, 1.5, 1.0 and 0.35 kg a.i. ha−1, respec-tively were mixed thoroughly and separately with 1 kg air-dried and sieved soil (≤2 mm) and were placed in earthenware pots. The water content of the soil was adjusted to 60% of water holding capacity and main-tained throughout the experiment by the periodic ad-dition of distilled water. To avoid photogradation of insecticides and evaporation loss of water from soil, the pots were kept covered with black polyethylene sheet and were incubated in the dark at 30±1◦C for 60 days. Treatments were replicated three times. Dur-ing incubation, soil samples (approximately 50 g) were collected at periodic intervals from the replicated pots of each treatment for analysis of growth and distribu-tion of microorganisms and the mineralizadistribu-tion of nu-trient elements. Soil moisture content was measured from subsamples.
2.2. Soil sampling and analysis
Soil samples were collected from each pot by tak-ing soil cores by pushtak-ing an open-ended glass tube (12.5 cm length, 1.8 cm inner diameter) into the soil to a depth of 10 cm. The subsamples were immedi-ately analyzed to determine microbial populations and chemical transformations.
2.2.1. Analysis of microbial population
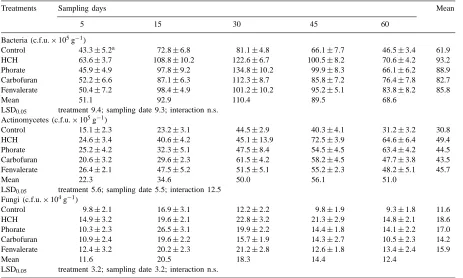
Table 2
Effects of insecticides on the populations of microorganisms in soil
Treatments Sampling days Mean
5 15 30 45 60
Bacteria (c.f.u.×105g−1) Control 43.3±5.2
a 72
.8±6.8 81.1±4.8 66.1±7.7 46.5±3.4 61.9
HCH 63.6±3.7 108.8±10.2 122.6±6.7 100.5±8.2 70.6±4.2 93.2
Phorate 45.9±4.9 97.8±9.2 134.8±10.2 99.9±8.3 66.1±6.2 88.9
Carbofuran 52.2±6.6 87.1±6.3 112.3±8.7 85.8±7.2 76.4±7.8 82.7
Fenvalerate 50.4±7.2 98.4±4.9 101.2±10.2 95.2±5.1 83.8±8.2 85.8
Mean 51.1 92.9 110.4 89.5 68.6
LSD0.05 treatment 9.4; sampling date 9.3; interaction n.s.
Actinomycetes (c.f.u.×105g−1)
Control 15.1±2.3 23.2±3.1 44.5±2.9 40.3±4.1 31.2±3.2 30.8
HCH 24.6±3.4 40.6±4.2 45.1±13.9 72.5±3.9 64.6±6.4 49.4
Phorate 25.2±4.2 32.3±5.1 47.5±8.4 54.5±4.5 63.4±4.2 44.5
Carbofuran 20.6±3.2 29.6±2.3 61.5±4.2 58.2±4.5 47.7±3.8 43.5
Fenvalerate 26.4±2.1 47.5±5.2 51.5±5.1 55.2±2.3 48.2±5.1 45.7
Mean 22.3 34.6 50.0 56.1 51.0
LSD0.05 treatment 5.6; sampling date 5.5; interaction 12.5
Fungi (c.f.u.×104g−1)
Control 9.8±2.1 16.9±3.1 12.2±2.2 9.8±1.9 9.3±1.8 11.6
HCH 14.9±3.2 19.6±2.1 22.8±3.2 21.3±2.9 14.8±2.1 18.6
Phorate 10.3±2.3 26.5±3.1 19.9±2.2 14.4±1.8 14.1±2.2 17.0
Carbofuran 10.9±2.4 19.6±2.2 15.7±1.9 14.3±2.7 10.5±2.3 14.2
Fenvalerate 12.4±3.2 20.2±2.3 21.2±2.8 12.6±1.8 13.4±2.4 15.9
Mean 11.6 20.5 18.3 14.4 12.4
LSD0.05 treatment 3.2; sampling date 3.2; interaction n.s.
aMean±s.d.; n.s.: not significant.
2.2.2. Isolation and identification of microorganisms
Individual colonies of bacteria, actinomycetes and fungi were isolated from and cultured on their re-spective agar media. Fourteen isolates of each type of microorganism were selected at random from the agar plates of each treatment and the isolates were trans-ferred to agar slants containing the same medium. After purification, the isolates were identified to generic level following the guide of Skerman (1967) as modified by Bowie et al. (1969) for bacteria, the key of Skerman (1975) for actinomycetes and proce-dure of Gilman (1957) for fungi.
2.2.3. Chemical analysis
Soil samples were also analyzed for changes in or-ganic C, total N and available N (NH4++NO3−)
fol-lowing the methods outlined by Jackson (1973). The water soluble P in soil was extracted in sodium bicar-bonate and quantified using a colorimetric procedure (Olsen and Dean, 1982).
2.2.4. Data analysis
The results were evaluated by analysis of variance (ANOVA) and the statistical significance (P=0.05) of difference between means within factors (insecticides and incubation time) was evaluated using Fisher’s pro-tected LSD method (Petersen, 1994).
3. Results and discussion
3.1. Effect on microbial population
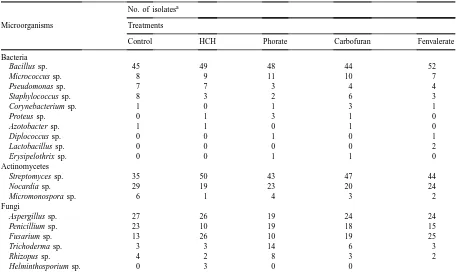
insecti-Table 3
Effects of insecticides on the occurrence of predominant genera of microorganisms in soil
No. of isolatesa
Microorganisms Treatments
Control HCH Phorate Carbofuran Fenvalerate
Bacteria
Bacillus sp. 45 49 48 44 52
Micrococcus sp. 8 9 11 10 7
Pseudomonas sp. 7 7 3 4 4
Staphylococcus sp. 8 3 2 6 3
Corynebacterium sp. 1 0 1 3 1
Proteus sp. 0 1 3 1 0
Azotobacter sp. 1 1 0 1 0
Diplococcus sp. 0 0 1 0 1
Lactobacillus sp. 0 0 0 0 2
Erysipelothrix sp. 0 0 1 1 0
Actinomycetes
Streptomyces sp. 35 50 43 47 44
Nocardia sp. 29 19 23 20 24
Micromonospora sp. 6 1 4 3 2
Fungi
Aspergillus sp. 27 26 19 24 24
Penicillium sp. 23 10 19 18 15
Fusarium sp. 13 26 10 19 25
Trichoderma sp. 3 3 14 6 3
Rhizopus sp. 4 2 8 3 2
Helminthosporium sp. 0 3 0 0
aFor each group of microorganisms, 14 isolates were identified at each sampling. Therefore, during five sampling periods the total
isolates under any treatment was 14×5=70.
cides. Population sizes of bacteria were greatest 30 days following application of all four insecticides. Stimulation of actinomycete and fungal populations was less pronounced, with maximum increases oc-curring between 15 and 60 days for different insecti-cides. This indicated greater utilization of insecticidal residues as well as their degraded products (Bhuyan et al., 1993) by the soil microorganisms to derive energy and other nutrient elements for their cellular metabolism (El-Shahaat et al., 1987). Another source of nutrients to the microorganisms was that applica-tion of insecticides had an effect on a certain part of the microbial, and perhaps also the soil animal, biomass of the soil resulting in death of that sensitive part and consequently released nutrients. Microor-ganisms not so sensitive to the insecticides utilized the released nutrients and increased in their numbers. It was also revealed that among the microorganisms, actinomycetes and fungi were highly responsive to the application of HCH (60.4 and 60.3%) and phorate
(44.5 and 46.6%) and this trend was also followed for actinomycetes with carbofuran (41.2%) and fenvaler-ate (48.4%) as compared to that of untrefenvaler-ated control soil.
3.2. Effect on the distribution of microorganisms
Most of the insecticides did not have marked ef-fects on the distribution of Bacillus and Micrococcus, the most dominant bacteria present in soil (Table 3). This trend was also recorded with HCH and carbofu-ran in regard to the proportions of Pseudomonas and
Staphylococcus, respectively, while the distribution of
the latter genera was retarded with phorate and fen-valerate and that of Pseudomonas with carbofuran and
Staphylococcus with HCH. On the other hand, Bacil-lus with fenvalerate, Corynebacterium with
Konopka, 1990; Sakata et al., 1992) who pointed out that different bacteria utilized the degraded products of the insecticides to derive energy, carbon and other nutrients for their growth and development resulting in an increase in their populations in soil.
Sustaining the earlier report (Das et al., 1995),
Streptomyces followed by Nocardia and Micromono-spora were the predominant genera of actinomycetes
isolated from soil (Table 3). The results of the present experiment clearly indicated that the proportions of
Streptomyces were highly increased due to the
incor-poration of insecticides while those of Nocardia and
Micromonospora were reduced. This indicated that
Streptomyces was the most active actinomycete to utilize the insecticidal residues in soil.
Aspergillus followed by Penicillium and Fusarium
were the most dominant fungi in soil treated with different insecticides (Table 3). This was also in ac-cordance with the report of Srimathi et al. (1986). Application of HCH, carbofuran and fenvalerate did not have marked effects on the distribution of
Aspergillus while the proportion of Fusarium was
highly increased by these insecticides and more so with HCH and fenvalerate, indicating that Fusarium utilized chlorinated hydrocarbon (HCH) and synthetic pyrethroid (fenvalerate) insecticides more effectively for their growth and metabolism (Levavon, 1993). Similar response was also shown by Trichoderma to phorate and carbofuran, and by Rhizopus to phorate. On the other hand, no insecticide was beneficial to
Penicillium in soil. This was also true for HCH and
fenvalerate in the case of Rhizopus and for phorate in the case of Aspergillus and Fusarium.
From the results (Table 3), it was also revealed that most of the predominant genera of microorganisms remained unchanged due to the application of insecti-cides while some secondary genera were more sensi-tive to the insecticides and were repressed in soil. The nutrients released due to the death of the secondary genera as well as the degraded products of the insecti-cides were utilized by the predominant and occasional genera of microorganisms resulting in an increase in their proportions in soil.
3.3. Effect on organic C and total N
The mineralization rate of organic C in soils treated with different insecticides was higher compared with
that of the control (Table 4). This indicated that in-secticides stimulated the growth and activities of heterotrophic microorganisms (Murthy et al., 1991) which in turn, mineralized the soil organic matter to derive energy and carbon for their cellular constituents (Debnath et al., 1994) resulting in lower retention of organic C in soil. Among the insecticides, the increase in mineralization of organic C was highest under HCH (18.8%) followed by phorate (15.2%) and fen-valerate (13.5%), reflecting trends in the populations of microorganisms discussed earlier (Table 2).
Similar trends were recorded for total N content (Table 4), reflecting denitrification following enhanced microbial mineralization of organic N in soil. The stimulating influence of the insecticides on micro-bial mineralization of organic N (Singh and Prasad, 1991; Rangaswamy and Venkateswarlu, 1993) brought about a significant reduction of total N content which was more pronounced with HCH (22.7%) followed by phorate (19%) and fenvalerate (18.1%). Similar results were also demonstrated by Gaikawad et al. (1973) with chlorinated hydrocarbon and organophosphate insec-ticides.
3.4. Effect on available N and P
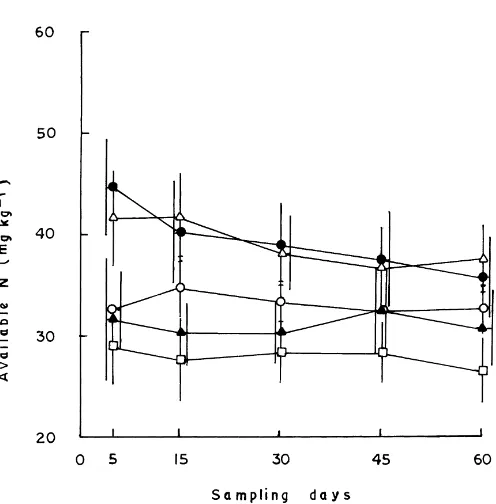
The greater mineralization of N following the ap-plication of insecticides resulted in an increase in the amounts of mineralized N (NH4+ and NO3−) in soil
(Fig. 1). The increase was most pronounced with HCH and phorate. This indicated that insecticides in gen-eral, and HCH and phorate in particular, stimulated the growth and activities of ammonifying and nitri-fying bacteria which were mainly responsible for the mineralization of organic N to NH4+ and oxidation
of NH4+ to NO3−, respectively (Rangaswamy and
Table 4
Effects of insecticides on organic C and total N in soil
Treatments Sampling days Mean
5 15 30 45 60
Amount of organic C (g kg−1)
Control 2.25±0.02a 2.23±0.04 2.20±0.07 2.15±0.02 2.12±0.02 2.19
HCH 2.23±0.02 1.95±0.05 1.91±0.06 1.81±0.03 1.81±0.02 1.94
Phorate 2.23±0.03 2.20±0.06 2.11±0.04 1.95±0.04 1.89±0.03 2.07 Carbofuran 2.21±0.11 2.18±0.06 2.09±0.04 1.98±0.04 1.94±0.03 2.08 Fenvalerate 2.22±0.04 2.16±0.02 2.05±0.02 1.94±0.02 1.92±0.02 2.05
Mean 2.23 2.14 2.07 1.97 1.94
LSD0.05 treatment 0.06; sampling date 0.05; interaction n.s.
Amount of total N (g kg−1)
Control 0.24±0.02 0.24±0.02 0.22±0.03 0.22±0.01 0.22±0.02 0.23
HCH 0.22±0.02 0.19±0.02 0.19±0.01 0.17±0.02 0.17±0.02 0.19
Phorate 0.21±0.03 0.20±0.02 0.19±0.02 0.19±0.02 0.17±0.01 0.19 Carbofuran 0.23±0.03 0.21±0.03 0.20±0.01 0.19±0.02 0.19±0.02 0.20 Fenvalerate 0.22±0.02 0.21±0.03 0.19±0.02 0.19±0.01 0.18±0.03 0.20
Mean 0.22 0.21 0.19 0.19 0.18
LSD0.05 treatment 0.02; sampling date n.s.; interaction n.s.
aMean±s.d.; n.s.: not significant.
Fig. 1. Effect of insecticides on the availability of N in soil. The treatments include control (h), HCH (△), phorate (d), carbofuran, (m)
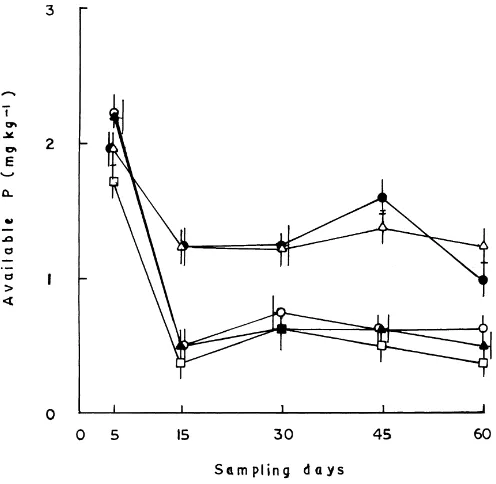
Fig. 2. Effect of insecticides on the availability of P in soil. For explanations, see Fig. 1.
therefore able to utilize the released nutrients for their growth and development. Similar observations were also recorded by earlier workers (Das and Mukherjee, 1994; Jana et al., 1998) with different types of insec-ticides in soil.
Incorporation of insecticides also brought about a significant rise in the availability of soluble P in soil (Fig. 2) suggesting that insecticides signifi-cantly increased the activities of phosphate mineral-izing/solubilizing microorganisms (Arora and Gaur, 1979; Das and Mukherjee, 1994). The availability of soluble P was greatly increased on the 5th day with carbofuran and fenvalerate and up to the 60th day with HCH and phorate, manifesting greater utiliza-tion of chlorinated hydrocarbon and organophosphate insecticides as well as their degraded products by the phosphate solubilizing/mineralizing microorganisms for their growth and metabolism (Ogunseitan and Odeymi, 1985).
4. Conclusions
The results of the present investigation clearly in-dicate that application of different insecticides, HCH
and phorate in particular, at their recommended field rates, killed part of the soil microbial, perhaps also soil animal, biomass resulting in an increase of avail-able N and P in soil. Some microorganisms, such as bacteria, actinomycetes and fungi not sensitive to the insecticides tested were able to utilize the nutrients with a resultant increase in their populations in soil. This also indicated that the tested insecticides at their recommended field rates did not have any deleterious effect on the growth and activities of the major soil microorganisms responsible for the transformations of C, N and P and thus the insecticides can be safely used to combat the insect pests for better crop production.
References
Arora, D., Gaur, A.C., 1979. Microbial solubilization of different inorganic phosphates. Ind. J. Exptl. Biol. 17, 1258–1261. Bhuyan, S., Sreedharan, B., Adhya, T.K., Sethunathan, N.,
1993. Enhanced biodegradation of g-hexachlorocyclohexane (g-HCH) in HCH (commerial) acclimatized flooded soil: factors affecting its development and persistence. Pestic. Sci. 38, 49– 55.
Environment. Soil Science Society of America, Madison, WI, USA, pp. 169–211.
Bowie, I.S., Loutit, M.W., Loutit, J.S., 1969. Identification of aerobic heterotrophic soil bacteria to the generic level by using multipoint inoculation techniques. Can. J. Microbiol. 15, 297– 302.
Das, A.C., Mukherjee, D., 1994. Effect of insecticides on the availability of nutrients, nitrogen fixation, and phosphate solubility in the rhizosphere soil of rice. Biol. Fertil. Soils 18, 37–41.
Das, A.C., Chakravarty, A., Sukul, P., Mukherjee, D., 1995. Insecticides: their effect on microorganisms and persistence in rice soil. Microbiol. Res 150, 187–194.
Debnath, A., Das, A.C., Mukherjee, D., 1994. Studies on the decomposition of non-conventional organic wastes in soil. Microbiol. Res. 149, 195–201.
El-Shahaat, M.S., Othman, M.A.S., Halfawym, E., Marei, A.S., 1987. Effect of carbamate and synthetic pyrethroid pesticides on some soil microbial activeities. Alexandria J. Agric. Res. 32, 427–438.
Gaikawad, S.T., Samantary, R.N., Patnaik, S., 1973. Effect of some soil application of biocides on N, P, K availability, K availability and C, N transformations in flooded rice soils. J. Ind. Soc. Soil Sci. 21, 263–269.
Gilman, J.C., 1957. A Manual of Soil Fungi. The Iowa State University Press, USA, 450 pp.
Jackson, M.L., 1973. Soil Chemical Analysis. Prentice-Hall, New Delhi, India, 498 pp.
Jana, T.K., Debnath, N.C., Basak, R.K., 1998. Effect of insecticides on decomposition of organic matter, ammonification and nitrification in a Fluventic Ustochrept. J. Ind. Soc. Soil Sci. 46, 133–134.
Jensen, H.L., 1930. Actonomycetes in Danish soils. Soil Sci. 30, 59–77.
Levavon, D., 1993. Roles of fungi and bacteria in the mineralization of the pesticides atrazine, alachlor, malathion and carbofuran in soil. Soil Biol. Biochem. 25, 1097–1105.
Martin, J.P., 1950. Use of acid, rose bengal and streptomycin in the plate method for estimating soil fungi. Soil Sci. 69, 215–232. Martinez-Toledo, M.V., Salmeron, V., Gonzalez-Lopez, J., 1992. Effect of an organophosphorus insecticide, phenophos on agricultural soil microflora. Chemosphere 24, 71–80. Matsumura, F., Boush, G.M., 1971. Metabolism of insecticides
by microorganisms. In: McLaren, A.D., Skujins, J. (Eds.), Soil Biochemistry, vol. 2. Marcel Dekker, New York, USA, pp. 320–336.
Moorman, T.B., 1989. A review of pesticide effects on microorganisms and microbial processes related to soil fertility. J. Prod. Agric. 2, 14–23.
Murthy, N.B.K., Kale, S.P., Raghu, K., 1991. Mineralization of
14C-labelled rice straw in aerobic and anaerobic clay soils as
influenced by insecticide treatment. Soil Biol. Biochem. 23, 857–859.
Ogunseitan, O.A., Odeymi, O., 1985. Effects of lindane, captan, and malathion on nitrification, sulphur oxidation, phosphate solubilization and respiration in tropical soil. Environ. Pollut., A 37, 343–354.
Olsen, S.R., Dean, L.A., 1982. Phosphorus. In: Black, C.A., Evans, D.D., White, J.L., Ensminger, L.E., Clark F.E., Dinauer, R.C., (Eds.), Methods of Soil Analysis, part 2. American Society of Agronomy, Madison, WI, USA, pp. 1035–1049.
Petersen, R.G., 1994. Agricultural Field Experiments: Design and Analysis. Marcel Dekker, New York, NY, USA, 409 pp. Rache, K.D., Coats, J., 1988. Comparative biodegradation of
organophosphorus insecticides in soil. Specificity of enhanced microbial biodegradation. J. Agric. Food Chem. 36, 193–199. Rangaswamy, V., Venkateswarlu, K., 1993. Ammonification and
nitrification in soils, and nitrogen fixation by Azospirillum sp. as influenced by cypermethrin and fenvalerate. Agric. Ecosyst. Environ. 45, 311–317.
Sakata, S., Mikami, N., Yamada, H., 1992. Degradation of pyrethroid optical isomers by soil microorganisms. J. Pestic. Sci. 17, 181–189.
Salle, A.J., 1973. Laboratory Manual on Fundamental Principles of Bacteriology. McGraw-Hill Book Company, New York, USA, 201 pp.
Simon-Sylvestre, G., Fournier, J.C., 1979. Effect of pesticides on soil microflora. Adv. Agron. 31, 1–92.
Singh, V.N., Prasad, C.R., 1991. Effect of phorate and gamma BHC on mineralization of nitrogen in soil. J. Ind. Soc. Soil Sci. 39, 183–185.
Skerman, V.B.D., 1967. A Guide to the Identification of the Genera of Bacteria. The Williams and Wilkins Company, Baltimore, USA, 303 pp.
Skerman, V.B.D., 1975. A key for the determination of the generic position of organisms listed in the manual. In : Buchanan, R.E., Gibbons, N.E., (Eds.), Bergy’s Manual of Determinative Bacteriology. The Williams and Wilkins Company, Baltimore, USA, pp. 1098–1146.
Srimathi, M.S., Karanth, N.G.K., Majumder, K., 1986. Influence of bromophos on some biological activities of the soil. J. Soil Biol. Ecol. 6, 9–15.
Thornton, H.G., 1922. On the development of a standardised agar medium for counting soil bacteria with a special regards to the respression of spreading colonies. Ann. Appl. Biol. 2, 241–274. Turco, R.F., Konopka, A., 1990. Biodegradation of carbofuran in enhanced and non-enhanced soil. Soil Biol. Biochem. 22, 195– 201.