Attentional Dysfunction: Adults with Schizophrenia,
ADHD, and a Normal Comparison Group
Randal G. Ross, Ann Olincy, Josette G. Harris, Bernadette Sullivan,
and Allen Radant
Background: Smooth pursuit eye movement (SPEM)
ab-normalities are found in schizophrenia. These deficits
often are explained in the context of the attentional and
inhibitory deficits central to schizophrenia
psychopathol-ogy. It remains unclear, however, whether these
attention-associated eye movement abnormalities are specific to
schizophrenia or are a nonspecific expression of
atten-tional deficits found in many psychiatric disorders. Adult
attention-deficit/hyperactivity disorder (ADHD) is an
al-ternative disorder with chronic attentional and inhibitory
dysfunction. Thus, a comparison of SPEM in adult
schizo-phrenia and adult ADHD will help assess the specificity
question.
Methods: SPEM is recorded during a 16.7° per second
constant velocity task in 17 adults with ADHD, 49 adults
with schizophrenia, and 37 normal adults; all groups
included individuals between ages 25–50 years.
Results: Smooth pursuit gain and the frequency of
antic-ipatory and leading saccades are worse in schizophrenic
subjects, with normal and ADHD subjects showing no
differences on these variables.
Conclusions: Many attention-associated SPEM
abnor-malities are not present in most subjects with ADHD,
supporting the specificity of these findings to the
atten-tional deficits seen in schizophrenia. Biol Psychiatry
2000;48:197–203 ©
2000
Society
of
Biological
Psychiatry
Key Words: Schizophrenia,
attention-deficit/hyperactiv-ity disorder, eye movements, smooth pursuit, saccades
Introduction
M
ore than 90 years ago, Diefendorf and Dodge
(Diefendorf and Dodge 1908) first reported that
individuals with schizophrenia had difficulty tracking a
predictably moving object. Abnormal eye tracking, one of
the most frequently studied and consistently reproduced
psychophysiologic abnormalities associated with
schizo-phrenia (Levy et al 1993), may help refine the
neurophys-iologic description of schizophrenia (Ross et al 1998b) and
facilitate genetic studies of schizophrenia (Clementz
1998). Ocular tracking of a moving target, commonly
referred to as smooth pursuit eye movements (SPEM),
requires the coordinated activation of both smooth pursuit
and saccadic systems (Gaymard and Pierrot-Deseilligny
1999). The roles of each of these eye-movement
abnor-malities in producing the eye-tracking abnorabnor-malities in
schizophrenia have yet to be fully determined.
SPEM abnormalities often can be at least partially
normalized
with
attention
enhancement
techniques
(Rosenberg et al 1997), and at least one of the pathologic
components of SPEM performance, task-inappropriate
intrusion of anticipatory saccades, has been
conceptual-ized as a failure of inhibitory control (Friedman et al 1992;
Rosenberg et al 1997; Ross et al 1996; Strik et al 1992).
Attentional and inhibitory control subjects thus appear to
play a major role in the SPEM abnormalities seen in
schizophrenia. Despite this focus on the interplay between
attentional and inhibitory processes, there has been only
minimal exploration of SPEM in another chronic
psychi-atric disorder of attentional and inhibitory dysfunction—
attention-deficit/hyperactivity disorder (ADHD). A
pri-mary deficit in ADHD is a failure in response inhibition
(Barkley 1997), a deficit that can be identified in saccadic
eye-movement tasks (Ross et al 1994) in a manner similar
to that found in schizophrenia (Ross et al 1998b). If SPEM
abnormalities associated with schizophrenia are due to
nonspecific attentional dysfunction, individuals with
ADHD should demonstrate similar SPEM abnormalities.
Reports have been contradictory as to whether
perfor-From the Departments of Psychiatry of the Denver Veterans Administration Medical Center (RGR, AO) and University of Colorado Health Sciences Center (RGR, AO, JGH, BS), Denver, Colorado, and the Seattle Veterans Adminis-tration Medical Center, Seattle, Washington (AR).
Address reprint requests to Randal G. Ross, M.D., University of Colorado Health Sciences Center, Box C268-71, 4200 E. 9th Ave., Denver CO 80262. Received September 20, 1999; revised February 3, 2000; accepted February 3,
2000.
© 2000 Society of Biological Psychiatry 0006-3223/00/$20.00
mance during a SPEM task is abnormal in children with
ADHD (Bala et al 1981; Bylsma and Pivik 1989; Jacobsen
et al 1996; Shapira et al 1980). There is no published
literature on SPEM performance in adult ADHD and no
published reports that include information on anticipatory
saccades in ADHD individuals of any age. This study
compares adults with schizophrenia, adults with ADHD,
and a comparison group of normal subjects on a smooth
pursuit eye movement task.
Methods and Materials
Subjects
Because normal aging can impact SPEM performance (Hutton et al 1993; Kanayama et al 1994; Kuechenmeister et al 1977; Larsby et al 1988; Ross et al 1999a; Sharpe and Sylvester 1978; Spooner et al 1980), all subjects were restricted to individuals between ages 25 and 50 years. Seventeen adult ADHD subjects were recruited from an adult outpatient clinic for ADHD. All subjects were recruited early in the diagnostic process. None had received stimulant medications for at least 10 years before enrollment in this study. All subjects were assessed using DSM-IV criteria (American Psychiatric Association 1994) via the structured clinical interview for DSM-IV (First et al 1997) and a semistructured clinical interview for ADHD developed by one of the authors of our study (RR). Exclusionary criteria included history of significant head injury, neurologic illness (e.g., seizures), substance abuse or dependence within the last 6 months, current major depression, any history of psychosis or bipolar disorder, or family history of psychosis or mania. ADHD subjects were identified as currently positive on 8.3 6 1.0 inattentive symptoms (range 6 –9) and 5.5 62.4 hyperactive-impulsive symptoms (range 2– 8). Six subjects (4 men and 2 women) were diagnosed with ADHD-combined subtype; 5 subjects (3 men and 2 women) were diagnosed with ADHD-inattentive subtype. All subjects had onset of symptoms before age 7 years. Wender Utah self-report scores (Ward et al 1993) were 55 6 16 with a range 37– 80 (all values greater than 2 standard deviations above reported values for gender-matched normal subjects). Four subjects (3 men and 1 woman) were diagnosed with concurrent social phobia. Past histories of psy-chiatric disorders in addition to ADHD were identified: major depression (n 5 3), obsessive-compulsive disorder (n 5 2), alcohol dependence (n 5 1), and marijuana abuse (n 5 1).
SPEM recordings from all age-appropriate schizophrenic and normal adults who have been recorded in our lab in the last 6 years were included as comparison groups. Normal adults were
excluded for history of significant head injury, neurologic illness (e.g., seizures), substance abuse or dependence within the last 6 months, current major depression, any history of psychosis or bipolar disorder, or family history of psychosis or mania. Adults with schizophrenia were recruited from outpatient psychiatric clinics. Diagnosis was confirmed with the use of the structured clinical interview for DSM-IV (First et al 1997). The recruitment process identified individuals with chronic schizophrenia who were maintained with stable symptoms on an outpatient basis. Schizophrenic subjects were excluded for neurologic illness (e.g., seizures), substance abuse or dependence within the last 6 months, and current major depression. This process produced SPEM recordings from 49 schizophrenic and 37 normal subjects for analysis. We have previously reported on many of these subjects (Ross et al 1998b, 1999a, 1999b).
ADHD, schizophrenic, and normal groups did not differ on age; however, there was a significant difference in gender (Table 1). No male-female differences exist on any eye-movement variable reported in this study.
Smooth Pursuit Eye Movements
Subjects were seated 43 cm in front of a video monitor on which a small target was displayed against a black background in an otherwise dark room. The subjects’ heads were stabilized with a bite bar and head rest. Horizontal eye movements were recorded using an infrared photoelectric limbus detection eye-tracking device, which is accurate to within 0.25° of visual angle and has a time constant of 4 msec. The analog output of the device was sampled at 500 Hz using a 12-bit analog-to-digital converter. There are no reported data assessing left-right differences in the dependent eye-movement measures employed in this study, but because disconjugate gaze is likely absent in most subjects, both eyes should be tracking the target in a similar manner. Our experience suggests that minimizing calibration time decreases the time subjects must maintain head position and improves the quality of the recording. Therefore, data were collected only from the eye for which the most rapid and accurate calibration could be obtained.
For the smooth pursuit eye-movement task, the target moved horizontally back and forth over 30° with a constant velocity of 16.7° per sec and a 1.4-sec fixation period between ramps, a “trapezoidal pattern.” Subjects were told to “keep your eyes on the target wherever it goes.”
All eye-movement data were analyzed with a computerized pattern-recognition program, and computer-analyzed results were confirmed with visual inspection by an experienced eye-movement evaluator (RR). Software-generated dependent mea-Table 1. Demographics for Adults with Attention-Deficit/Hyperactivity Disorder (ADHD), Schizophrenia, and a Normal
Comparison Group
ADHD group
Schizophrenic group
Normal
group Statistic Probability
N 17 49 37
Male/female 10/7 36/13 15/22 x259.5 .009
sures have excellent test-retest reliability (Roy-Byrne et al 1995). This analysis system has been described elsewhere (Radant and Hommer 1992; Ross et al 1996) and will be described briefly here. Raw data consist of eye position and target position for each 2 msec of recorded tracking. Eye movements were divided into discrete segments, and then each segment was classified as saccade, smooth pursuit, or artifact. Saccades were identified on the basis of peak velocity (greater than 35°/sec), initial acceler-ation (greater than 2000°/sec2
), and duration ($9 msec). Seg-ments not meeting velocity and acceleration criteria were con-sidered smooth pursuit or fixation. Artifactual segments caused by eye blinks and head movements show distinct morphology and were removed from the analysis by the pattern recognition software. Gain for a given interval of smooth pursuit was defined as mean eye velocity divided by target velocity. Global smooth pursuit gain was defined as the mean gain, weighted for time, of all intervals of true smooth pursuit (Abel et al 1991). Intervals defined as saccades are not included in computing smooth pursuit gain. During trapezoidal tasks, eye movements during fixation or within 250 msec of a change in target motion were discarded from the analysis because these movements may not represent normal pursuit (Lisberger and Pavelko 1989).
Lowered smooth pursuit gain can occur secondary to either impairment in the smooth pursuit system or as a compensatory mechanism to task-inappropriate saccades that intrude on other-wise normal pursuit (Abel and Ziegler 1988; Clementz and Sweeney 1990). Thus, it has been suggested that smooth pursuit gain is a global measure of task performance (Radant and Hommer 1992).
Catch-up saccades were used as the dependent measure of smooth pursuit system performance. Catch-up saccades function to significantly reduce error between foveal gaze and target location and compensate for poor smooth pursuit system perfor-mance (eye velocity below that of target velocity). Saccades, which are in the same direction as target motion and begin and end behind the target, are defined as catch-up saccades. In addition, saccades that are in the same direction as target motion but that begin behind target location and end ahead of target location are also classified as catch-up saccades if postsaccadic position error is#50% of presaccadic position error (i.e., the saccade functions to dramatically decrease the mismatch be-tween visual gaze and target location).
One type of task-inappropriate saccade, which intrudes on otherwise normal smooth pursuit, is the anticipatory saccade. Anticipatory saccades must 1) be in the direction of target motion, 2) either begin and end ahead of target location or increase position error by 100%, and 3) be followed by a 50 msec interval of eye velocity less than 50% of target velocity. This definition of anticipatory saccades is based on analyses of parameters that maximize differences between schizophrenic subjects and normal subjects (Ross et al 1999b, in press). Many authors also include a minimum amplitude criterion when defin-ing anticipatory saccades, generally greater than 4 –5° (Abel and Ziegler 1988; Clementz and Sweeney 1990). We traditionally have not included an amplitude criterion in our definition of anticipatory saccades and shall continue in this article to include anticipatory saccades of all amplitudes within the definition of anticipatory saccades. To allow comparison with other accounts,
however, we also shall subdivide anticipatory saccades into large anticipatory saccades (with amplitudes greater than 4°) and leading saccades (Ross et al 1999b).
Smooth pursuit, catch-up saccades, and anticipatory saccades account for more than 90% of eye movements during smooth pursuit tracking (Litman et al 1994; Radant and Hommer 1992). Other components (e.g., square wave jerks, backup saccades, etc.) constitute only a small portion of smooth pursuit tracking and generally do not differ between schizophrenic subjects and control subjects (Litman et al 1994; Radant and Hommer 1992). Some saccades begin behind the target, end in front of the target, and do not dramatically increase or decrease position error. These saccades do not meet the definition for either catch-up or anticipatory saccades and are not considered here.
Saccades generally are reported as frequency measures, that is, the number of each saccadic subtype divided by artifact-free recording time. The problem with this approach is that frequency measures do not include information about the amplitudes of saccades. A single large saccade may have a similar effect on global tracking as several small saccades will have. In addition to frequency data, we report the percentage of total eye movements accounted for by each saccadic subtype, a measure mathemati-cally identical to multiplying frequency by mean amplitude. Thus, the percentage of total eye movements accounted for by each saccadic subtype includes amplitude information along with frequency information in the same dependent measure (Olincy et al 1998; Ross et al 1993, 1996, 1997, 1998b). Because amplitude criteria are included in the definition of large anticipatory and leading saccades, these two variables shall only be reported as frequency variables.
Statistical Analysis
Comparisons between groups were made with an analysis of variance (ANOVA), with post hoc least-squared differences between groups. In addition, effect sizes (Cohen 1988) for comparisons between the schizophrenic and normal groups, the schizophrenic and ADHD groups, and the ADHD and normal groups were computed for each eye-movement variable. Effect sizes of 0.2– 0.49 were considered small, 0.5– 0.79 moderate, and greater than or equal to 0.8 large. Differences in effect size of greater than 0.2 were considered notable.
Results
Table 2 shows results for each eye-movement variable. A
significant effect of group was found for each
eye-movement measure, with post hoc analyses demonstrating
differences between the schizophrenic and normal groups
on all measures. Significant differences between
schizo-phrenic and ADHD groups were found in gain and the
frequencies of anticipatory and leading saccades.
Signifi-cant differences between the ADHD and normal groups
were only identified for the percentage of total eye
movements due to smooth pursuit.
saccades and the frequency of large anticipatory saccades)
to large (all other SPEM measures) effect sizes. The
largest effect sizes were for frequency of anticipatory
saccades (1.53), frequency of leading saccades (1.46), gain
(1.24), and the percentage of total eye movements due to
smooth pursuit (1.11).
Five comparisons between the schizophrenic and
ADHD groups produced effect sizes that would be
con-sidered small (
,
0.5) or below: frequency of catch-up
saccades, percentage of total eye movements due to
smooth pursuit, percentage of total eye movements due to
catch-up saccades, percentage of total eye movements due
to anticipatory saccades, and frequency of large
anticipa-tory saccades. Moderate effect sizes were produced for
smooth pursuit gain. The frequency of anticipatory
sac-cades and the frequency of leading sacsac-cades produced
large effect sizes for comparisons between the
schizo-phrenic and ADHD groups.
With one exception, effect sizes were notably lower
(difference in effect size
.
0.2) for schizophrenic and ADHD
group comparisons than for the schizophrenic and normal
group comparisons. The one exception is frequency of
leading saccades, which produced similar large effect sizes
(1.31–1.46) for comparisons between both the schizophrenic
and normal groups and the schizophrenic and ADHD groups.
Discussion
Schizophrenic adults performed more poorly than did
normal adults on each of the SPEM measures examined.
This sample contains some individuals who have been
discussed in previous reports and thus cannot be
consid-ered a replication; however, this is the largest sample of
schizophrenic subjects and normal subjects we have
pub-lished in this age range, suggesting these differences
remain even within larger groups. Additionally, the broad
range of eye-movement deficits is consistent with what
has been reported elsewhere (Clementz and Sweeney
1990; Litman et al 1994; Radant and Hommer 1992;
Sweeney et al 1994).
Impaired motion perception (Chen et al 1999a, 1999b)
and impaired ability to match eye velocity to target
velocity (Ross et al 1996; Farber et al 1997) may
contrib-ute to impaired SPEM abnormalities in schizophrenia;
however, a major contributor to schizophrenia-associated
SPEM abnormalities is, by experimental and theoretical
argument, associated with attentional deficits. One focus
of this study was to determine whether these attentional
findings are associated with the specific types of
atten-tional deficits found in schizophrenia or with nonspecific
attentional deficits that might be present in any adult with
attentional and inhibitory deficits. Adults with ADHD
were chosen as a comparison group with chronic deficits
in these areas.
Three eye-movement measures were significantly more
abnormal among the schizophrenic patients than among
the ADHD patients: gain, frequency of anticipatory
sac-cades, and frequency of leading saccades. The ADHD
group did not differ from the normal group on any of these
three measures. This suggests that the general attentional
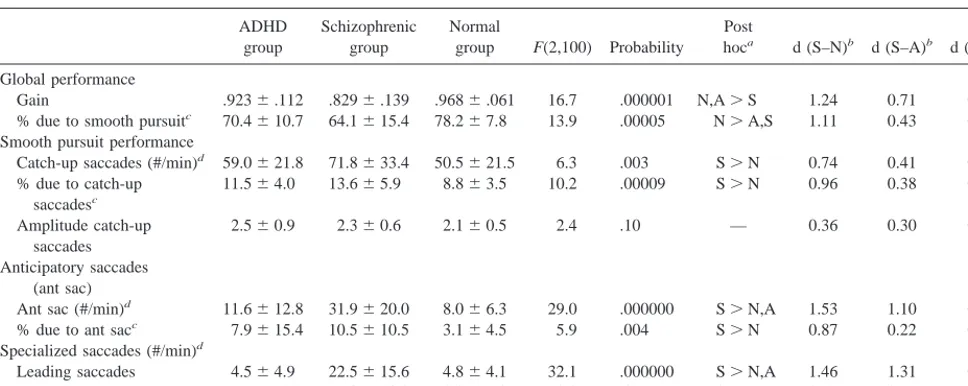
Table 2. Smooth Pursuit Performance in Adults with Attention-Deficit/Hyperactivity Disorder (ADHD), Schizophrenia, and a Normal Comparison Group
ADHD group
Schizophrenic group
Normal
group F(2,100) Probability Post
hoca d (S–N)b d (S–A)b d (A–N)b
Global performance
Gain .9236.112 .8296.139 .9686.061 16.7 .000001 N,A.S 1.24 0.71 0.56 % due to smooth pursuitc 70.4610.7 64.1615.4 78.267.8 13.9 .00005 N.A,S 1.11 0.43 0.89
Smooth pursuit performance
Catch-up saccades (#/min)d 59.0621.8 71.8633.4 50.5621.5 6.3 .003 S.N 0.74 0.41 0.40
% due to catch-up saccadesc
11.564.0 13.665.9 8.863.5 10.2 .00009 S.N 0.96 0.38 0.74
Amplitude catch-up saccades
2.560.9 2.360.6 2.160.5 2.4 .10 — 0.36 0.30 0.62
Anticipatory saccades (ant sac)
Ant sac (#/min)d 11.6612.8 31.9620.0 8.066.3 29.0 .000000 S.N,A 1.53 1.10 0.41
% due to ant sacc 7.9615.4 10.5610.5 3.164.5 5.9 .004 S.N 0.87 0.22 0.52
Specialized saccades (#/min)d
Leading saccades 4.564.9 22.5615.6 4.864.1 32.1 .000000 S.N,A 1.46 1.31 0.07 Large ant sac 6.6611.9 7.8610.3 3.065.0 3.0 .05 S.N 0.57 0.11 0.46
All values are means6SD.
aPost hoc tests are least-squared differences: S, schizophrenia, A, ADHD, N, normal.
bEffect size (d) as defined by Cohen (1988): S-N, Schizophrenic group versus normal group comparison; S-A, schizophrenic group versus ADHD group comparison;
A-N, ADHD group versus normal group comparison.
and inhibitory dysfunction associated with ADHD does
not translate into major SPEM abnormalities in the
major-ity of ADHD patients. Nonetheless, for two of these three
measures (gain and frequency of anticipatory saccades),
the effect sizes are notably larger for the comparisons
between the schizophrenic and normal groups than for
comparisons between the schizophrenic and ADHD
groups. This is primarily attributable to a subgroup of
ADHD subjects who performed abnormally (for example,
see results for gain in Figure 1). Heterogeneity of eye
movement performance also has been found among
schizophrenic subjects (Holzman et al 1984, 1988).
One SPEM measure, the frequency of leading saccades,
produced similar effect sizes for both
schizophrenic-normal comparisons (1.46) and schizophrenic-ADHD
comparisons (1.31). Leading saccades are a subset of
anticipatory saccades. While tracking a moving object,
there is a natural tendency to generate a saccade and move
the eye ahead of current target location. For example,
while watching a car skid across an icy intersection, an
individual frequently will generate a saccade along the
projected path of the car to determine if and what the car
might hit. These anticipations of car movement are termed
“anticipatory saccades.” The target tracking task employed
in this study is simple and highly predictable. As phase lag
during this task approaches zero, this task elicits predictive
smooth pursuit. Anticipatory saccades, although rare in
normal adult subjects, do occur during this task. This
suggests that even in this simple task, saccadic anticipation
of target motion is a normal process. The SPEM tasks used
in the study of schizophrenia includes the instruction to
“keep your eye on the target,” thus requiring the subject to
inhibit the tendency to use saccades to anticipate target
motion. The failure to inhibit anticipatory saccades has
been proposed as a marker of genetic risk for
schizophre-nia because it is present in adults with schizophreschizophre-nia and
their relatives (Whicker et al 1985; Rosenberg et al 1997;
Ross et al 1998b). The lack of an increase in anticipatory
saccades in relatives of depressed patients (Rosenberg et al
1997) has argued for the possible specificity of this
marker. One of the major problems on research with this
marker, however, has been some disagreement on whether
amplitude criteria should be included in the definition.
Although a number of authors have argued for a minimum
amplitude criteria of 4 –5° (Abel et al 1991; Clementz and
Sweeney 1990), other labs have suggested that there is no
a priori reason to assume that anticipatory saccades cannot
include saccades of smaller amplitudes (Radant and
Hom-mer 1992; Ross et al 1999b). Fortunately, Strik et al
(1992) provided a possible solution to this issue when they
classified saccades solely based on amplitude criteria; they
found that saccades less than 4° were closely related to
another pathophysiologic dysfunction characterized in
schizophrenia: reduced amplitude of the auditory N100
event-related potential. They argued that larger saccades
represented a nonspecific attentional dysfunction. This
finding raised the possibility that saccades with amplitudes
greater than 4° represent a different physiologic process
than those with amplitudes less than 4°. The results from
the current study support the application of this hypothesis
to anticipatory saccades. Large anticipatory saccades
(am-plitudes
.
4°) occur significantly more often in
schizo-phrenic subjects than in normal subjects, replicating
pre-vious work (Litman et al 1994; Rosenberg et al 1997);
however, they do not differ between schizophrenic
sub-jects and ADHD subsub-jects, and the schizophrenic-ADHD
effect size, at 0.11, is below what is considered to be even
a small physiologic effect (Cohen 1998). Thus, as
pre-dicted by Strik et al (1992), the frequency of large
anticipatory saccades appears to be a nonspecific effect of
attentional dysfunction. Conversely, leading anticipatory
saccades (anticipatory saccades with amplitudes 1– 4°) are
significantly elevated in schizophrenia relative to both
ADHD and normal subjects, and there appears to be no
notable difference between ADHD subjects and normal
subjects (effect size 0.07, see Figure 2) on this variable.
For leading saccades, the effect size is similar in
schizo-phrenic-normal comparisons and schizophrenic-ADHD
comparisons, supporting the specificity of this finding to
schizophrenia.
In summary, as a group, ADHD subjects did not
significantly differ from normal subjects on the majority
of SPEM measures used in this study. Nonetheless, for
most measures, a few ADHD subjects performed very
poorly. ADHD subjects were identified based on a
structured evaluation by a single investigator (RGR),
without interrater reliability assessment; however, subjects
identified as meeting criteria for ADHD also scored poorly
on the Wender self-report scale, a scale associated with
ADHD in adults (Ward et al 1993), suggesting that a
majority of these subjects likely had ADHD. Additionally,
ADHD subjects and schizophrenic subjects were not
matched for severity of attentional dysfunction, and it can
be assumed that, on average, the schizophrenic subjects
demonstrated more severe psychopathology. The
gener-ally lower level of psychopathology in the ADHD subjects
(relative to the schizophrenic subjects) cannot be entirely
ruled out as a contributor to the generally normal range of
SPEM performance exhibited by the ADHD subjects.
Nonetheless, nonpsychotic relatives of schizophrenic
pro-bands also show SPEM abnormalities, and in particular
anticipatory saccade abnormalities, similar to the
psy-chotic probands (Whicker et al 1985; Ross et al 1998b).
Thus, SPEM and anticipatory saccade abnormalities
ap-pear to reflect genetic vulnerability rather than severity of
disease.
One of the goals of determining the specificity of SPEM
abnormalities to schizophrenia is assessing the possible
utility of SPEM abnormalities as a genetic risk marker in
family linkage studies. ADHD is a common disorder in the
population (Biederman 1998) and is likely to occur in
many families used for linkage analyses. The present
results suggest that the percentage of leading saccades is
unlikely to be elevated as the result of general attentional
dysfunction, thus allowing the inclusion of ADHD family
members in genetic studies.
This research was funded by a NARSAD Junior Investigator Award (RGR), by The Veterans Administration Medical Research Service, and by USPHS Grants Nos. MH56539, MH44212, and MH38321.
References
Abel L, Friedman L, Jesberger J, Malki A, Meltzer HY (1991): Quantitative assessment of smooth pursuit gain and catch-up saccades in schizophrenia and affective disorders. Biol
Psy-chiatry 29:1063–1072.
Abel L, Ziegler A (1988): Smooth pursuit eye movements in schizophrenics—what constitutes quantitative assessment?
Biol Psychiatry 24:747–761.
American Psychiatric Association (1994): Diagnostic and
Sta-tistical Manual of Mental Disorders, (4th ed). Washington,
DC: American Psychiatric Association Press.
Bala SP, Cohen B, Morris AG, Atkin A, Gittelman R, Kates W (1981): Saccades of hyperactive and normal boys during ocular pursuit. Dev Med Child Neurol 23:323–336. Barkley RA (1997): Behavioral inhibition, sustained attention,
and executive functions: Constructing a unifying theory of ADHD. Psychol Bull 121:65–94.
Biederman J (1998): Attention-deficit/hyperactivity disorder: A life-span perspective. J Clin Psychiatry 59(suppl 7):4 –16. Bylsma FW, Pivik RT (1989): The effects of background
illumination and stimulant medication on smooth pursuit eye movements of hyperactive children. J Abnorm Psychol 17: 73–90.
Chen Y, Levy DL, Nakayama K, Matthysse S, Palafox G, Holzman PS (1999a): Dependence of impaired eye tracking on deficient velocity discrimination in schizophrenia. Arch
Gen Psychiatry 56:155–161.
Chen Y, Palafox GP, Nakayama K, Levy D, Mattthysse S, Holzman PS (1999b): Motion perception in schizophrenia.
Arch Gen Psychiatry 56:149 –154.
Clementz BA (1998): Psychophysiological measures of (dis)in-hibition as liability indicators for schizophrenia.
Psychophys-iology 35:648 – 668.
Clementz BA, Sweeney JA (1990): Is eye movement dysfunction a biological marker for schizophrenia: A methodological review. Psychol Bull 108:77–92.
Cohen J (1988): Statistical Power Analysis for the Behavioral
Sciences. Hillsdale, NJ: Lawrence Erlbaum.
Diefendorf AR, Dodge R (1908): An experimental study of the ocular reaction on the insane from photographic records.
Brain 31:451– 489.
Farber RH, Clementz BA, Swerdlow NR (1997): Characteristics of open- and closed-loop smooth pursuit responses among obsessive-compulsive disorder, schizophrenia, and nonpsy-chiatric individuals. Psychophysiology 34:157–162. First MB, Spitzer RL, Gibbon M, Wingerson DK (1997):
Structured Clinical Interview for DSM-IV Axis I Disorders— Patient Edition. New York: New York State Psychiatric
Institute.
Friedman L, Abel LA, Jesberge JA, Malki A, Meltzer HY (1992): Saccadic intrusions into smooth pursuit in patients with schizophrenia or affective disorder and normal controls.
Biol Psychiatry 31:1110 –1118.
Gaymard B, Pierrot-Deseilligny C (1999): Neurology of sac-cades and smooth pursuit. Curr Opin Neurol 12:13–19. Holzman PS, Kringlen E, Matthysse S, Flanagan SD, Lipton RB,
Cramer G, et al (1988): A single dominant gene can account for eye tracking dysfunction and schizophrenia in offspring of discordant twins. Arch Gen Psychiatry 45:641– 647. Holzman PS, Solomon CM, Levin S, Waternaux CS (1984):
Pursuit eye movement dysfunctions in schizophrenia. Arch
Gen Psychiatry 41:136 –139.
Hutton JT, Nagel JA, Loewenson RB (1993): Variables affecting eye tracking performance. Electroencephalogr Clin
Neuro-physiol 56:414 – 419.
Jacobsen LK, Hong WL, Hommer DW, Hamgurger SD, Castel-lanos FX, Frazier JA, et al (1996): Smooth pursuit eye movements in childhood onset schizophrenia: Comparison with attention-deficit hyperactivity disorder and normal con-trols. Biol Psychiatry 40:1144 –1154.
Kanayama R, Nakamura T, Sano R, Ohki M, Okuyama T, Kimura Y, Koike Y (1994): Effect of aging on mooth pursuit eye movement. Acta Otolaryngol Suppl (Stockh) 511:131– 134.
Kuechenmeister CA, Linton PH, Mueller TV, White HB (1977): Eye tracking in relation to age, sex, and illness. Arch Gen
Psychiatry 34:578 –579.
Larsby B, Thell J, Moller C, Odkvist L (1988): The effect of stimulus predictability and age on human tracking eye move-ments. Acta Otolaryngol (Stockh) 105:21–30.
Levy DL, Holzman PS, Matthysse S, Mendell NR (1993): Eye dysfunction and schizophrenia: A critical review. Schizophr
Res 19:461–536.
Lisberger SG, Pavelko TA (1989): Topographic and directional organization of visual motion inputs for initiation of horizon-tal and vertical smooth pursuit eye movements in monkeys.
J Neurophysiol 61:173–185.
Litman R, Hommer DW, Radant AD, Clem T, Pickar D (1994): Quantitative effects of typical and atypical neuroleptics on smooth pursuit eye tracking in schizophrenia. Schizophr Res 12:107–120.
Olincy A, Ross RG, Young DA, Roath M, Freedman R (1998): Improvement in smooth pursuit eye movements after ciga-rette smoking in schizophrenic patients.
Neuropsychophar-macology 18:175–185.
Radant AD, Hommer DW (1992): A quantitative analysis of saccades and smooth pursuit during visual pursuit tracking: A comparison of schizophrenics with normals and substance abusing controls. Schizophr Res 6:225–235.
Rosenberg DR, Sweeney JA, Squires-Wheeler E, Keshavan MS, Cornblatt BA, Erlenmeyer-Kimling L (1997): Eye tracking dysfunction in offspring from the New York High Risk Project: Diagnostic specificity and the role of attention.
Psychiatry Res 66:121–130.
Ross DE, Buchanan RW, Medoff D, Lahti AC, Thaker GK (1998): Association between eye tracing disorder in schizo-phrenia and poor sensory integration. Am J Psychiatry 155: 1352–1357.
Ross RG, Harris JG, Cullum CM, Rilling LM, Radant AD, Adler LE, Freedman R (1997): Smooth pursuit eye movements in parents of probands with schizophrenia: An obligate carrier approach. Soc Neurosci Abstr 20:1262.
Ross RG, Harris JG, Olincy A, Radant AD, Adler LE, Freedman R (1998a): Familial transmission of two independent saccadic abnormalities in schizophrenia. Schizophr Res 30:59 –70. Ross RG, Hommer DW, Breiger D, Varley C, Radant AD
(1994): Eye movement task related to frontal lobe functioning in children with attention deficit disorder. J Am Acad Child
Adolesc Psychiatry 33:869 – 874.
Ross RG, Hommer DW, Radant AD, Roath M, Freedman R (1996): Early expression of smooth pursuit eye movement abnormalities in children of schizophrenic parents. J Am Acad
Child Adolesc Psychiatry 35:941–949.
Ross RG, Olincy A, Harris JG, Radant A, Adler LE, Compagnon N, Freedman R (1999a): The effects of age on a smooth pursuit tracking task in adults with schizophrenia and nor-mals. Biol Psychiatry 46:383–391.
Ross RG, Olincy A, Harris JG, Radant AD, Adler LE, Freedman R (1998b): Anticipatory saccades during smooth pursuit eye movements and familial transmission of schizophrenia. Biol
Psychiatry 8:690 – 697.
Ross RG, Olincy A, Radant A (in press): What duration of post-saccadic slowing identifies anticipatory saccades during smooth pursuit eye movements? Psychophysiology.
Ross RG, Olincy A, Radant AD (1999b): Amplitude criteria and anticipatory saccades during smooth pursuit eye movements in schizophrenia. Psychophysiology 36:464 – 468.
Ross RG, Radant AD, Hommer DW (1993): A developmental study of smooth pursuit eye movements in normal children from 7 to 15 years of age. J Am Acad Child Adolesc
Psychiatry 32:783–791.
Roy-Byrne PP, Radant AD, Wingerson DK, Cowley DS (1995): Human oculomotor function: Reliability and diurnal varia-tion. Biol Psychiatry 38:92–97.
Shapira YA, Jones MH, Sherman SP (1980): Abnormal eye movements in hyperkinetic children with learning disability.
Neuropa¨diatrie 11:36 – 44.
Sharpe JA, Sylvester TO (1978): Effect of aging on horizontal smooth pursuit. Invest Ophthalmol Vis Sci 17:465– 468. Spooner JW, Sakala SM, Baloh RW (1980): Effect of aging on
eye tracking. Arch Neurol 37:575–576.
Strik WK, Dierks T, Boning J, Osterheider M, Caspari A, Korber J (1992): Disorders of smooth pursuit eye movements and auditory N100 in schizophrenic patients. Psychiatry Res 41:227–235.
Sweeney JA, Clementz BA, Haas GL, Escobar MD, Drake K, Frances AJ (1994): Eye tracking dysfunction in schizophre-nia: Characterization of component eye movement abnormal-ities, diagnostic specificity, and the role of attention. J
Abnorm Psychol 103:222–230.
Ward MF, Wender PH, Reimherr FW (1993): The Wender Utah Rating Scale: An aid in the retrospective diagnosis of child-hood attention deficit disorder. Am J Psychiatry 150:885– 890.
Whicker L, Abel LA, Dell’Osso LF (1985): Smooth pursuit eye movements in the parents of schizophrenics.