L
Journal of Experimental Marine Biology and Ecology 245 (2000) 155–169
www.elsevier.nl / locate / jembe
Interpreting large-scale experiments on effects of trawling on
benthic fauna: an empirical test of the potential effects of
spatial confounding in experiments without replicated control
and trawled areas
a ,* b,c b
Mats Lindegarth , Daniel Valentinsson , Maria Hansson , b
Mats Ulmestrand
a
Centre for Research on Ecological Impacts of Coastal Cities, Marine Ecology Laboratories, A11,
University of Sydney, Sydney, NSW 2006, Australia
b
Institute of Marine Research, Box 4, S-453 21 Lysekil, Sweden
c
¨ ¨ ¨
Tjarno Marine Biological Laboratory, S-452 96 Stromstad, Sweden
Received 12 May 1999; received in revised form 11 October 1999; accepted 12 October 1999
Abstract
Disturbances due to trawling and dredging is a serious threat to assemblages of benthic marine animals. We tested hypotheses about effects of trawling on benthic assemblages in a manipulative field experiment, using gear and intensities relevant to future management of trawling in a Swedish fjord. Three trawled and three control sites were sampled at several times before and after trawling was initiated. This paper describes how conclusions about effects of trawling might differ between experiments involving replicate sites and experiments using only one trawled and one control site, as in several recent studies. Analyses of selected taxa showed that abundances of many species changed differently among control sites. Differences in temporal change between pairs of single trawled and control sites were also frequent. Neither the quantitative nor the qualitative nature of differences between treatments could, however, be coherently interpreted among the different combinations of trawled and control sites. This is consistent with results obtained from analyses using all sites, which showed no consistent effects of trawling on any of these taxa. These results provide empirical evidence that spatial confounding may cause serious problems to formal interpretation of experiments, which use only one control and one trawled area. Such potential problems can best be solved by ensuring that the study incorporates more than
one control site. 2000 Elsevier Science B.V. All rights reserved.
Keywords: BACI; Benthic assemblages; Confounding; Large-scale experiment; Pseudoreplication; Trawling
*Corresponding author. Tel.: 161-2-9351-4933; fax: 161-2-9351-6713.
E-mail address: [email protected] (M. Lindegarth)
1. Introduction
Physical disturbance due to mobile fishing gear is a serious and growing threat to diversity and production in marine benthic habitats (e.g. De Groot, 1984; Jones, 1992; Lindeboom, 1995; Kaiser, 1998; Lindeboom and De Groot, 1998). Gullmarsfjorden on the West Coast of Sweden was closed to trawling for shrimp, Pandalus borealis (Krøyer) in 1990. The ban was introduced to protect the fjord as an important reference area for scientific study and to protect areas deemed valuable for recruitment of commercial fish stocks. To guide future management, regional government authorities decided in 1995 that a large-scale experiment on potential effects of trawling on benthic fauna should be done in the area.
This experiment is one example of many recent studies on potential effects of mobile fishing gear on benthic diversity and production (e.g. Bergman and Hup, 1992; Brylinsky et al., 1994; Thrush et al., 1995; Currie and Parry, 1996; Kaiser and Spencer, 1996; Tuck et al., 1998). Most of these experiments involve monitoring biological response variables before and after trawling. Abundances of organisms may, however, change naturally. Therefore tests of hypotheses about effects of mobile fishing gear also require contrasting the magnitude of temporal change in fished areas to change in undisturbed, control areas (Green, 1979; Bernstein and Zalinski, 1983; Stewart-Oaten et al., 1986; Underwood, 1992). Several recent experiments on effects of trawling and dredging have involved pair-wise comparisons of one ‘control’- and one ‘treatment’-site (e.g. Thrush et al., 1995; Currie and Parry, 1996; Tuck et al., 1998). These investigators recognise that such experimental designs lack independent replicates of the treatments and therefore changes in response variables can not logically be attributed to the experimental treatment (Hurlbert, 1984; Underwood, 1991, 1992). Nevertheless, differ-ences in temporal change found between sites have been interpreted from these experiments as if they were caused by the experimental treatment. Such conclusions are not justified unless explicit comparisons show that repeated experimentation demonstrate similar types of effects.
The validity of experimental designs using one control and one potentially impacted area (Impact (‘ BACI’, Green, 1979) and Before-After-Control-Impact-Paired-Series (‘BACIPS’, Bernstein and Zalinski, 1983; Stewart-Oaten et al., 1986) rests on the assumption that what happens in the control area would also have happened in the potentially impacted area, were it not for the experimental treatment (Stewart-Oaten, 1996). Unless more than one control area is monitored, it is however not possible to evaluate the plausibility of this assumption and how potential violations affect quantitative and qualitative conclusions about effects of experimental treatments (Underwood, 1992, 1994). It is therefore very important to investigate the extent of variability in temporal change among potential control areas and what practical consequences such variability may have for the interpretation of experiments on effects of mobile fishing gear on benthic soft-sediment fauna.
M. Lindegarth et al. / J. Exp. Mar. Biol. Ecol. 245 (2000) 155 –169 157
observed in this experiment are reported elsewhere (Lindegarth et al., in press, Hansson et al., in review). In this paper, we explore problems associated with interpretation of results that are obtained from experiments using unreplicated treatment and control sites. This is done by comparing conclusions from analyses of the whole experiment (i.e. including all sites) and from analyses of pairs of sites. Analyses of total abundance, number of species and abundances of the most abundant species of polychaetes, echinoderms and bivalves are used as examples.
2. Materials and methods
2.1. Study area and sampling procedures
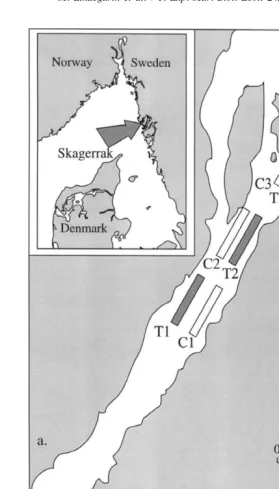
The experiment was done in Gullmarsfjorden on the west coast of Sweden (Fig. 1a). Gullmarsfjorden is approximately 35 km long, has a maximum depth of 120 m, and is separated from the deeper parts of the Skagerrak and the North Sea by a sill approximately 50 m deep. The fjord had supported a minor trawling fishery for the shrimp, Pandalus borealis since 1902. The 6.5 year closure to trawling prior to the experiment may or may not have been sufficient for abundances of benthic species to recover. Observations following another type of environmental disturbance in the area (Rosenberg, 1971) do, however, suggest recovery of abundances the majority of species and the diversity of assemblages can take place in this period of time.
Six sites were established as 1.5 km long transects in the inner part of the fjord (Fig. 1a), where benthic sampling and trawling were subsequently done. The ship’s position (62.5 m) was determined by a differential global positioning system. Each site was sampled four times before (July to November 1996) and four times after (July to November 1997) trawling had begun in the trawled sites (T1, T2 and T3). Trawling was done repeatedly after November 1996. Three sites (C1, C2 and C3) remained untrawled during the experiment and served as controls (Fig. 1b). At each time, ten samples of macrofauna were taken randomly in each site with a Smith-McIntyre bottom grab (0.1
2
m , 70 kg) and sieved through 4 mm and 1 mm mesh. The residue was preserved in 4% buffered formalin in seawater. In the laboratory, macrofauna larger than 4 mm were sorted, identified to the lowest practical taxonomic level, counted and preserved in 80% ethanol. The decision to primarily sort only the coarse fraction led to loss of some information but allowed a larger number of replicates to be sorted with available resources.
2.2. Intensity of trawling
M. Lindegarth et al. / J. Exp. Mar. Biol. Ecol. 245 (2000) 155 –169 159
local fishermen suggested that eighty hauls per site per year would be an appropriate intensity of trawling. This was the intensity, which could be considered if trawling would be allowed again in the fjord. Trawling effort was evenly distributed throughout the study period (two hauls per week) except for a break of two months (January and February 1997) due to ice in the fjord. Dates for sampling the benthic fauna were randomly selected and were independent of the trawling dates.
Trawling was done repeatedly along fixed transects (sites T1–T3). It was not possible to control the exact position of the trawl, because of the depth (75–90 m) and the necessary length of the trawl wire (300 m). The intensity of physical disturbance to the sediment was estimated with a sediment profile camera (Nilsson and Rosenberg, 1997). This was done using an experimental design, which was similar to that used to sample macrofauna. Physical damages to sediments were observed in 17% of the photos (15315 cm) at the trawled sites after trawling had started (Hansson et al., 1997). There was no difference in physical disturbance among the three trawled sites and no disturbances related to fishing in the control sites.
2.3. Statistical analyses
The experiment was designed to test hypotheses about effects on overall means following one year of trawling and effects, which occurred on some sampling dates. As specified by the sampling design, there were four factors in the analysis: ‘Before /After’ (fixed); ‘Treatment’ (fixed); ‘Site’ (random and nested within ‘Treatment’) and ‘Time’ (random and nested within ‘Before /After’). Effects of trawling on means and on temporal variability are detected by the interactions ‘B /A3Tr’ and ‘Tr3Ti(B /A)’ respectively. The effect of the interaction ‘B /A3Tr’ can be estimated only if the variability due to one, or both interactions ‘B /A3S(Tr)’ and ‘Tr3Ti(B /A)’ is very small (by convention P.0.25; Winer et al., 1991; Underwood, 1997a). Consequently,
post hoc elimination and pooling procedures were applied wherever appropriate. Student-Newman-Keuls’ (SNK) tests were used to elucidate the nature of significant effects. All analyses were done using log (X11) transformed data. This was done to
10
actually are). We used data from this experiment to test whether spatial confounding would have been a serious problem, had we only included a single control and trawled site in our experiment. We did this by comparing change from before to after trawling started in pairs of sites. If pairs of control sites differ as frequently as pairs made up of one control and one trawled site, and if conclusions about qualitative and quantitative effects of trawling differ depending on which pair of control and trawled site is selected, an experiment without replicated sites must be considered very unreliable.
3. Results
3.1. Analyses of whole experiment
Analyses of total number of individuals of all species, number of species and the numbers of the most abundant species of polychaete, Spiophanes kroeyeri (Grube),
¨ ¨
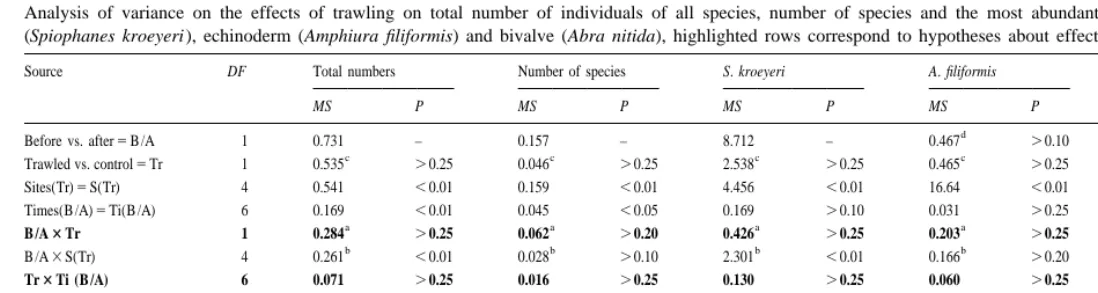
echinoderm Amphiura filiformis (O. F. Muller) and bivalve Abra nitida (O. F. Muller) did not reveal any significant effects of trawling (Table 1). All variables, however, showed significant interactive temporal and spatial patterns of variability, i.e. changes from ‘Before’ to ‘After’ or changes among ‘Times’ differed among sites (Table 1). This shows that sample sizes and mesh size were sufficient for detection of natural temporal and spatial patterns.
The total number of individuals, the number of species, and the abundances of S.
kroeyeri and A. nitida changed significantly from before to after trawling started (Table
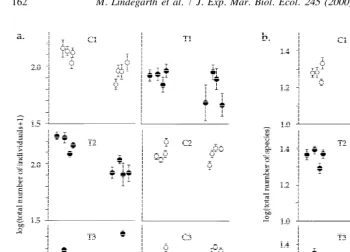
1). These changes varied among sites and were not consistent within treatments (Figs. 2 and 3). Therefore, this component of variability (‘B /A3S(Tr)’) could not be eliminated and the tests for consistent differences between controls and trawled areas had to be done with few degrees of freedom (Table 1). Assuming that the estimated means reflected ‘true’ means, statistical tests had low power to detect differences between treatments as significant. Furthermore, if the variation among sites and times was ignored (and pooling is done even if P,0.25) changes in abundances of S. kroeyeri, A.
filiformis and A. nitida would still not be significantly different between trawled and
untrawled sites (F ,F ). This indicates that the failure to reject the
null-obs 1,466,0.05
hypothesis is not simply because few sites were sampled, but to a larger degree because changes were qualitatively different among sites (Figs. 2 and 3).
Tests of the hypothesis that trawling affects the temporal dynamics of abundances and the number of species (i.e. the interaction ‘Tr3Ti(B /A)’) did not reveal differences between treatments for any of the variables (Table 1). The estimated effect of this interaction was negligible (P.0.25) for all variables and could therefore be eliminated, allowing tests for the interaction ‘B /A3Tr’.
3.2. Comparisons between pairs of sites
M
Analysis of variance on the effects of trawling on total number of individuals of all species, number of species and the most abundant species of polychaete (Spiophanes kroeyeri ), echinoderm (Amphiura filiformis) and bivalve (Abra nitida), highlighted rows correspond to hypotheses about effects of trawling Source DF Total numbers Number of species S. kroeyeri A. filiformis A. nitida
MS P MS P MS P MS P MS P
Residual 432 0.031 0.009 0.120 0.059 0.120
Cochrans test 0.16* 0.15* 0.084* 0.058 0.054
a,c,d b
F-ratio against MS , MS and MS respectively following elimination, F-ratio against pooled error-term (SS 1SS ) / B /A3S( Tr) S( Tr) B /A3S( Tr) Tr3Ti( B /A) Ti( B /A)3S( Tr)
(df 1df ).
Fig. 2. Temporal change in log of (a) total abundance of individuals of all species and (b) number of species in samples from three trawled sites (T1–T3) and the three control sites (C1–C3). Layout corresponds to relative geographic positions of sites with C1 in the south-west end and C3 in the north-east end of the fjord (Mean6S.E.; n510).
the initiation of trawling differed among all control locations for total number of individuals and for the polychaete S. kroeyeri (Table 2a and c). Comparisons of temporal change in the number of species and abundance of the bivalve A. nitida both indicated differences between one pair of control sites (Table 2b and d). No differences between pairs of control sites were detected for the echinoderm, A. filiformis (not shown). Thus, the number of species and abundances of animals exhibit diverse and unpredictable temporal dynamics in the absence of disturbances due to trawling (Figs. 2 and 3). These natural changes differed among variables and often differed among parts of the fjord (Table 2).
M. Lindegarth et al. / J. Exp. Mar. Biol. Ecol. 245 (2000) 155 –169 163
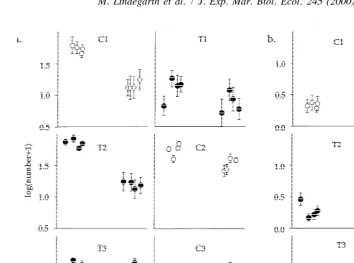
Fig. 3. Temporal change in numbers of (a) Spiophanes kroeyeri and (b) Abra nitida in samples from the six different areas (Mean6S.E.; n510).
Table 2
Tests of hypotheses of differences in change in means from before to after in comparisons between pairs of
a
sites, i.e. ‘S3B /A’
(a) Total C1 C2 C3 (b) Number C1 C2 C3
number of C1 – – – of species C1 – – –
individuals C2 ,0.01 – – C2 ,0.01 – –
C3 ,0.01 ,0.01 – C3 .0.10 .0.50
T1 .0.50 .0.20 ,0.05 T1 .0.50 ,0.05 .0.10
T2 .0.10 ,0.05 ,0.01 T2 .0.10 ,0.00 ,0.05
T3 .0.10 .0.50 .0.20 T3 .0.25 .0.50 .0.25
(c) Spiphanes C1 C2 C3 (d) Abra C1 C2 C3
kroeyeri C1 – – – nitida C1 – – –
C2 ,0.01 – – C2 .0.50 – –
C3 ,0.01 ,0.01 – C3 ,0.05 .0.10 –
T1 ,0.05 .0.50 ,0.00 T1 ,0.05 .0.05 .0.25
T2 .0.25 ,0.01 ,0.00 T2 .0.50 .0.25 ,0.01
T3 ,0.01 .0.20 ,0.00 T3 ,0.05 .0.25 .0.50
a
between pairs of trawled and control sites (Table 2c). The total number of individuals of all species, the number of species and numbers of the bivalve A. nitida differed between sites in three out of nine comparisons (Table 2a, b and d).
The design of most unreplicated experiments consists of a control site adjacent to the experimentally disturbed site. This design attempts to minimize natural (non-experimen-tal) variation between sites. Thus, the most relevant comparisons in this study are T1 vs. C1 or C2, T2 vs. C1 or C2, or T3 vs. C3 (Fig. 1). Three of five response variables investigated were significantly different between T2 and C2, while none were sig-nificantly different between T2 and C1. Thus, conclusions about the severity of impacts from trawling would differ strongly depending on which sites were selected for an unreplicated experiment. It also follows that quantitative estimates of the magnitude of effects vary greatly, depending on which combination of control and trawled site was selected.
Comparisons of different combinations of controls and trawled sites also resulted in qualitatively different conclusions about effects of trawling. There were significant differences in change of abundance of the polychaete, S. kroeyeri, in three of the five combinations of adjacent controls and trawled sites (Table 3). The abundance of S. kroeyeri decreased in C1, C2, T1 and T2, increased in C3 but did not change significantly in T3 (SNK, Table 3). Although the abundance of S. kroeyeri decreased significantly in both sites located close to the mouth of the fjord, the abundances decreased significantly more in C1 (Fig. 3a). If the experiment had included only these sites (T1 vs. C1), we would have concluded that trawling had a ‘positive effect’ on the abundance of S. kroeyeri. In the middle sites (T2 vs. C2), numbers in both sites decreased. Because the decrease is larger in T2, the conclusion from this comparison would have been that trawling had a ‘negative effect’ on the abundance of S. kroeyeri (Fig. 3a). Finally, an unreplicated experiment in the sites located in the inner parts of the
Table 3
Analysis of variance for tests of hypotheses about differences in change of mean abundances of the most abundant polychaete Spiophanes kroeyeri, between pairs of adjacent controls and trawled sites
Source DF T1 vs. C1 T2 vs. C2 T3 vs. C3 T2 vs. C1 T1 vs. C2
Residual 144 0.23 0.07 0.06 0.14 0.16
Cochrans test 0.13 ns 0.20 * 0.14 ns 0.14 ns 0.19 *
SNK of S3B /A C1: Aft,Bef C2: Aft,Bef C3: Bef,Aft – –
T1: Aft,Bef T2: Aft,Bef T3: Aft5Bef – – a
F-ratio against pooled MS. P-values shown only for hypotheses relevant to tests of hypotheses about
M. Lindegarth et al. / J. Exp. Mar. Biol. Ecol. 245 (2000) 155 –169 165
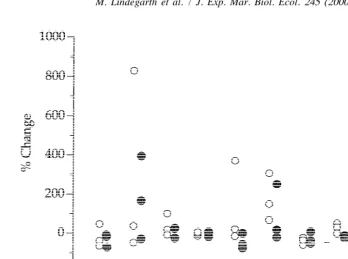
Fig. 4. Percent change in abundance of various species from before trawling to after trawling started. Each point represents one site. Empty symbols denote control sites and shaded symbols denote trawled sites. P5polychaete, E5echinoderm and B5bivalve.
fjord would also have suggested ‘negative effect’ of trawling. Because the abundance increased in C3 but not in T3, trawling would have been interpreted as counteracting recruitment of S. kroeyeri. Similar inconsistencies in the interpretation of the qualitative nature of effects of trawling were apparent also for A. nitida (Fig. 3b).
The three individual species discussed here were selected because of their large abundances and because they were taxonomically and ecologically diverse. Differences in temporal change among sites were, however, common for most of the abundant species (Fig. 4). For many species, abundances increased in some sites and decreased in others. In general, there were no obvious differences in rank order between the two types of sites (Fig. 4).
4. Discussion
similar to those of this experiment (e.g. Thrush et al., 1995; Currie and Parry, 1996; Tuck et al., 1998). We contrasted conclusions about effects of trawling on selected variables from analyses of the whole experiment, with conclusions based on com-parisons between individual sites. Analyses of the whole experiment did not reveal any patterns of temporal change in abundances of these variables, which could unambiguous-ly be interpreted as effects of the continuous trawling. These results were generalunambiguous-ly consistent with observations of relatively small physical disturbances on the bottom. Nevertheless, analyses of pairs of sites revealed a large number of significant differences in the way that abundances changed in individual sites. This is consistent with findings from previous experiments using one disturbed and one control site. Because significant differences were detected in comparisons between control sites, and because ‘effects’ varied quantitatively and qualitatively among combinations of sites, it is however clear that impacts of trawling could not have been assessed in a useful way, had this experiment contained only one trawled and one control site.
The implications of this result for interpretations of experiments on effects of trawling using one trawled and one control area (and assessments of environmental impacts in general) are potentially very important but will presumably differ among types of experiments. One important aspect is the temporal pattern, in which the experimental treatment is applied. Human-induced or natural disturbances may be characterized as either short-term (‘pulse’) or continuous (‘press’) (Bender et al., 1984; Glasby and Underwood, 1996). Manipulative experiments testing for effects of mobile fishing gear mimic either types of disturbances: some studies monitor effects of a single disturbance event (e.g. Bergman and Hup, 1992; Kaiser and Spencer, 1996; Hall and Harding, 1997), others quantify cumulative effects of repeated disturbances (e.g. Tuck et al., 1998; this study). Conclusions from experiments on effects of short-term, ‘pulse’ disturbances may be less affected by the type of confounding confounding observed here because abundances of animals may not be naturally variable at these temporal scales. Information based on ‘press’ type experiments is, however, generally more useful for long-term management of soft-sediment ecosystems. This is because experimental treatments must resemble the disturbing activity in intensity as well as spatial and temporal scope to be relevant (e.g. Jones, 1992; Walters, 1993; Underwood, 1996; Currie and Parry, 1996; Hall and Harding, 1997).
M. Lindegarth et al. / J. Exp. Mar. Biol. Ecol. 245 (2000) 155 –169 167
would have been informative for suggesting models to explain the observed patterns (Humphrey et al., 1995). The autumn and winter in which our experiment was conducted were exceptional because there was no inflow of water from the Skagerrak into the fjord. The resulting oxygen deficiencies (H. Nilsson, pers. comm.) may have caused variability in abundance to be unusually large. Nevertheless, some species (e.g. A. nitida) increased in abundance even in the deepest sites, where the lowest concentrations of oxygen would be expected to occur. This problem underscores the need to incorporate replicate trawl and / or control sites in studies of effects of trawling. The variability in temporal change among control sites raises issues over the relative probabilities of committing type I- and type II-errors (e.g. Fairweather, 1991; Mapstone, 1995; Underwood, 1997b). Because of this variability, there have to be larger effects of trawling before the null hypothesis of no difference in temporal change among trawled and control sites will be rejected (Table 1). Possible solutions to this problem include incorporating additional sites or allowing a larger probability to make type I-errors, e.g. a 50.1 or 0.2 (Peterman, 1990; Underwood, 1994). Post hoc pooling did not generally
affect interpretation of the results. This indicates that differences in temporal change among sites, rather than the small number of degrees of freedom cause results to be inconclusive with respect to the effects of trawling. In contrast, the frequent detection of differences in temporal change between pairs of sites indicates that tests of these hypotheses were comparatively powerful in relation to the observed differences. As emphasised here and discussed elsewhere, such comparisons are confounded and can not be used to estimate effects of trawling (e.g. Hurlbert, 1984; Underwood, 1991, 1992). While there are disagreements over analytical methods, there is current consensus regarding the desirability of using multiple controls for the detection of environmental impacts in experiments or observations (e.g. Underwood, 1991, 1992, 1994; Humphrey et al., 1995; Osenberg and Schmitt, 1996). This is the first experiment, which evaluates effects of insufficient replication at these sorts of spatial and temporal scales. We conclude that our experiment could not have been interpreted in a useful way, had it only involved one trawled and on control site. The consequences to the interpretation of other experiments are potentially large but have to be assessed individually. In general, however, experimental analyses of effects of trawling that use only one trawled and one control area are very difficult to interpret unless some independent evidence is available to convincingly demonstrate that the difference between these two areas is larger than between two areas, which are not trawled. Probably, it will usually be more efficient to replicate the areas than to seek such independent information.
Acknowledgements
Earlier drafts of this manuscript were greatly improved by comments from T. Glasby, ¨
A.J. Underwood and two anonymous referees. We would like to thank S. Soderberg and J. Olofsson for excellent cooperation and assistance during the trips. We also want to thank A. Lindahl, K. Nagel, K. Ringdahl and T. Carlsson for technical assistance. This
´
study was funded by the EUs Financial Instrument for Fisheries Guidance, the Nordic
¨ ¨
Centre for Research on Ecological Impacts of Coastal Cities, University of Sydney, Australia. [AU]
References
Bender, E.A., Case, T.J., Gilpin, M.E., 1984. Perturbation experiments in community ecology: theory and practice. Ecology 65, 1–13.
Bergman, M.J.N., Hup, M., 1992. Direct effects of beam trawling on macrofauna in a sandy sediment in the southern North Sea. Ices J. Mar. Sci. 49 (1), 5–11.
Bernstein, B.B., Zalinski, J., 1983. An optimum sampling design and power tests for environmental biologists. J. Environ. Manage. 16, 335–343.
Brylinsky, M., Gibson, J., Gordon, Jr. D.C., 1994. Impacts of flounder trawls on the intertidal habitat and community of the Minas Basin, Bay of Fundy. Can. Fish. Aquat. Sci. 51 (3), 650–661.
Currie, D.R., Parry, G.D., 1996. Effects of scallop dredging on a soft sediment community: A large-scale experimental study. Mar. Ecol. Prog. Ser. 134 (1-3), 131–150.
De Groot, S.J., 1984. The impact of bottom trawling on benthic fauna of the North Sea. Ocean. Manage. 9, 177–190.
Fairweather, P.G., 1991. Statistical power and design requirements for environmental monitoring. Aust. J. Freshwater Res. 42, 555–567.
Glasby, T.M., Underwood, A.J., 1996. Sampling to differentiate between pulse and press perturbations. Env. Monit. Assess. 42 (3), 241–252.
Green, R.H., 1979. Sampling Design and Statistical Methods for Environmental Biologists, 1st Edition, John Wiley and Sons, New York.
Hall, S.J., Harding, M.J., 1997. Physical disturbance and marine benthic communities: The effects of mechanical harvesting of cockles on non-target benthic infauna. J. Appl. Ecol. 34 (2), 497–517. Hansson, M., Valentinsson, D., Ulmestrand, M., Lindahl, A., Lindegarth, M., Nilsson, H.C., Rosenberg, R.,
¨ ˚ ¨
1997. Raktralningens effekter i Gullmarsfjorden. Report to the Local Government of Goteborgs and Bohus ¨
Lan, in Swedish.
Humphrey, C.L., Faith, D.P., Dostine, P.L., 1995. Baseline requirements for assessment of mining impact using biological monitoring. Aust. J. Ecol. 20, 150–166.
Hurlbert, S.H., 1984. Pseudoreplication and the design of ecological field experiments. Ecol. Monogr. 54 (2), 187–211.
Hurlbert, S.H., White, M.D., 1993. Experiments with freshwater invertebrate zooplanktivores: quality of statistical analyses. Bull. Mar. Sci. 53 (1), 128–153.
Jones, J.B., 1992. Environmental impact of trawling on the seabed: a review. NZ J. Mar. Fresh. Res. 26, 59–67.
Kaiser, M.J., Spencer, B.E., 1996. The effects of beam-trawl disturbance on infaunal communities in different habitats. J. Anim. Ecol. 65 (3), 348–358.
Kaiser, M.J., 1998. Significance of bottom-fishing disturbance. Cons. Biol. 12 (6), 1230–1235.
Lindeboom, H.J., 1995. Protected areas in the North Sea: An absolute need for future marine research. Helgol. Meeresunters. 49 (1-4), 591–602.
Lindeboom, H.J., de Groot, S.J. 1998. The effects of different types of fisheries on the North Sea and Irish Sea benthic ecosystems. NIOZ-RAPPORT 1998-1 / RIVO-DLO REPORT C003 / 98. Den Bourg / Ijmuiden. Mapstone, B.D., 1995. Scalable decision rules for environmental impact studies: effect size, type I and type II
errors. Ecol. Appl. 5 (2), 402–410.
McArdle, B.H., Gaston, K.J., 1995. The temporal variability of densities: back to basics. OIKOS 74 (1), 165–171.
Morrisey, D.J., Underwood, A.J., Howitt, L., Stark, J.S., 1992. Temporal variation in soft-sediment benthos. J. Exp. Mar. Biol. Ecol. 164, 233–245.
M. Lindegarth et al. / J. Exp. Mar. Biol. Ecol. 245 (2000) 155 –169 169 Osenberg, C.W., Schmitt, R.J., 1996. Detecting ecological impacts caused by human activities. In: Schmitt, R.J., Osenberg, C.W. (Eds.), Detecting Ecological Impacts: Concepts and Applications in Coastal Habitats, Academic Press, San Diego, pp. 3–16.
Peterman, R.M., 1990. Statistical power analysis can improve fisheries research and management. Can. J. Fish. Aquat. Sci. 47, 2–15.
¨
Rosenberg, R., 1971. Recovery of the littoral fauna in Saltkallefjord subsequent to discontinued operations of a sulphite pulp mill. Thalass. Jugosl. 7, 341–351.
Stewart-Oaten, A., 1996. Problems in the analysis of environmental monitoring data. In: Schmitt, R.J., Osenberg, C.W. (Eds.), Detecting Ecological Impacts: Concepts and Applications in Coastal Habitats, Academic Press, San Diego, pp. 109–131.
Stewart-Oaten, A., Murdoch, W.W., Parker, K.R., 1986. Environmental impact assessment: ‘pseudoreplication’ in time? Ecology 67 (4), 929–940.
Thrush, S.F., Pridmore, R.D., Hewitt, J.E., 1994. Impacts on soft-sediment macrofauna: the effects of spatial variation on temporal trends. Ecol. Appl. 4 (1), 31–41.
Thrush, S.F., Hewitt, J.E., Cummings, V.J., Dayton, P.K., 1995. The impact of habitat disturbance by scallop dredging on marine benthic communities: What can be predicted from the results of experiments? Mar. Ecol. Prog. Ser. 129 (1-3), 141–150.
Tuck, I.D., Hall, S.J., Robertson, M.R., Armstrong, E., Basford, D.J., 1998. Effects of physical trawling disturbance in a previously unfished sheltered Scottish sea loch. Mar. Ecol. Prog. Ser. 162, 227–242. Turner, S.J., Thrush, S.F., Pridmore, R.D., Hewitt, J.E., Cummings, V.J., Maskery, M., 1995. Are soft-sediment
communities stable?. An example from a windy harbour. Mar. Ecol. Prog. Ser. 120 (1-3), 219–230. Underwood, A.J., 1991. Beyond BACI: experimental designs for detecting human environmental impacts on
temporal variations in natural populations. Aust. J. Freshwater Res. 42, 569–587.
Underwood, A.J., 1992. Beyond BACI: the detection of environmental impacts on populations in the real, but variable, world. J. Exp. Mar. Biol. Ecol. 161, 145–178.
Underwood, A.J., 1994. On beyond BACI: sampling designs that might reliably detect environmental disturbances. Ecol. Appl. 4, 3–15.
Underwood, A.J., 1996. Detection, interpretation, prediction and management of environmental disturbances: some roles for experimental marine ecology. J. Exp. Mar. Biol. Ecol. 200, 1–27.
Underwood, A.J., 1997a. Environmental decision-making and the precautionary principle: what does this principle mean in environmental sampling practice? Landsc. Urb. Plann. 37, 137–146.
Underwood, A.J., 1997b. Experiments in Ecology – Their Logical Design and Interpretation using Analysis of Variance, Cambridge University Press, Cambridge.
Walters, C.J., 1993. Dynamic models and large scale field experiments in environmental impact assessment and management. Austr. J. Ecol. 18, 53–61.